Initial Characterization of 3D Culture of Yolk Sac Tissue
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
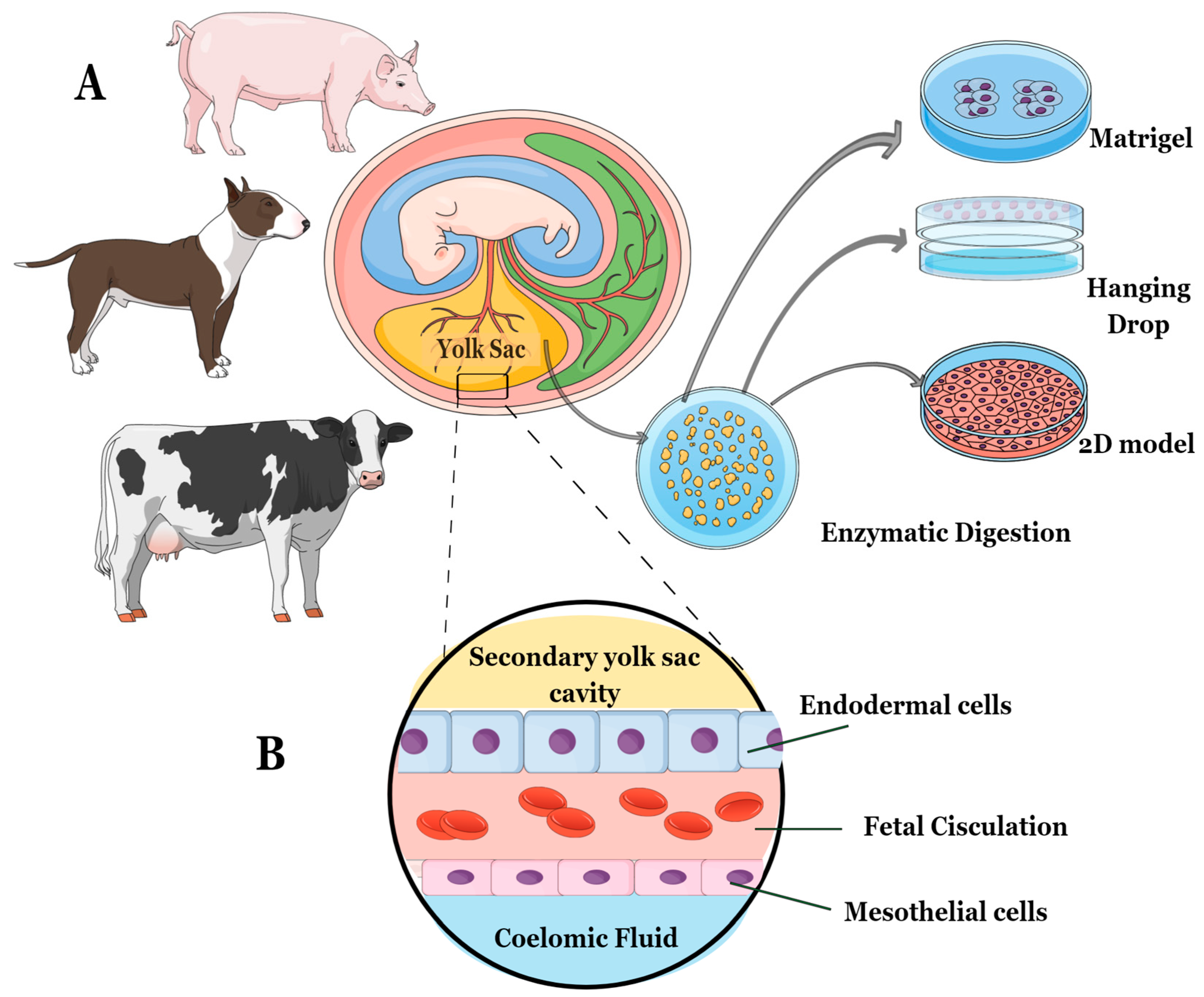
2.1. Animal Sample Collection
2.2. Isolation Protocol and Study Design
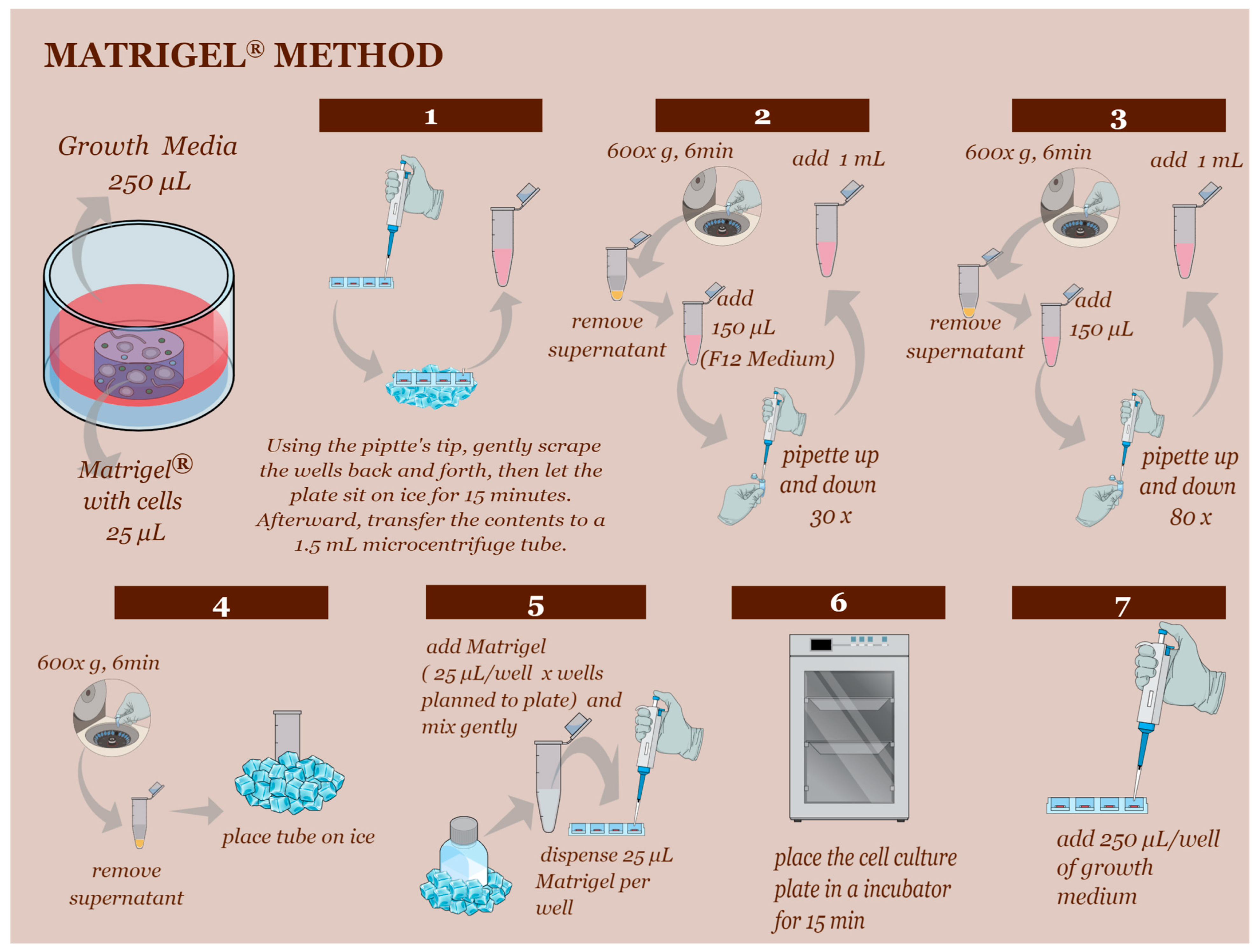
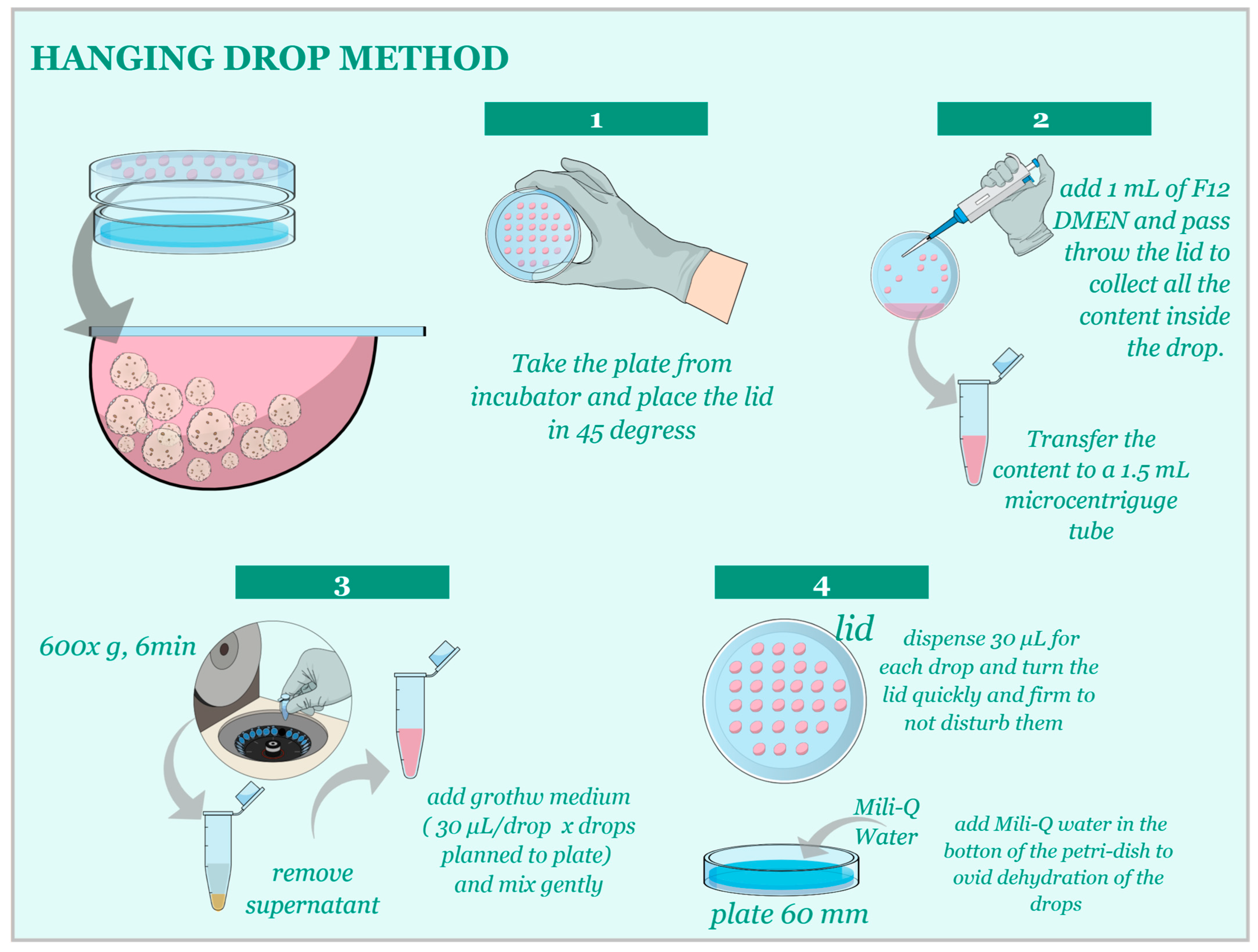
2.3. D Cell Culture Establishment—Matrigel® Method and Hanging-Drop Method
2.4. D Cell Culture Establishment—Media Used
2.5. D Cell Culture—Control Method of YS Tissue Culture
2.6. Morphological Characterization—Optical Microscopy
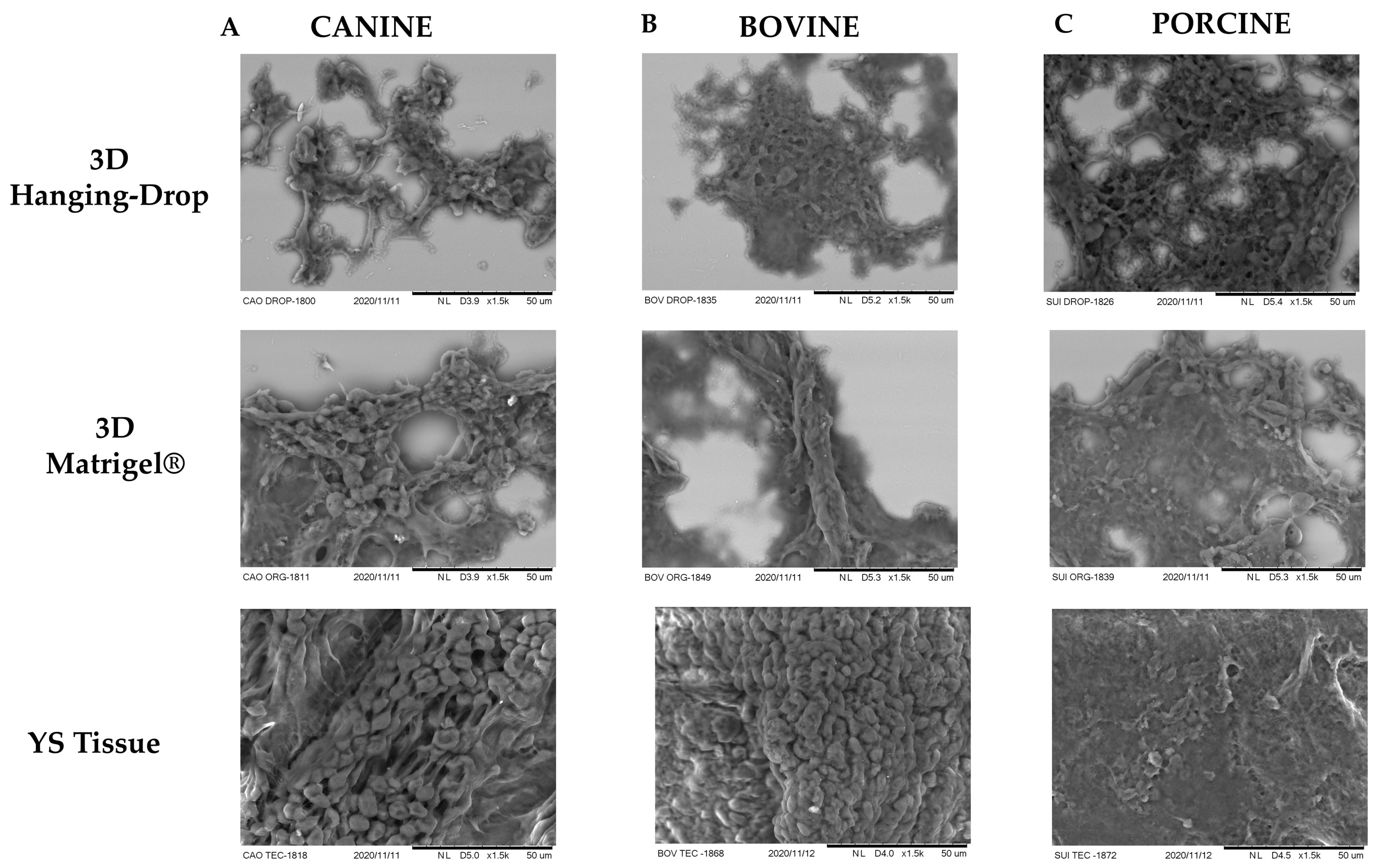
2.7. Morphological Characterization—Scanning Electron Microscopy
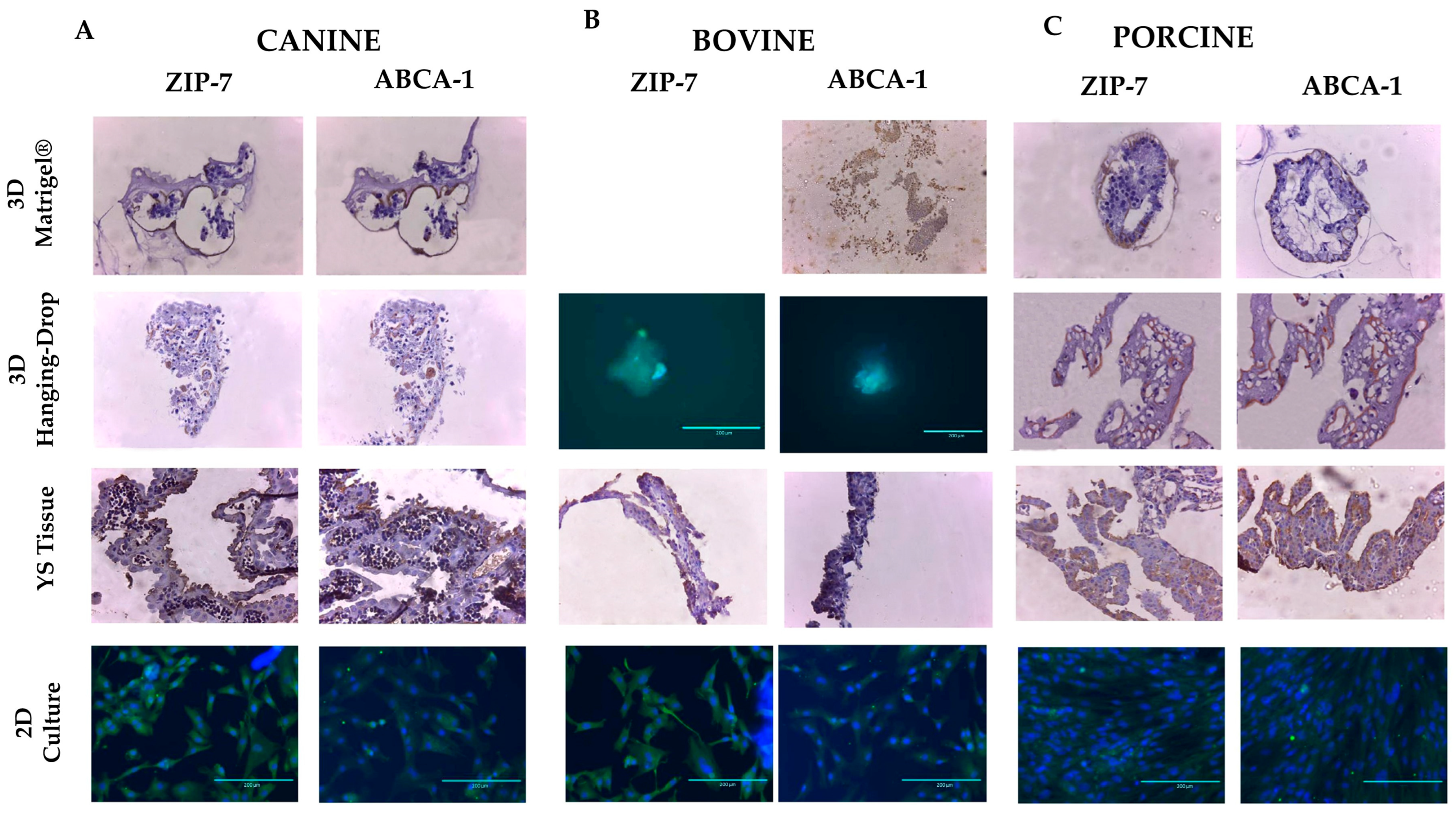
2.8. Morphological Characterization—Immunohistochemistry
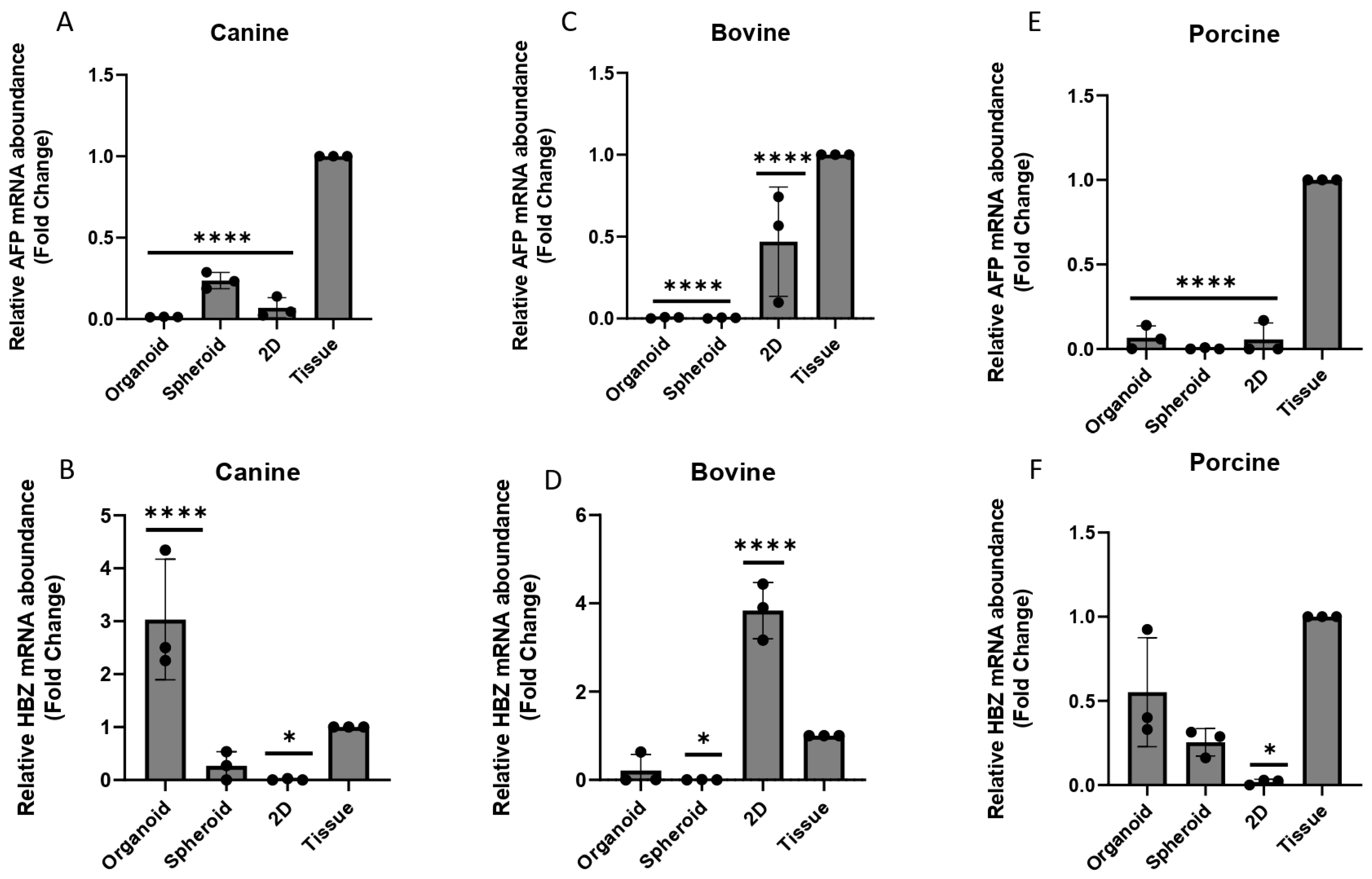
2.9. Characterization—Genetic Expression
2.10. Statistical Analysis
3. Results
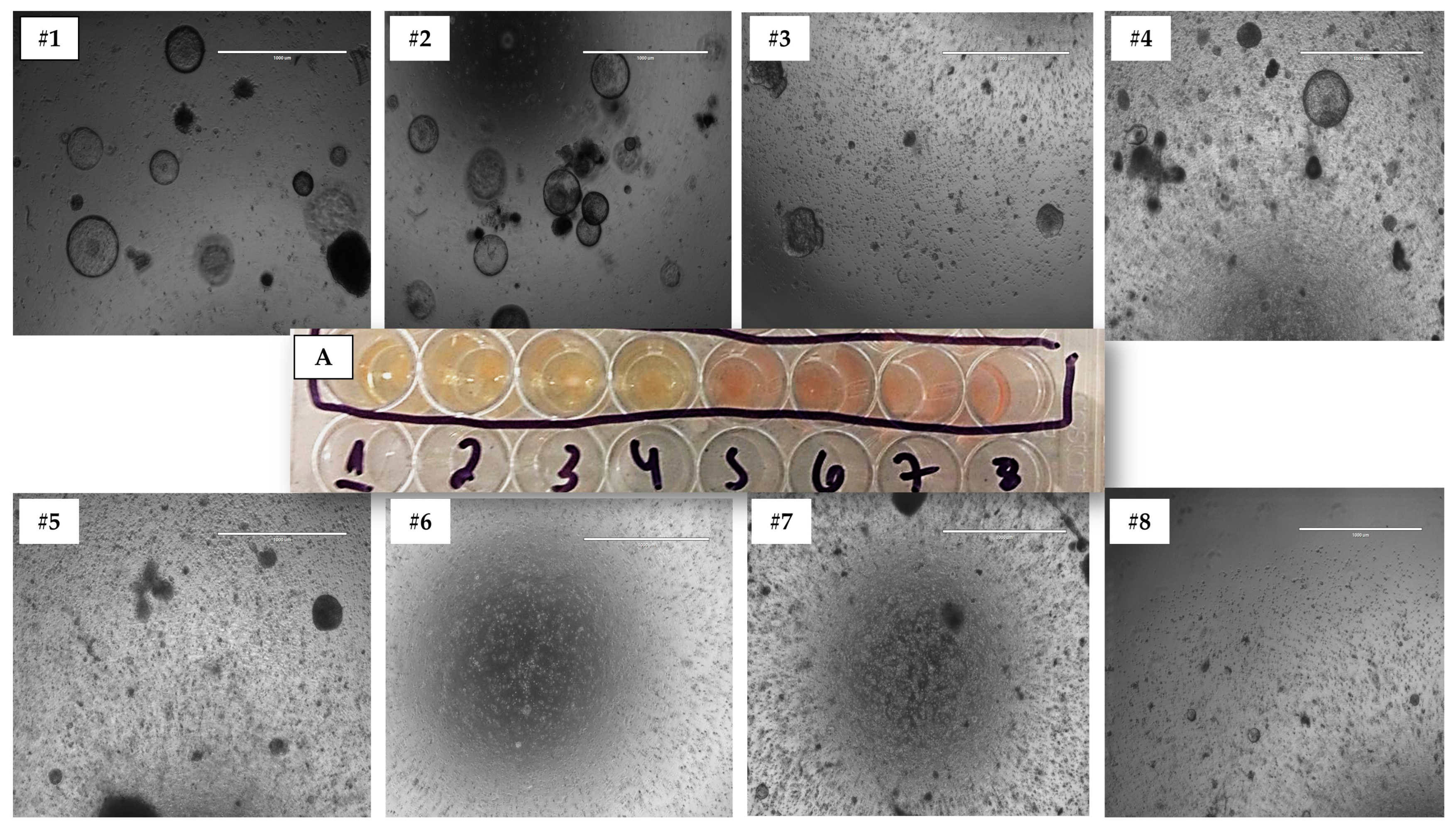
3.1. 3D Model Culture Establishment
3.2. Morphology and Cellular Conformation
3.3. Characterization—Transport Markers
3.4. Characterization—Genetic Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, G.J.; Watson, A.L.; Hempstock, J.; Skepper, J.N.; Jauniaux, E. Uterine Glands Provide Histiotrophic Nutrition for the Human Fetus during the First Trimester of Pregnancy. J. Clin. Endocrinol. Metab. 2002, 87, 2954–2959. [Google Scholar] [CrossRef] [PubMed]
- King, B.F.; Enders, A.C. Protein Absorption and Transport by the Guinea Pig Visceral Yolk Sac Placenta. Am. J. Anat. 1970, 129, 261–287. [Google Scholar] [CrossRef] [PubMed]
- Zohn, I.E.; Sarkar, A.A. The Visceral Yolk Sac Endoderm Provides for Absorption of Nutrients to the Embryo during Neurulation. Birth Defects Res. Part A Clin. Mol. Teratol. 2010, 88, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Brent, R.L.; Fawcett, L.B. Nutritional Studies of the Embryo during Early Organogenesis with Normal Embryos and Embryos Exhibiting Yolk Sac Dysfunction. J. Pediatr. 1998, 132, S6–S16. [Google Scholar] [CrossRef]
- Palis, J.; Yoder, M.C. Yolk-Sac Hematopoiesis: The First Blood Cells of Mouse and Man. Exp. Hematol. 2001, 29, 927–936. [Google Scholar] [CrossRef]
- Stremmel, C.; Schuchert, R.; Wagner, F.; Thaler, R.; Weinberger, T.; Pick, R.; Mass, E.; Ishikawa-Ankerhold, H.C.; Margraf, A.; Hutter, S.; et al. Yolk Sac Macrophage Progenitors Traffic to the Embryo during Defined Stages of Development. Nat. Commun. 2018, 9, 75. [Google Scholar] [CrossRef]
- Nikolic, A.; Volarevic, V.; Armstrong, L.; Lako, M.; Stojkovic, M. Primordial Germ Cells: Current Knowledge and Perspectives. Stem Cells Int. 2016, 2016, 1741072. [Google Scholar] [CrossRef]
- Huch, M.; Koo, B.K. Modeling Mouse and Human Development Using Organoid Cultures. Development 2015, 142, 3113–3125. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a Dish: Modeling Development and Disease Using Organoid Technologies. Science 2014, 345, 1247125. [Google Scholar] [CrossRef]
- Almeida, F.R.C.L.; Dias, A.L.N.A. Pregnancy in Pigs: The Journey of an Early Life. Domest. Anim. Endocrinol. 2022, 78, 106656. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.J.; Torres-Rovira, L.; Barbero, A.; Ayuso, M.; Garcia-Contreras, C.; Vazquez-Gomez, M. Developmental Origins of Health and Disease in Swine: Implications for Animal Production and Biomedical Research. Theriogenology 2016, 86, 110–119. [Google Scholar] [CrossRef]
- Roberts, J.F.; Jeff Huang, C.C. Bovine Models for Human Ovarian Diseases. Prog. Mol. Biol. Transl. Sci. 2022, 189, 101–154. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Wolf, E.; Braun, J.; Kolb, H.J.; Adler, H. Canine Embryo-Derived Stem Cells and Models for Human Diseases. Hum. Mol. Genet. 2008, 17, R42–R47. [Google Scholar] [CrossRef]
- Starling, M.J.; Branson, N.; Thomson, P.C.; McGreevy, P.D. Age, Sex and Reproductive Status Affect Boldness in Dogs. Vet. J. 2013, 197, 868–872. [Google Scholar] [CrossRef]
- Wenceslau, C.V.; Miglino, M.A.; Martins, D.S.; Ambrósio, C.E.; Lizier, N.F.; Pignatari, G.C.; Kerkis, I. Mesenchymal Progenitor Cells from Canine Fetal Tissues: Yolk Sac, Liver, and Bone Marrow. Tissue Eng. Part A 2011, 17, 2165–2176. [Google Scholar] [CrossRef]
- Mançanares, C.A.F.; Oliveira, V.C.; Oliveira, L.J.; Carvalho, A.F.; Sampaio, R.V.; Mançanares, A.C.F.; Souza, A.F.; Perecin, F.; Meirelles, F.V.; Miglino, M.A.; et al. Isolation and Characterization of Mesenchymal Stem Cells from the Yolk Sacs of Bovine Embryos. Theriogenology 2015, 84, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Machado Bertassoli, B.; Santos, A.C.; Fratini, P.; Alves De Lima Will, S.E.; Rodrigues, M.N.; Chaves, A.; Neto, A. Isolation and characterization of stem cells from yolk sac domestic porcine (Sus scrofa). Arch. Vet. Sci. 2015, 20, 1–10. [Google Scholar]
- Gjesdal, F. Age Determination of Bovine Foetuses. Acta Vet. Scand. 1969, 10, 197–218. [Google Scholar] [CrossRef]
- Lopate, C. Estimation of Gestational Age and Assessment of Canine Fetal Maturation Using Radiology and Ultrasonography: A Review. Theriogenology 2008, 70, 397–402. [Google Scholar] [CrossRef]
- Lee, S.Y.; Anderson, J.W.; Scott, G.L.; Mossman, H.W. Ultrastructure of the Placenta and Fetal Membranes of the Dog: II. The Yolk Sac. Am. J. Anat. 1983, 166, 313–327. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement Membrane Matrix with Biological Activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards Organoid Culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef] [PubMed]
- Turco, M.Y.; Gardner, L.; Kay, R.G.; Hamilton, R.S.; Prater, M.; Hollinshead, M.S.; McWhinnie, A.; Esposito, L.; Fernando, R.; Skelton, H.; et al. Trophoblast Organoids as a Model for Maternal–Fetal Interactions during Human Placentation. Nature 2018, 564, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Han, X.; Uyunbilig, B.; Zhang, M.; Duo, S.; Zuo, Y.; Zhao, Y.; Yun, T.; Tai, D.; Wang, C.; et al. Establishment of Bovine Trophoblast Stem-like Cells from in Vitro-Produced Blastocyst-Stage Embryos Using Two Inhibitors. Stem Cells Dev. 2014, 23, 1501–1514. [Google Scholar] [CrossRef]
- Wang, L.M.; Feng, H.L.; Ma, Y.Z.; Cang, M.; Li, H.J.; Yan, Z.; Zhou, P.; Wen, J.X.; Bou, S.; Liu, D.J. Expression of IGF Receptors and Its Ligands in Bovine Oocytes and Preimplantation Embryos. Anim. Reprod. Sci. 2009, 114, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Kautz, E.; Gram, A.; Aslan, S.; Ay, S.S.; Selçuk, M.; Kanca, H.; Koldaş, E.; Akal, E.; Karakaş, K.; Findik, M.; et al. Expression of Genes Involved in the Embryo-Maternal Interaction in the Early-Pregnant Canine Uterus. Reproduction 2014, 147, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Vassiliev, I.; Nottle, M.B. Isolation and Culture of Porcine Embryonic Stem Cells. Methods Mol. Biol. 2013, 1074, 85–95. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Cindrova-Davies, T.; Jauniaux, E.; Elliot, M.G.; Gong, S.; Burton, G.J.; Charnock-Jones, D.S. RNA-Seq Reveals Conservation of Function among the Yolk Sacs of Human, Mouse, and Chicken. Proc. Natl. Acad. Sci. USA 2017, 114, E4753–E4761. [Google Scholar] [CrossRef]
- Tomasi, T.B. Structure and Function of Alpha-Fetoprotein. Annu. Rev. Med. 1977, 28, 453–465. [Google Scholar] [CrossRef]
- Manning, L.R.; Russell, J.E.; Padovan, J.C.; Chait, B.T.; Popowicz, A.; Manning, R.S.; Manning, J.M. Human Embryonic, Fetal, and Adult Hemoglobins Have Different Subunit Interface Strengths. Correlation with Lifespan in the Red Cell. Protein Sci. 2007, 16, 1641–1658. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of Organoids from Mouse and Human Endometrium Showing Endometrial Epithelium Physiology and Long-Term Expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-Term, Hormone-Responsive Organoid Cultures of Human Endometrium in a Chemically Defined Medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef]
- Miese-Looy, G.; van den Heuvel, M.J.; Edwards, A.K.; Lamarre, J.; Tayade, C. Expression of Insulin-like Growth Factor (IGF) Family Members in Porcine Pregnancy. J. Reprod. Dev. 2012, 58, 51–60. [Google Scholar] [CrossRef]
- Blitek, A.; Morawska, E.; Ziecik, A.J. Regulation of Expression and Role of Leukemia Inhibitory Factor and Interleukin-6 in the Uterus of Early Pregnant Pigs. Theriogenology 2012, 78, 951–964. [Google Scholar] [CrossRef] [PubMed]
- Tiedemann, K.; Minuth, W.W. The Pig Yolk Sac I. Fine Structure of the Posthaematopoietic Organ. Histochemistry 1980, 68, 133–146. [Google Scholar] [CrossRef]
- Galdos-Riveros, A.; Rezende, L.C.; Pessolato, A.; Zogno, M.A.; Rici, R.E.M.; Miglino, A. The Structure of the Bovine Yolk Sac: A Study Microscopic. In Current Microscopy Contributions to Advances in Science and Technology, 5th ed.; Formatex Research Center: Basking Ridge, NJ, USA, 2012; pp. 508–515. [Google Scholar]
- Mançanares, C.A.F.; de Oliveira, V.C.; Oliveira, L.J.; Miglino, M.A.; Meirelles, F.V.; Ambrósio, C.E. Morphological and Molecular Analysis of In Vitro Tubular Structures from Bovine Yolk Sac-Derived MSCs. Stem Cells Int. 2019, 2019, 5073745. [Google Scholar] [CrossRef]
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC Transporters as Therapeutic Targets: Emerging Opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560. [Google Scholar] [CrossRef]
- Potapova, I.A.; Gaudette, G.R.; Brink, P.R.; Robinson, R.B.; Rosen, M.R.; Cohen, I.S.; Doronin, S.V. Mesenchymal Stem Cells Support Migration, Extracellular Matrix Invasion, Proliferation, and Survival of Endothelial Cells in Vitro. Stem Cells 2007, 25, 1761–1768. [Google Scholar] [CrossRef]
- Foty, R. A Simple Hanging Drop Cell Culture Protocol for Generation of 3D Spheroids. J. Vis. Exp. JoVE 2011, 51, 2720. [Google Scholar] [CrossRef]
- Jeong, Y.; Tin, A.; Irudayaraj, J. Flipped Well-Plate Hanging-Drop Technique for Growing Three-Dimensional Tumors. Front. Bioeng. Biotechnol. 2022, 10, 898699. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.S.; Fraser, S.T.; Eakin, G.S.; Mangano, M.; Isern, J.; Sahr, K.E.; Hadjantonakis, A.K.; Baron, M.H. Tg(Afp-GFP) Expression Marks Primitive and Definitive Endoderm Lineages during Mouse Development. Dev. Dyn. 2006, 235, 2549–2558. [Google Scholar] [CrossRef] [PubMed]
- Santise, G.; Maselli, D.; Malanga, D.; di Vito, A.; Mandarino, N.; Boccadamo, G.; Zeppa, P.; Amorosi, A.; Viglietto, G.; Rizzuto, A.; et al. Identification of Mesothelial Cells in Intraoperative Blood Salvage. Am. J. Transl. Res. 2019, 11, 1771. [Google Scholar] [PubMed]
- Yagi, S.; Shiojiri, N. Identification of Novel Genetic Markers for Mouse Yolk Sac Cells by Using Microarray Analyses. Placenta 2017, 49, 68–71. [Google Scholar] [CrossRef]
- Corrò, C.; Novellasdemunt, L.; Li, V.S.W. A Brief History of Organoids. Am. J. Physiol. Cell Physiol. 2020, 319, C151–C165. [Google Scholar] [CrossRef]

| Compound | Concentration | Vendor | Cat # |
|---|---|---|---|
| Supplement minus vitamin A (B27) | 1% | Gibco | 12587010 |
| N2 | 1% | Gibco | 17502048 |
| Nicotinamide | 10 nM | Sigma | N0636 |
| Glutamine | 1% | Sigma | G7513 |
| L-Nac | 1% | Sigma | A9165 |
| F-12 culture medium | - | Gibco | 21127022 |
| Recombinant Human Noggin (NOG) | 100 ng/mL | Peprotech | 120-10C |
| Recombinant Human R-Spondin-1 (RPOS-1) | 500 ng/mL | Peprotech | 120-38 |
| Recombinant Human FGF-10 (FGF-10) | 100 ng/mL | Peprotech | 100-26 |
| Recombinant Murine HGF (HGF) | 50 ng/mL | Peprotech | 315-23 |
| Recombinant Murine EGF (EGF) | 50 ng/mL | Peprotech | 315-09 |
| ALK-4, -5, -7 inhibitor (A83-01) | 500 nM | Sigma | SML0788 |
| Recombinant Human IGF-II (IGF-2) | 50 ng/mL | Peprotech | 100-12 |
| Recombinant Human LIF (LIF) | 50 ng/mL | Peprotech | 300-05 |
| Matrigel® | 25 µL/well | Corning | 356231 |
| Genes | ID Access | Sequence | |
|---|---|---|---|
| Canis lupus familiaris | |||
| Alpha-fetoprotein (AFP) | NM_001003027.1 | forward | TTCCAAGTTGCAGAACCCGT |
| reverse | CCATAGTGGGCAGCCAAAGA | ||
| hemoglobin subunit zeta-like (HBZ) | XM_003639130.3 | forward | TCCCACTCAGCTCCACCAT |
| reverse | ATCTTGCCCCACATGGACAG | ||
| beta-2-microglobulin (B2M) | NM_001284479.1 | forward | CTGGCGACGGCTGGTTT |
| reverse | TCTGCTGGGTGTCGTGAGTA | ||
| Bos taurus | |||
| Alpha-fetoprotein (AFP) | NM_001034262.2 | forward | GGGAAAATTTGGACCCCGGA |
| reverse | CCAGCACGTTTCCTTTGCAG | ||
| hemoglobin subunit zeta (HBZ) | XM_002683810.5 | forward | AAGTTCCTGTCTCACTGCCTG |
| reverse | GACGCCGGATACAATCGACA | ||
| actin beta (ACTB) | XM_005225005.1 | forward | CTTCCTGGGTGATCTGCCTT |
| reverse | CCGTGTTGGCGTAGAGGTC | ||
| Sus scrofa | |||
| Alpha-fetoprotein (AFP) | NM_214317.1 | forward | AGAGGAAACGTGCTGGAGTG |
| reverse | TCAAGTGTGGTGGGCAACTT | ||
| hemoglobin subunit zeta (HBZ) | XM_003481082.4 | forward | ATATAAGGGGACCACGGGGG |
| reverse | AATTGTCCTCTCGGCCTTGG | ||
| actin beta (ACTB) | XM_021086047.1 | forward | TGTGGATCAGCAAGCAGGAG |
| reverse | CTGCAGGTCCCGAGAGAATG |
| Media # | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Specie | Passage # | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 |
| Canine | Passage 1 | 11.0 ± 1.2 | 12.0 ± 1.1 | 10.0 ± 1.1 | 8.5 ± 1.1 | 0.0 ± 0.0 | 2.8 ± 1.1 | 0.8 ± 0.7 | 2.0 ± 1.1 |
| Passage 2 | 8.6 ± 1.7 | 11.2 ± 2.3 | 7.5 ± 1.6 | 7.2 ± 1.7 | 0.0 ± 0.0 | 2.0 ± 1.3 | 0.4 ± 0.5 | 2.2 ± 1.9 | |
| Passage 3 | 7.7 ± 1.5 | 11.8 ± 1.5 | 7.4 ± 2.1 | 6.7 ± 1.9 | 0.0 ± 0.0 | 1.3 ± 0.8 | 0.3 ± 0.7 | 0.0 ± 1.0 | |
| Bovine | Passage 1 | 2.5 ± 1.2 | 1.0 ± 0.8 | 1.8 ± 1.4 | 9.7 ± 1.7 | 0.0 ± 0.0 | 5.8 ± 0.9 | 10.2 ± 1.0 | 1.5 ± 1.0 |
| Passage 2 | 1.6 ± 0.7 | 0.5 ± 0.7 | 1.5 ± 1.2 | 9.8 ± 1.4 | 0.0 ± 0.0 | 4.0 ± 1.5 | 9.1 ± 1.0 | 1.0 ± 0.5 | |
| Passage 3 | 0.5 ± 0.7 | 0.3 ± 0.5 | 1.1 ± 0.9 | 10.1 ± 1.1 | 0.0 ± 0.0 | 3.2 ± 1.8 | 5.4 ± 1.5 | 0.0 ± 0.5 | |
| Porcine | Passage 1 | 10.0 ± 1.2 | 2.8 ± 1.1 | 13.4 ± 1.4 | 3.4 ± 1.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.4 ± 1.0 |
| Passage 2 | 8.1 ± 1.1 | 2.2 ± 0.6 | 13.5 ± 1.5 | 3.2 ± 1.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.1 ± 0.6 | |
| Passage 3 | 7.4 ± 1.0 | 2.1 ± 1.7 | 13.5 ± 1.2 | 2.5 ± 1.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.8 ± 0.7 | |
| Specie | 3D Media | 2D Media |
|---|---|---|
| Canine | #2—Expansion Media + A83-01 + IGF-2 | α-Men + 15% FBS + 1% P/S + 1% glut + 1% AANE |
| Bovine | #4—Expansion Media + A83-01 | α-Men + 10% FBS + 1% P/S + 1% AANE + 1% EAA + 1% BME |
| Porcine | #3—Expansion Media + A83-01 + LIF | α-Men + 15% FBS + 1% P/S + 2% glut + 25 µg AMB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, V.M.; Pinto, P.A.F.; Motta, L.C.B.; Almeida, M.F.; de Andrade, A.F.C.; Pavaneli, A.P.P.; Ambrósio, C.E. Initial Characterization of 3D Culture of Yolk Sac Tissue. Animals 2023, 13, 1435. https://doi.org/10.3390/ani13091435
Pereira VM, Pinto PAF, Motta LCB, Almeida MF, de Andrade AFC, Pavaneli APP, Ambrósio CE. Initial Characterization of 3D Culture of Yolk Sac Tissue. Animals. 2023; 13(9):1435. https://doi.org/10.3390/ani13091435
Chicago/Turabian StylePereira, Vitória Mattos, Priscila Avelino Ferreira Pinto, Lina Castelo Branco Motta, Matheus F. Almeida, André Furugen Cesar de Andrade, Ana Paula Pinoti Pavaneli, and Carlos Eduardo Ambrósio. 2023. "Initial Characterization of 3D Culture of Yolk Sac Tissue" Animals 13, no. 9: 1435. https://doi.org/10.3390/ani13091435
APA StylePereira, V. M., Pinto, P. A. F., Motta, L. C. B., Almeida, M. F., de Andrade, A. F. C., Pavaneli, A. P. P., & Ambrósio, C. E. (2023). Initial Characterization of 3D Culture of Yolk Sac Tissue. Animals, 13(9), 1435. https://doi.org/10.3390/ani13091435






