Neuropathology of Central and Peripheral Nervous System Lymphoma in Dogs and Cats: A Study of 92 Cases and Review of the Literature
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Caseload
2.2. Histopathology and Immunohistochemistry
2.3. Pathological Patterns
3. Results
3.1. Intracranial Lymphoma
3.2. Intraspinal Lymphoma
3.3. Peripheral Nervous System Lymphoma
4. Discussion
4.1. Intracranial Lymphoma
4.1.1. Intraparenchymal Patterns
4.1.2. Lymphomatosis Cerebri
4.1.3. Intravascular Lymphoma
4.1.4. Extraparenchymal Patterns
4.1.5. Leptomeningeal Lymphomatosis
4.2. Intraspinal Lymphoma
4.2.1. Extradural Lymphoma
4.2.2. Intradural-Extramedullary Lymphoma
4.2.3. Intramedullary Lymphoma
4.3. Peripheral Nervous System Lymphoma
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| I | Primary form |
| II | Secondary/multicentric form |
| C | Cervical spinal cord |
| cn | Canine |
| CNS | Central nervous system |
| CNSL | Central nervous system lymphoma |
| CP | Choroid plexus |
| CT | Cervicothoracic spinal cord |
| EAL | Extra-axial lymphoma |
| ED | Extradural |
| ELH | European Longhair cat |
| ESH | European Shorthair cat |
| F | Female |
| FL | Forelimbs |
| fn | Feline |
| HL | Hindlimbs |
| IC | Intracranial |
| ID-EM | Intradural-extramedullary |
| IM | Intramedullary |
| IP | Intraparenchymal mass |
| IPL | Intraparenchymal lymphoma |
| IVL | Intravascular lymphoma |
| L | Lumbar spinal cord |
| LC | Lymphomatosis cerebri |
| LL | Leptomeningeal lymphomatosis |
| M | Male |
| MM | Meningeal mass |
| NL | Neurolymphomatosis |
| NM | Neutered male |
| NSL | Nervous system lymphoma |
| PG | Pituitary gland |
| PNS | Peripheral nervous system |
| PNSL | Peripheral nervous system lymphoma |
| S | Sacral spinal cord |
| SC | Intraspinal |
| SCL | Intraspinal lymphoma |
| SF | Spayed female |
| T | Thoracic spinal cord |
| TL | Thoracolumbar spinal cord |
References
- Snyder, J.M.; Shofer, F.S.; Van Winkle, T.J.; Massicotte, C. Canine intracranial primary neoplasia: 173 cases (1986–2003). J. Vet. Intern. Med. 2006, 20, 669–675. [Google Scholar] [PubMed]
- Rissi, D.R.; McHale, B.J.; Miller, A.D. Primary nervous system lymphoma in cats. J. Vet. Diagn. Investig. 2022, 34, 712–717. [Google Scholar] [CrossRef]
- Snyder, J.; Lipitz, L.; Skorupski, K.; Shofer, F.; Van Winkle, T. Secondary intracranial neoplasia in the dog: 177 cases (1986–2003). J. Vet. Intern. Med. 2008, 22, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.E.; Uchida, K.; Chambers, J.K.; Kok, M.K.; Son, N.V.; Shiga, T.; Hirabayashi, M.; Ushio, N.; Nakayama, H. A retrospective survey on canine intracranial tumors between 2007 and 2017. J. Vet. Med. Sci. 2020, 82, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Troxel, M.T.; Vite, C.H.; Winkle, T.J.; Newton, A.L.; Tiches, D.; Dayrell-Hart, B.; Kapatkin, A.S.; Shofer, F.S.; Steinberg, S.A. Feline intracranial neoplasia: Retrospective review of 160 cases (1985–2001). J. Vet. Intern. Med. 2003, 17, 850–859. [Google Scholar]
- Song, R.; Vite, C.; Bradley, C.; Cross, J. Postmortem evaluation of 435 cases of intracranial neoplasia in dogs and relationship of neoplasm with breed, age, and body weight. J. Vet. Intern. Med. 2013, 27, 1143–1152. [Google Scholar] [CrossRef]
- Marioni-Henry, K.; Van Winkle, T.J.; Smith, S.H.; Vite, C.H. Tumors affecting the spinal cord of cats: 85 cases (1980–2005). J. Am. Vet. Med. Assoc. 2008, 232, 237–243. [Google Scholar] [CrossRef]
- Mandara, M.T.; Domini, A.; Giglia, G. Feline lymphoma of the nervous system. Immunophenotype and anatomical patterns in 24 cases. Front. Vet. Sci. 2022, 9, 959466. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, A.M.; March, P.A.; Fishman, C. Acute bilateral trigeminal neuropathy associated with nervous system lymphosarcoma in a dog. J. Am. Anim. Hosp. Assoc. 2000, 36, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, P.A.; Charles, J.B.; Tzipory, L.; Ficociello, J.E.; Marvel, S.J.; Barrera, J.; Spraker, T.R.; Ehrhart, E.J. Neurolymphomatosis in a dog with B-cell lymphoma. Vet. Pathol. 2012, 49, 771–774. [Google Scholar] [CrossRef]
- Rupp, A.; Ives, E.; Rudorf, H.; Constantino-Casas, F. Sciatic T-cell neurolymphomatosis in a dog. Vet. Rec. Case Rep. 2014, 2, e000050. [Google Scholar] [CrossRef]
- Ueno, H.; Miyoshi, K.; Fukui, S.; Kondo, Y.; Matsuda, K.; Uchide, T. Extranodal lymphoma with peripheral nervous system involvement in a dog. J. Vet. Med. Sci. 2014, 76, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Nakagun, S.; Horiuchi, N.; Watanabe, K.; Matsumoto, K.; Tagawa, M.; Shimbo, G.; Kobayashi, Y. CD3 and CD20 co-expression in a case of canine peripheral T-cell lymphoma with prominent cardiac and peripheral nerve involvement. J. Vet. Diagn. Investig. 2018, 30, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.B.; Kornegay, J.N.; Duncan, J.R.; Oliver, J.E., Jr. Feline spinal lymphosarcoma: A retrospective evaluation of 23 cats. J. Vet. Intern. Med. 1994, 8, 99–104. [Google Scholar] [CrossRef]
- Higgins, M.A.; Rossmeisl, J.H.; Saunders, G.K.; Hayes, S.; Kiupel, M. B-cell lymphoma in the peripheral nerves of a cat. Vet. Pathol. 2008, 45, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Linzmann, H.; Brunnberg, L.; Gruber, A.D.; Klopfleisch, R. A neurotropic lymphoma in the brachial plexus of a cat. J. Feline Med. Surg. 2009, 11, 522–524. [Google Scholar] [CrossRef]
- Bray, K.Y.; Muñana, K.R.; Meichner, K.; White, L.A.; Seiler, G. Eosinophilic meningomyelitis associated with T-cell lymphoma in a cat. Vet. Clin. Pathol. 2016, 45, 698–702. [Google Scholar] [CrossRef]
- Mello, L.S.; Leite-Filho, R.V.; Panziera, W.; Bandinelli, M.B.; Sonne, L.; Driemeier, D.; Pavarini, S.P. Feline lymphoma in the nervous system: Pathological, immunohistochemical, and etiological aspects in 16 cats. Pes. Vet. Bras. 2019, 39, 393–401. [Google Scholar] [CrossRef]
- LeCouteur, R.A. Update on feline neuromuscular diseases. J. Feline Med. Surg. 2003, 5, 109–115. [Google Scholar] [CrossRef]
- LaRue, M.K.; Taylor, A.; Back, A.R.; Lindley, S.E.; Boudreaux, B.L.; Almond, G.T.; Shores, A.; Brawner, W.R.; Smith, A.N. Central nervous system lymphoma in 18 dogs (2001 to 2015). J. Small Anim. Pract. 2018, 59, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Keenihan, E.; Schweizer, D.; Maiolini, A.; Guevar, J.; Oevermann, A.; Gutierrez-Quintana, R. Clinical and mag-netic resonance imaging features of lymphoma involving the nervous system in cats. J. Vet. Intern. Med. 2022, 36, 679–693. [Google Scholar] [CrossRef]
- Rosin, A. Neurologic diseases associated with lymphosarcoma in ten dogs. J. Am. Vet. Med. Assoc. 1982, 181, 50–53. [Google Scholar]
- Britt, J.O., Jr.; Simpson, J.G.; Howard, E.B. Malignant lymphoma of the meninges in two dogs. J. Comp. Pathol. 1984, 94, 45–53. [Google Scholar] [CrossRef]
- Vernau, K.M.; Higgins, R.J.; Bollen, A.W.; Jimenez, D.F.; Anderson, J.V.; Koblik, P.D.; Lecouteur, R.A. Primary canine and feline nervous system tumors: Intraoperative diagnosis using the smear technique. Vet. Pathol. 2001, 38, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-H.; Ciesielski, T.; Kim, J.H.; Yhee, J.-Y.; Im, K.-S.; Nam, H.-M.; Kim, I.-H.; Kim, J.-H.; Sur, J.-H. Primary central nervous system B-cell lymphoma in a young dog. Am. Jew. Hist. 2012, 53, 559–564. [Google Scholar]
- Sisó, S.; Marco-Salazar, P.; Moore, P.F.; Sturges, B.K.; Vernau, W.; Wisner, E.R.; Bollen, A.W.; Dickinson, P.J.; Higgins, R.J. Canine nervous system lymphoma subtypes display characteristic neuroanatomical patterns. Vet. Pathol. 2016, 54, 53–60. [Google Scholar] [CrossRef]
- Lampe, R.; Levitin, H.A.; Hecht, S.; Vieson, M.D.; Selting, K.A.; Hague, D.W.; Foss, K.D. MRI of CNS lymphoma with choroid plexus involvement in five dogs and one cat. J. Small Anim. Pract. 2021, 62, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Czasch, S.; Risse, K.; Baumgärtner, W. Central nervous system metastasis of a cutaneous epitheliotropic lymphosarcoma in a dog. J. Comp. Pathol. 2000, 123, 59–63. [Google Scholar] [CrossRef]
- Kent, M.; Delahunta, A.; Tidwell, A.S. MR imaging findings in a dog with intravascular lymphoma in the brain. Vet. Radiol. Ultrasound 2001, 42, 504–510. [Google Scholar] [CrossRef]
- Long, S.N.; Johnston, P.E.J.; Anderson, T.J. Primary T-cell lymphoma of the central nervous system in a dog. J. Am. Vet. Med. Assoc. 2001, 218, 719–722. [Google Scholar] [CrossRef]
- Thomovsky, S.A.; Packer, R.A.; Burcham, G.N.; Heng, H.G. Imaging diagnosis-magnetic resonance imaging features of met-astatic cerebral lymphoma in a dog. Vet. Radiol. Ultrasound. 2011, 52, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.; Castillo-Alcala, F. Hind-limb paresis in a dog with paralumbar solitary T-cell lymphoma. Am. Jew. Hist. 2010, 51, 480–484. [Google Scholar]
- Veraa, S.; Dijkman, R.; Meij, B.P.; Voorhout, G. Comparative imaging of spinal extradural lymphoma in a Bordeaux dog. Am. Jew. Hist. 2010, 51, 519–521. [Google Scholar]
- Mattei, C.; Oevermann, A.; Schweizer, D.; Guevar, J.; Maddox, T.W.; Fleming, K.L.; Ricci, E.; Rosati, M.; Biserni, R.; Iv, J.F.G.; et al. MRI ischemic and hemorrhagic lesions in arterial and venous territories characterize central nervous system intravascular lymphoma in dogs. Vet. Radiol. Ultrasound 2022. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Fales-Williams, A. Hypernatremia associated with intracranial B-cell lymphoma in a cat. Vet. Clin. Pathol. 2006, 35, 362–365. [Google Scholar] [CrossRef]
- Nakamoto, Y.; Ozawa, T.; Uchida, K.; Omori, K.; Hase, K.; Nakaichi, M. Primary Intra-Axial B-Cell Lymphoma in a Cat. J. Vet. Med. Sci. 2009, 71, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.J.; Mansfield, C.S.; Milne, M.E.; Hodge, P.J. Central diabetes insipidus in a cat with central nervous system B cell lymphoma. J. Feline Med. Surg. 2011, 13, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Akiyoshi, H.; Shimazaki, H.; Kawakami, R.; Mie, K.; Yamada, Y.; Ohashi, F. Apparent diffusion coefficient value for a B-cell central nervous system lymphoma in a cat. J. Feline Med. Surg. Open Rep. 2018, 4, 2055116917750762. [Google Scholar] [CrossRef]
- Leite-Filho, R.V.; Panziera, W.; Bandinelli, M.B.; Henker, L.C.; Monteiro, K.C.; Corbellini, L.G.; Driemeier, D.; Sonne, L.; Pavarini, S.P. Epidemiological, pathological and immunohistochemical aspects of 125 cases of feline lymphoma in Southern Brazil. Vet. Comp. Oncol. 2019, 18, 224–230. [Google Scholar] [CrossRef]
- Pardo, A.P.; Gómez, M.A.; González, C.M. Cerebellar cortical degeneration associated with feline leukemia virus infection and cerebellar lymphoma in a young cat. Open Vet. J. 2019, 9, 246–252. [Google Scholar] [CrossRef]
- Fondevila, D.; Vilafranca, M.; Pumarola, M. Primary central nervous system T-cell lymphoma in a cat. Vet. Pathol. 1998, 35, 550–553. [Google Scholar] [CrossRef]
- Morita, T.; Kondo, H.; Okamoto, M.; Park, C.; Sawashima, Y.; Shimada, A. Periventricular spread of primary central nervous system T-cell lymphoma in a cat. J. Comp. Pathol. 2008, 140, 54–58. [Google Scholar] [CrossRef]
- Tsuboi, M.; Uchida, K.; Park, E.S.; Kotera, Y.; Seki, T.; Takahashi, M.; Nakayama, H. Systemic T-cell large granular lymphocyte lymphoma with multifocal white matter degeneration in the brain of a Japanese domestic cat. J. Vet. Med. Sci. 2010, 72, 795–799. [Google Scholar] [CrossRef]
- Valli, V.E.; Myint, M.S.; Barthel, A.; Bienzle, D.; Caswell, J.; Colbatzky, F.; Durham, A.; Ehrhart, E.J.; Johnson, Y.; Jones, C.; et al. Classification of canine malignant lymphomas according to the World Health Organization criteria. Vet. Pathol. 2010, 48, 198–211. [Google Scholar] [CrossRef]
- Degl’Innocenti, S.; Della Camera, N.; Falzone, C.; Cantile, C. Canine cerebral intravascular lymphoma: Neuropathological and immunohistochemical findings. Vet. Pathol. 2018, 56, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Callanan, J.; Jones, B.A.; Irvine, J.; Willett, B.J.; McCandlish, I.A.P.; Jarrett, O. Histologic classification and immunophenotype of lymphosarcomas in cats with naturally and experimentally acquired feline immunodeficiency virus infections. Vet. Pathol. 1996, 33, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Tomek, A.; Cizinauskas, S.; Doherr, M.; Gandini, G.; Jaggy, A. Intracranial neoplasia in 61 cats: Localisation, tumour types and seizure patterns. J. Feline Med. Surg. 2006, 8, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Palus, V.; Volk, H.A.; Lamb, C.R.; Targett, M.P.; Cherubini, G.B. MRI features of cns lymphoma in dogs and cats. Vet. Radiol. Ultrasound 2011, 53, 44–49. [Google Scholar] [CrossRef]
- Mandara, M.T.; Motta, L.; Calò, P. Distribution of feline lymphoma in the central and peripheral nervous systems. Vet. J. 2016, 216, 109–116. [Google Scholar] [CrossRef]
- Yoshino, Y.; Chambers, J.K.; Nakamori, T.; Goto-Koshino, Y.; Nishigaki, K.; Tsujimoto, H.; Matsuki, N.; Nakayama, H.; Uchida, K. Primary cerebellar lymphoma with Hodgkin lymphoma–like morphology in a cat. J. Vet. Diagn. Investig. 2017, 29, 707–710. [Google Scholar] [CrossRef]
- Vail, D.M.; Withrow, S.; Page, R. Feline lymphoma and leukemia. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Vail, D.M., Ed.; Saunders Elsevier: St. Louis, MO, USA, 2020; pp. 715–730. [Google Scholar]
- Yu, H.; Gao, B.; Liu, J.; Yu, Y.-C.; Shiroishi, M.S.; Huang, M.-M.; Yang, W.-X.; Guan, Z.-Z. Lymphomatosis cerebri: A rare variant of primary central nervous system lymphoma and MR imaging features. Cancer Imaging 2017, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.E.; Kleinschmidt-DeMasters, B.; Corboy, J.R.; Damek, D.M.; Filley, C.M. Lymphomatosis cerebri as a cause of white matter dementia. Hum. Pathol. 2005, 36, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Luna, S.G.; Carrasco, L.; Gómez-Laguna, J.; Hilbe, M.; Mínguez, J.J.; Köhler, K.; Mulas, J.M.D.L. Primary central nervous system T-cell lymphoma mimicking meningoencephalomyelitis in a cat. Can. Vet. J. 2013, 54, 602–605. [Google Scholar]
- Bakshi, R.; Mazziotta, J.C.; Mischel, P.S.; Jahan, R.; Seligson, D.B.; Vinters, H.V. Lymphomatosis cerebri presenting as a rapidly progressive dementia: Clinical, neuroimaging and pathologic findings. Dement. Geriatr. Cogn. Disord. 1999, 10, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, C.; Velasco, R.; Vidal, N.; Sánchez, J.J.; Argyriou, A.A.; Besora, S.; Graus, F.; Bruna, J. Lymphomatosis cerebri: A rare form of primary central nervous system lymphoma. Analysis of 7 cases and systematic review of the literature. Neuro Oncol. 2015, 18, 707–715. [Google Scholar] [CrossRef]
- Pfleger, L.; Tappeiner, J. Zur Kenntnis der systemisierten Endotheliomastose der cutanen Blutgefdsse. Hautzarzt 1959, 10, 359–363. [Google Scholar]
- Ponzoni, M.; Campo, E.; Nakamura, S. Intravascular large B-cell lymphoma: A chameleon with multiple faces and many masks. Blood 2018, 132, 1561–1567. [Google Scholar] [CrossRef]
- McDonough, S.P.; Van Winkle, T.J.; Valentine, B.A.; van Gessel, Y.A.; Summers, B.A. Clinicopathological and immunophenotypical features of canine intravascular lymphoma (malignant angioendotheliomatosis). J. Comp. Pathol. 2002, 126, 277–288. [Google Scholar] [CrossRef]
- Ródenas, S.; Pumarola, M.; Gaitero, L.; Zamora, À.; Añor, S. Magnetic resonance imaging findings in 40 dogs with histologically confirmed intracranial tumours. Vet. J. 2011, 187, 85–91. [Google Scholar] [CrossRef]
- Henrich, M.; Huisinga, M.; Bauer, N.; Reinacher, M. A case of intravascular lymphoma with mixed lineage antigen expression in a cat. J. Vet. Med. Ser. A 2007, 54, 575–578. [Google Scholar] [CrossRef]
- Guillén, A.; Rossanese, M.; Ricci, E.; German, A.J.; Blackwood, L. Gastric intravascular lymphoma in a dog: Case report and literature review. J. Am. Anim. Hosp. Assoc. 2020, 56, 185. [Google Scholar] [CrossRef] [PubMed]
- Bush, W.W.; Throop, J.L.; McManus, P.M.; Kapatkin, A.S.; Vite, C.H.; Van Winkle, T.J. Intravascular lymphoma involving the central and peripheral nervous systems in a dog. J. Am. Anim. Hosp. Assoc. 2003, 39, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, H. Multisystem angiotropic lymphoma (malignant angioendotheliomatosis) involving the humerus in a dog. J. Vet. Diagn. Investig. 1996, 8, 502–505. [Google Scholar] [CrossRef]
- Lane, L.V.; Allison, R.W.; Rizzi, T.R.; Stern, A.W.; Snider, T.; Moore, P.F.; Vernau, W. Canine intravascular lymphoma with overt leukemia. Vet. Clin. Pathol. 2012, 41, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Zanoguera, V.L.; Motta, L. Multicentric intravascular lymphoma with particular involvement of the cervical spinal cord in a dog. J. Small Anim. Pract. 2022, 63, 82. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, J.M.; Higgins, R.J.; Kortz, G.D.; Bailey, C.S.; Moore, P.F. Intravascular malignant T-cell lymphoma (malignant angi-oendotheliomatosis) in a cat. Vet. Pathol. 1997, 34, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Zaki, F.A.; Hurvitz, A.I. Spontaneous neoplasms of the central nervous system of the cat. J. Small Anim. Pract. 1976, 17, 773–782. [Google Scholar] [CrossRef]
- Pavlou, G.; Pal, D.; Bucur, S.; Chakrabarty, A.; Van Hille, P.T. Intracranial Non-Hodgkin’s MALT lymphoma mimicking a large convexity meningioma. Acta Neurochir. 2006, 148, 791–793. [Google Scholar] [CrossRef]
- Matmati, K.S.; Matmati, N.; Hannun, Y.A.; Rumboldt, Z.; Patel, S.; Lazarchick, J.; Stuart, R.; Giglio, P. Dural MALT lymphoma with disseminated disease. Hematol. Rep. 2010, 2, e10. [Google Scholar] [CrossRef]
- Green, K.; Hogg, J.P. Central Nervous System Lymphoma. In StatPearls [Internet]. Treasure Island (FL); StatPearls Publishing: Tampa, Fl, USA, 2022. [Google Scholar]
- Mandara, M.T.; Rossi, F.; Lepri, E.; Angeli, G. Cerebellar leptomeningeal carcinomatosis in a dog. J. Small Anim. Pract. 2007, 48, 504–507. [Google Scholar] [CrossRef]
- Giordano, C.; Giudice, C.; Bellino, C.; Borrelli, A.; D’Angelo, A.; Gianella, P. A case of oculo-cerebral B-cell lymphoma in a cat. Vet. Ophthalmol. 2012, 16, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Grossman, S.A.; Krabak, M.J. Leptomeningeal carcinomatosis. Cancer Treat. Rev. 1999, 25, 103–119. [Google Scholar] [CrossRef]
- Dallman, M.J.; Saunders, G.K. Primary spinal cord lymphosarcoma in a dog. J. Am. Vet. Med. Assoc. 1986, 189, 1348–1349. [Google Scholar]
- Waters, D.J.; Hayden, D.W. Intramedullary spinal cord metastasis in the dog. J. Vet. Intern. Med. 1990, 4, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Spodnick, G.J.; Berg, J.; Moore, F.M.; Cotter, S.M. Spinal lymphoma in cats: 21 cases (1976–1989). J. Am. Vet. Med. Assoc. 1992, 200, 373–376. [Google Scholar]
- Bagley, R.S.; Wheeler, S.J.; Klopp, L.; Sorjonen, D.C.; Thomas, W.B.; Wilkens, B.E.; Gavin, P.R.; Dennis, R. Clinical features of trigeminal nerve-sheath tumor in 10 dogs. J. Am. Anim. Hosp. Assoc. 1998, 34, 19–25. [Google Scholar] [CrossRef]
- Auger, M.; Hecht, S.; Springer, C.M. Magnetic resonance imaging features of extradural spinal neoplasia in 60 dogs and seven cats. Front. Vet. Sci. 2021, 7, 610490. [Google Scholar] [CrossRef]
- Turner, J.L.; Luttgen, P.J.; VanGundy, T.E.; Roenigk, W.J.; Hightower, D.; Frelier, P.F. Multicentric osseous lymphoma with spinal extradural involvement in a dog. J. Am. Vet. Med. Assoc. 1992, 200, 196–198. [Google Scholar]
- Lamagna, B.; Lamagna, F.; Meomartino, L.; Paciello, O.; Fatone, G. Polyostotic lymphoma with vertebral involvement and spinal extradural compression in a dog. J. Am. Anim. Hosp. Assoc. 2006, 42, 71–76. [Google Scholar] [CrossRef]
- Cho, J.-H.; Cho, D.-C.; Sung, J.-K.; Kim, K.-T. Primary malignant lymphoma in a spinal cord presenting as an epidural mass with myelopathy: A case report. Korean J. Spine 2012, 9, 265–268. [Google Scholar] [CrossRef]
- Maccauro, G.; Spinelli, M.S.; Mauro, S.; Perisano, C.; Graci, C.; Rosa, M.A. Physiopathology of Spine Metastasis. Int. J. Surg. Oncol. 2011, 2011, 107969. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.A. The pathological features associated with spinal tumours in 29 dogs. J. Comp. Pathol. 1985, 95, 549–557. [Google Scholar] [CrossRef]
- Kippenes, H.; Gavin, P.R.; Bagley, R.S.; Silver, G.M.; Tucker, R.L.; Sande, R.D. Magnetic resonance imaging features of tumors of the spine and spinal cord in dogs. Vet. Radiol. Ultrasound 1999, 40, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Allett, B.; Hecht, S. Magnetic resonance imaging findings in the spine of six dogs diagnosed with lymphoma. Vet. Radiol. Ultrasound 2016, 57, 154–161. [Google Scholar] [CrossRef]
- Marioni-Henry, K.; Vite, C.H.; Newton, A.L.; Van Winkle, T.J. Prevalence of diseases of the spinal cord of cats. J. Vet. Intern. Med. 2005, 18, 851–858. [Google Scholar] [CrossRef]
- Bradshaw, J.; Pearson, G.; Gruffydd-Jones, T. A retrospective study of 286 cases of neurological disorders of the cat. J. Comp. Pathol. 2004, 131, 112–120. [Google Scholar] [CrossRef]
- Northington, J.W.; Juliana, M.M. Extradural lymphosarcoma in six cats. J. Small Anim. Pract. 1978, 19, 409–416. [Google Scholar] [CrossRef]
- Flatland, B.; Fry, M.M.; Newman, S.J.; Moore, P.F.; Smith, J.R.; Thomas, W.B.; Casimir, R.H. Large anaplastic spinal B-cell lymphoma in a cat. Vet. Clin. Pathol. 2008, 37, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Laisse, C.J.M.; Oliveira, E.C.; Rolim, V.M.; Negreiros, D.O.; Driemeier, D.; Pavarini, S.P. Hemorrhagic myelomalacia in a cat with extradural T-cell lymphoma. Semin. Ciências Agrárias 2017, 38, 327–334. [Google Scholar] [CrossRef]
- De Lahunta, A.; Glass, E. Small animal spinal cord diseases. In Veterinary Neuroanatomy and Clinical Neurology, 3rd ed.; Saunders, W.B., Ed.; Elsevier: St Louis, MO, USA, 2009; pp. 243–284. [Google Scholar]
- Vail, D.M.; Pinkerton, M.; Young, K.M. Canine lymphoma and lymphocytic leukemia. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; Vail, D.M., Ed.; Saunders Elsevier: St. Louis, MO, USA, 2020; pp. 688–715. [Google Scholar]
- Brehm, D.M.; Vite, C.H.; Steinberg, H.S.; Haviland, J.; Van Winkle, T. A retrospective evaluation of 51 cases of peripheral nerve sheath tumors in the dog. J. Am. Anim. Hosp. Assoc. 1995, 31, 349–359. [Google Scholar] [CrossRef]
- Chijiwa, K.; Uchida, K.; Tateyama, S. Immunohistochemical evaluation of canine peripheral nerve sheath tumors and other soft tissue sarcomas. Vet. Pathol. 2004, 41, 307–318. [Google Scholar] [CrossRef]
- Schulman, F.Y.; Johnson, T.O.; Facemire, P.R.; Fanburg-Smith, J.C. Feline peripheral nerve sheath tumors: Histologic, im-munohistochemical, and clinicopathologic correlation (59 tumors in 53 cats). Vet. Pathol. 2009, 46, 1166–1180. [Google Scholar] [CrossRef] [PubMed]
- Luttgen, P.J.; Braund, K.G.; Brawner, W.R.; Vandevelde, M. A retrospective study of twenty-nine spinal tumours in the dog and cat. J. Small Anim. Pract. 1980, 21, 213–226. [Google Scholar] [CrossRef]
- Fankhauser, R.; Vandevelde, M. Zur klinik der tumoren des Nervensystems bei Hund und Katze. Schweiz Arch Tierheilkd 1981, 123, 553. [Google Scholar]
- Bradley, R.L.; Withrow, S.J.; Snyder, S.P. Nerve sheath tumors in the dog. J. Am. Anim. Hosp. Assoc. 1982, 18, 915. [Google Scholar]
- Fox, J.G.; Gutnick, M.J. Horner’s syndrome and brachial paralysis due to lymphosarcoma in a cat. J. Am. Vet. Med. Assoc. 1972, 160, 977–980. [Google Scholar]
- Allen, J.G.; Amis, T. Lymphosarcoma involving cranial nerves in a cat. Aust. Vet. J. 1975, 51, 155–158. [Google Scholar] [CrossRef]
- Hughes, R.A.C.; Britton, T.; Richards, M. Effects of lymphoma on the peripheral nervous system. J. R. Soc. Med. 1994, 87, 526–530. [Google Scholar] [CrossRef]
- Mandrioli, L.; Morini, M.; Biserni, R.; Gentilini, F.; Turba, M.E. A case of feline neurolymphomatosis: Pathological and molecular investigations. J. Vet. Diagn. Investig. 2012, 24, 1083–1086. [Google Scholar] [CrossRef]
- Del Grande, A.; Sabatelli, M.; Luigetti, M.; Conte, A.; Granata, G.; Rufini, V.; Del Ciello, A.; Gaudino, S.; Fernández, E.; Hohaus, S.; et al. Primary multifocal lymphoma of peripheral nervous system: Case report and review of the literature. Muscle Nerve 2014, 50, 1016–1022. [Google Scholar] [CrossRef]
- Mori, M.; Izawa, T.; Sasaki, H.; Sonoyama, J.; Nishimura, S.; Shimamura, S.; Shimada, T.; Hasegawa, T.; Kuwamura, M.; Yamate, J. A case of feline T-cell lymphoma with tropism for striated muscle and peripheral nerve. J. Comp. Pathol. 2019, 168, 8–12. [Google Scholar] [CrossRef]
- Henke, D.; Vandevelde, M.; Oevermann, A. Polyganglioradiculoneuritis in a young cat: Clinical and histopathological findings. J. Small Anim. Pract. 2009, 50, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Mellanby, R.J.; Jeffery, N.D.; Baines, E.A.; Woodger, N.; Herrtage, M.E. Magnetic resonance imaging in the diagnosis of lym-phoma involving the brachial plexus in a cat. Vet. Radiol. Ultrasound 2003, 44, 522–525. [Google Scholar] [CrossRef]
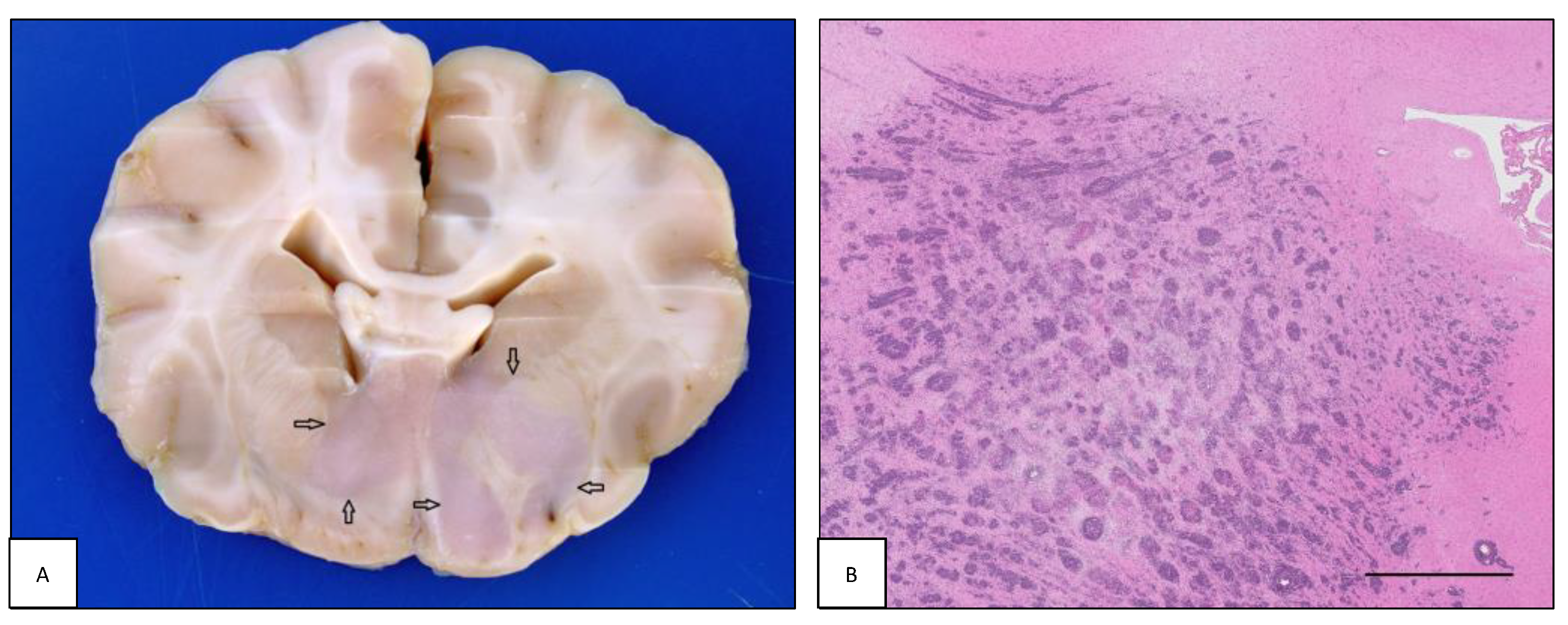


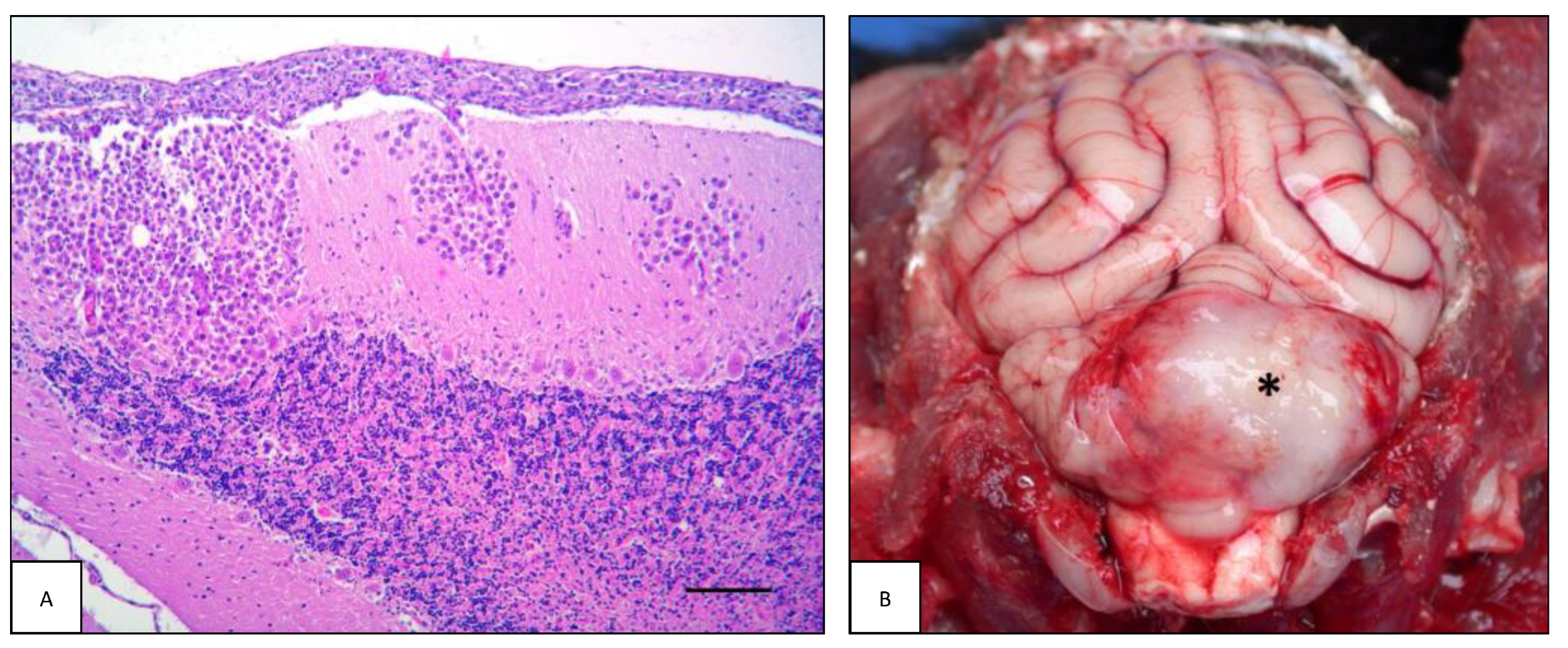
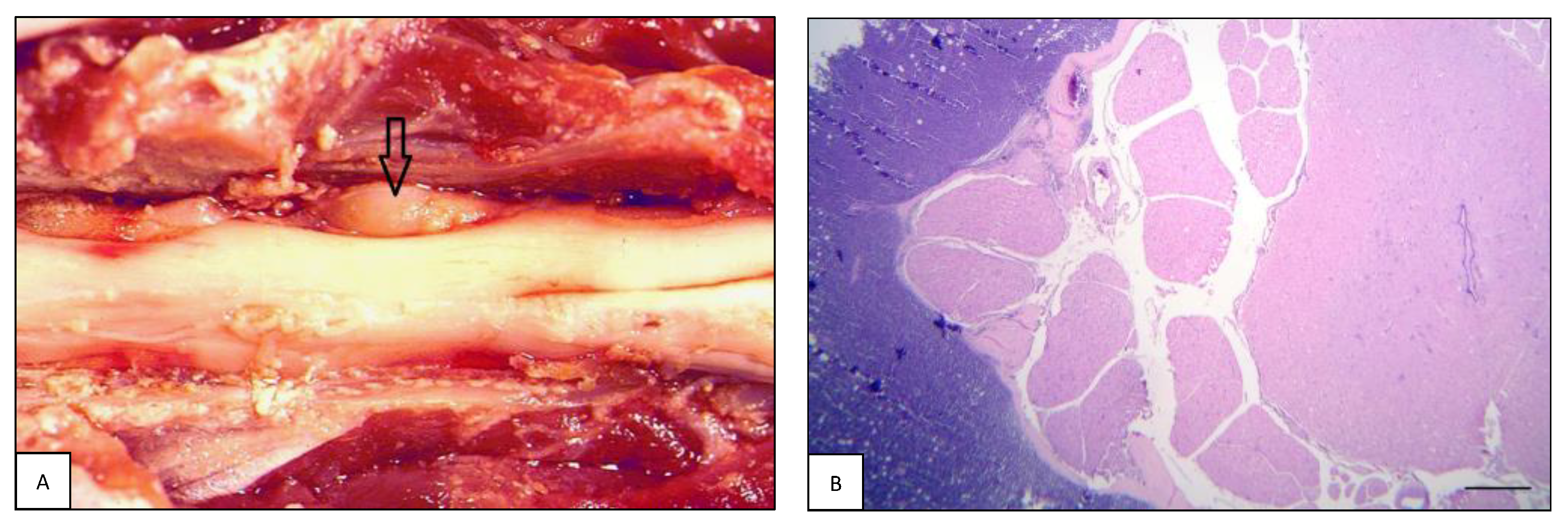

| Case# | Species | Breed | Sex | Age | Neurological Signs | Tissue Origin |
|---|---|---|---|---|---|---|
| 1 | cn | Border Collie | F | 2 y | no menace response, nystagmus, trigeminal paralysis | necropsy |
| 2 | cn | Mixed breed | M | 10 m | paraparesis, back pain | biopsy |
| 3 | cn | German Shepherd | F | 8 y | acute paraparesis | necropsy |
| 4 | cn | Cane Corso | SF | 5 y | ataxia, depression, strabismus, left hemiparesis | necropsy |
| 5 | cn | Great Dane | M | 2.5 y | monoparesis, cauda equina syndrome | necropsy |
| 6 | cn | Mixed breed | F | 6 y | monoparesis | biopsy |
| 7 | cn | Cane Corso | M | 1 y | paraparesis | biopsy |
| 8 | cn | Epagneul Breton | M | 2 y | absent menace response, mydriasis, ataxia, head tilt | necropsy |
| 9 | cn | Hound | F | 7 y | progressive weakness, ataxia, ascending paresis | necropsy |
| 10 | cn | Golden Retriever | M | 7 y | multifocal intracranial syndrome | necropsy |
| 11 | cn | Shih-tzu | M | 8 y | ataxia, tetraparesis, no menace response, absence of pupillary light reflex | necropsy |
| 12 | cn | Bulldog | M | 4 y | right HL monoplegia | biopsy |
| 13 | cn | Mixed breed | SF | 7 y | acute and progressive paraparesis | biopsy |
| 14 | cn | West Highland White Terrier | M | 4 y | forebrain syndrome, blindness | necropsy |
| 15 | cn | Labrador Retriever | NM | 12 y | paraplegia | necropsy |
| 16 | cn | Labrador Retriever | M | 6 y | tetraparesis | necropsy |
| 17 | cn | Yorkshire Terrier | M | 7 y | paraparesis | necropsy |
| 18 | cn | Border Collie | SF | 13 y | right head tilt, brainstem syndrome | necropsy |
| 19 | cn | Beagle | M | 7 y | acute paraparesis | necropsy |
| 20 | cn | Mixed breed | M | 10 y | cervical pain, tetraparesis | necropsy |
| 21 | cn | Staffordshire Terrier | F | 6.5 y | ↓ menace response, masticatory muscles atrophy, ↓ facial sensitivity | necropsy |
| 22 | cn | Labrador Retriever | F | 3 y | left HL monoplegia, right HL monoparesis | necropsy |
| 23 | cn | Mixed breed | M | 4.5 y | paraparesis | biopsy |
| 24 | cn | Rottweiler | M | 2 y | depression, ataxia, no menace response, anisocoria, ↓ facial sensitivity | necropsy |
| 25 | cn | Labrador Retriever | M | 7 y | forebrain syndrome | necropsy |
| 26 | cn | Bergamasco Shepherd | M | 7 y | paraparesis, severe depression | necropsy |
| 27 | cn | Boxer | F | 3.5 y | forebrain syndrome | necropsy |
| 28 | cn | English Cocker Spaniel | M | 11 y | forebrain syndrome | necropsy |
| 29 | cn | Boxer | F | 4 y | intracranial multifocal syndrome, seizures | necropsy |
| 30 | cn | Pointer | M | 13 y | paraparesis, depression | necropsy |
| 31 | cn | German Shepherd | F | 9 y | tetraparesis, depression | necropsy |
| 32 | cn | Mixed breed | SF | 12 y | right hemiparesis | necropsy |
| 33 | cn | Dachshund | F | 2.5 y | depression, seizures | necropsy |
| 34 | cn | Boxer | F | 10.5 y | seizures | necropsy |
| 35 | cn | Mixed breed | F | 4 y | tetraplegia | necropsy |
| 36 | cn | Mixed breed | M | 14 y | left HL monoparesis | necropsy |
| 37 | cn | Mixed breed | M | 9 y | tetraparesis | necropsy |
| 38 | cn | German Shepherd | F | 3 y | paraparesis | necropsy |
| 39 | cn | Hound | F | 2 y | severe depression, tetraparesis | necropsy |
| 40 | cn | Boxer | F | 7 y | neck pain, intracranial syndrome | necropsy |
| 41 | cn | Mixed breed | M | 14 y | central vestibular syndrome | necropsy |
| 42 | cn | Amstaff | M | 3 y | right HL progressive lameness | biopsy |
| 43 | cn | Cane Corso | F | 7 y | monoplegia | biopsy |
| 44 | cn | Rottweiler | F | 4.5 y | right HL monoparesis | biopsy |
| 45 | cn | Labrador Retriever | M | 10 y | tetraparesis, paraplegia | biopsy |
| 46 | fn | ESH | F | 9 y | bilateral blindness, depression, seizures, | necropsy |
| 47 | fn | ESH | F | 5 y | ataxia, left HL monoparesis, strangury | biopsy |
| 48 | fn | Persian | M | 11 y | seizures, ataxia, blindness | necropsy |
| 49 | fn | ESH | M | 6 y | progressive paraparesis, ataxia | necropsy |
| 50 | fn | ESH | F | 14 y | ataxia, depression | necropsy |
| 51 | fn | ESH | M | 9 y | ataxia, depression, vertical nystagmus | biopsy |
| 52 | fn | ESH | NM | 14 y | depression, compulsive gait | necropsy |
| 53 | fn | ESH | M | 11 y | paraplegia | necropsy |
| 54 | fn | ESH | M | 8 y | forebrain syndrome | biopsy |
| 55 | fn | ESH | NM | 17 y | seizures, depression, tetraparesis, bilateral mydriasis | necropsy |
| 56 | fn | ESH | M | 8 y | left FL monoplegia and muscle atrophy | necropsy |
| 57 | fn | ESH | SF | 5 y | paraplegia | biopsy |
| 58 | fn | ESH | F | 12 y | forebrain syndrome | necropsy |
| 59 | fn | ESH | M | 3 y | paraplegia | necropsy |
| 60 | fn | ESH | M | 1 y | back pain, paraparesis | biopsy |
| 61 | fn | ESH | F | 10 y | prosencephalic syndrome | necropsy |
| 62 | fn | ESH | NM | 11 y | depression, right drifting, compulsive gait | biopsy |
| 63 | fn | ESH | NM | 12 y | compulsive gait, left circling | necropsy |
| 64 | fn | ESH | NM | 4 y | seizures | necropsy |
| 65 | fn | ESH | SF | 12 y | progressive tetraparesis | necropsy |
| 66 | fn | ESH | M | 11 y | left blindness, forebrain syndrome | necropsy |
| 67 | fn | ESH | M | 4 y | paraparesis | necropsy |
| 68 | fn | ESH | SF | 5 y | progressive paraparesis | biopsy |
| 69 | fn | ESH | SF | 15 y | blindness, forebrain syndrome | necropsy |
| 70 | fn | ESH | SF | 8 y | ataxia, behavioral changes, mydriasis, left pleurothotonus | necropsy |
| 71 | fn | ESH | NM | 9 y | brainstem syndrome | necropsy |
| 72 | fn | ESH | SF | 4 y | paraplegia | biopsy |
| 73 | fn | ESH | NM | 1 y | paraplegia, loss of deep pain sensation | biopsy |
| 74 | fn | Ragdoll | F | 3 m | seizures | necropsy |
| 75 | fn | ESH | SF | 3 y | paraplegia | necropsy |
| 76 | fn | ESH | M | 10 y | paraplegia, back pain | necropsy |
| 77 | fn | Maine Coon | F | 13 y | depression, paraparesis | necropsy |
| 78 | fn | ESH | SF | 8 m | paraparesis | biopsy |
| 79 | fn | ESH | M | 8 m | paraplegia, loss of deep pain sensation | necropsy |
| 80 | fn | Norwegian Forest cat | SF | 11 y | depression, weakness, ↓ left menace response and left pupillary light reflex | necropsy |
| 81 | fn | ESH | F | 12.5 y | left circling, anisocoria, left miosis, right FL monoparesis | necropsy |
| 82 | fn | Carthusian | M | 6 y | progressive paraparesis | necropsy |
| 83 | fn | ESH | SF | 10 y | paraparesis | necropsy |
| 84 | fn | ESH | F | 2 y | acute paraplegia | necropsy |
| 85 | fn | ESH | SF | 11 y | tetraparesis | necropsy |
| 86 | fn | Siamese | SF | 7 y | paraparesis, hyperesthesia, ↓ pain sensation, ataxia, urinary loss | necropsy |
| 87 | fn | ESH | F | 7 y | severe paraparesis | biopsy |
| 88 | fn | ESH | M | 14 y | right hemiparesis | necropsy |
| 89 | fn | ESH | SF | 7 y | acute tetraplegia, dysphagia, right Horner syndrome | necropsy |
| 90 | fn | ESH | M | 14 y | progressive tetraparesis | biopsy |
| 91 | fn | ELH | SF | 12 y | right HL monoplegia | biopsy |
| 92 | fn | ESH | SF | 11 y | lameness and monoparesis | biopsy |
| N° | Species | Type | Anatomical Site | Location and Distribution | Pathological Pattern | Nuclear Size | Grading | Phenotype |
|---|---|---|---|---|---|---|---|---|
| 1 | cn | II | IC | occipital lobe | IP | large | medium | B |
| 2 | cn | I | SC | T10-T13 | ED | intermed. | low | B |
| 3 | cn | II | SC | L3-L5 | ED | large | high | B |
| 4 | cn | II | IC | occipito-temporal lobe | IP, MM | large | high | T |
| 5 | cn | II | SC, PNS | cauda equina, sciatic nerve | IM, NL | small | low | B |
| 6 | cn | I | PNS | L6 nerve root | NL | large | high | B |
| 7 | cn | I | SC | T9 | ED | large | high | B |
| 8 | cn | II | IC | occipital lobe | IP | large | high | non-B, non-T |
| 9 | cn | II | SC, PNS | LS, sciatic nerves | IM, NL | large | high | T |
| 10 | cn | I | IC, PNS | parieto-occipital lobes, V nerve root | IP, NL | large | high | B |
| 11 | cn | II | IC | basal nuclei | IP | large | high | T |
| 12 | cn | II | PNS | ischiatic nerve | NL | large | high | B |
| 13 | cn | II | PNS | L1 nerve root | NL | large | high | B |
| 14 | cn | II | IC | occipital lobe | IP | large | high | B |
| 15 | cn | II | SC | L | ED | large | high | B |
| 16 | cn | II | SC | C3-C6 | ED | large | medium | B |
| 17 | cn | II | SC | T11-T13 | ED | small | low | T |
| 18 | cn | II | IC | thalamus, cerebellum, m. obl., CP, PG | IP, MM | large | high | B |
| 19 | cn | I | SC | T6-T7 | IM | large | high | non-B, non-T |
| 20 | cn | I | SC | C3-C4 | IM | intermed. | low | B |
| 21 | cn | I | IC, PNS | trigeminal nerves, CP | NL | intermed. | low | B |
| 22 | cn | I | SC | L4-L7 | ED | large | high | B |
| 23 | cn | II | SC | T12-L1 | ED | large | high | B |
| 24 | cn | I | IC | thalamus | IP | large | high | B |
| 25 | cn | I | IC | multifocal | IVL | large | ND | B |
| 26 | cn | I | IC | multifocal | IVL | large | ND | non-B, non-T |
| 27 | cn | I | IC | multifocal | IVL | large | ND | T |
| 28 | cn | I | IC | multifocal, CP | IVL | large | ND | non-B, non-T |
| 29 | cn | I | IC | multifocal | IVL | large | ND | B |
| 30 | cn | I | IC | multifocal, CP | IVL | large | ND | B |
| 31 | cn | I | IC | multifocal | IVL | large | ND | T |
| 32 | cn | II | IC | multifocal | IVL | large | ND | non-B, non-T |
| 33 | cn | I | IC | multifocal | IVL | large | ND | T |
| 34 | cn | I | IC | multifocal | IVL | large | ND | non-B, non-T |
| 35 | cn | II | SC | C, CT, TL, LS | ID-EM, LL | small | medium | B |
| 36 | cn | I | PNS | L femoral nerve | NL | large | high | T |
| 37 | cn | II | PNS | multiple spinal nerves | NL | intermed. | low | T |
| 38 | cn | II | SC | T | ED | small | medium | T |
| 39 | cn | I | IC | diffuse | LC | intermed. | medium | T |
| 40 | cn | II | IC | frontal, temporal, cerebellar lobes | LL | large | high | T |
| 41 | cn | II | IC | ponto-cerebellar angle | IP | large | high | B |
| 42 | cn | I | PNS | L5 nerve root | NL | large | low | B |
| 43 | cn | I | PNS | sciatic nerve | NL | large | low | T |
| 44 | cn | I | PNS | sciatic nerve | NL | large | medium | T |
| 45 | cn | I | PNS | sciatic nerves | NL | small | low | T |
| 46 | fn | I | IC | optic nerves, diencephalon | IP | intermed. | medium | B |
| 47 | fn | I | SC | L4-L5 | ED | intermed. | low | B |
| 48 | fn | II | IC | parietal lobe | IP | intermed. | medium | T |
| 49 | fn | II | IC, SC | cerebellar, T | LL, ED | large | medium | B |
| 50 | fn | I | IC | frontal lobe | IP, MM | large | high | non-B, non-T |
| 51 | fn | I | IC | frontal lobe | IP, MM | large | high | B |
| 52 | fn | II | IC | frontal lobe, CP | IP, MM | large | high | B |
| 53 | fn | II | SC | LS | ED | small | medium | T |
| 54 | fn | I | IC | parietal lobe | IP, MM | large | high | B |
| 55 | fn | I | IC | diencephalon, thalamus | IP, MM | small | low | T |
| 56 | fn | II | PNS | brachial plexus | NL | large | medium | T |
| 57 | fn | I | SC | T12-L1 | ED | small | low | B |
| 58 | fn | I | IC | frontal lobe, diencephalon | IP, MM | large | high | T |
| 59 | fn | I | SC | L5-L6 | ED | large | medium | B |
| 60 | fn | II | SC | L1 | ED | large | high | B |
| 61 | fn | II | IC | frontal lobe | MM | large | medium | non-B, non-T |
| 62 | fn | I | IC | frontal lobe | IP | intermed. | medium | T |
| 63 | fn | I | IC | frontal lobe | IP, MM | large | high | B |
| 64 | fn | I | IC | frontal lobe | IP, MM | large | high | B |
| 65 | fn | I | SC | CT | IM | large | high | non-B, non-T |
| 66 | fn | II | IC | diencephalon | IP, MM | intermed. | high | B |
| 67 | fn | II | SC | T8 | ED | large | high | B |
| 68 | fn | I | SC | L3 | ED | large | medium | B |
| 69 | fn | II | IC | diencephalon | IP, MM | large | high | B |
| 70 | fn | II | IC | medulla oblongata | IP | intermed. | low | B |
| 71 | fn | II | IC | medulla oblongata | IP | large | low | T |
| 72 | fn | I | SC | L3-L4 | ED | small | medium | B |
| 73 | fn | II | SC | T11 | ED | small | medium | T |
| 74 | fn | I | PNS | cervical spinal nerves | NL | large | low | B |
| 75 | fn | I | SC | TL | ED | small | low | T |
| 76 | fn | II | SC | TL | ID-EM | intermed. | low | T |
| 77 | fn | II | IC, SC | cerebral cortex, m. obl., T | ID-EM, MM | small | medium | T |
| 78 | fn | I | SC | T5-T7 | ED | intermed. | medium | B |
| 79 | fn | II | IC, SC | TL, CP | MM, ED | small | low | B |
| 80 | fn | I | IC | olfactory lobe | IP, MM | large | high | B |
| 81 | fn | I | IC | parietal lobe | IP, MM | intermed. | medium | T |
| 82 | fn | II | IC, PNS | C and TL nerve roots | NL | small | low | B |
| 83 | fn | II | SC | TL | ED | small | low | T |
| 84 | fn | II | SC | TL | ED | small | low | B |
| 85 | fn | II | SC | CT | ED | intermed. | medium | T |
| 86 | fn | I | SC | LS | ED | intermed. | low | non-B, non-T |
| 87 | fn | I | SC | T11-T12 | ID-EM | large | medium | T |
| 88 | fn | I | IC | forebrain, midbrain | LL | large | high | T |
| 89 | fn | I | IC, PNS | II, V cranial, brachial, sciatic nerves | NL | intermed. | low | B |
| 90 | fn | I | PNS | C7 nerve root | NL | small | low | T |
| 91 | fn | I | PNS | sciatic nerve | NL | small | medium | T |
| 92 | fn | I | PNS | sciatic nerve | NL | large | medium | B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonti, N.; Parisi, F.; Aytaş, Ç.; Degl’Innocenti, S.; Cantile, C. Neuropathology of Central and Peripheral Nervous System Lymphoma in Dogs and Cats: A Study of 92 Cases and Review of the Literature. Animals 2023, 13, 862. https://doi.org/10.3390/ani13050862
Fonti N, Parisi F, Aytaş Ç, Degl’Innocenti S, Cantile C. Neuropathology of Central and Peripheral Nervous System Lymphoma in Dogs and Cats: A Study of 92 Cases and Review of the Literature. Animals. 2023; 13(5):862. https://doi.org/10.3390/ani13050862
Chicago/Turabian StyleFonti, Niccolò, Francesca Parisi, Çağla Aytaş, Sara Degl’Innocenti, and Carlo Cantile. 2023. "Neuropathology of Central and Peripheral Nervous System Lymphoma in Dogs and Cats: A Study of 92 Cases and Review of the Literature" Animals 13, no. 5: 862. https://doi.org/10.3390/ani13050862
APA StyleFonti, N., Parisi, F., Aytaş, Ç., Degl’Innocenti, S., & Cantile, C. (2023). Neuropathology of Central and Peripheral Nervous System Lymphoma in Dogs and Cats: A Study of 92 Cases and Review of the Literature. Animals, 13(5), 862. https://doi.org/10.3390/ani13050862





