The Preservation of the Effects of Preweaning Nutrition on Growth, Immune Competence and Metabolic Characteristics of the Developing Heifer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatments
2.2. Animal Management
2.2.1. Preweaning Animal Management
2.2.2. Postweaning Animal Management
2.3. Immune Challenge
2.3.1. Acquired Immune Response
2.3.2. Cell Mediated Immune Response
- A = mean test site thickness at 48 h post injection;
- B = mean test site thickness prior to injection;
- C = mean control site thickness at 48 h post injection;
- D = mean control site thickness prior to injection.
2.4. Biomarker Analysis
2.5. Statistical Analysis
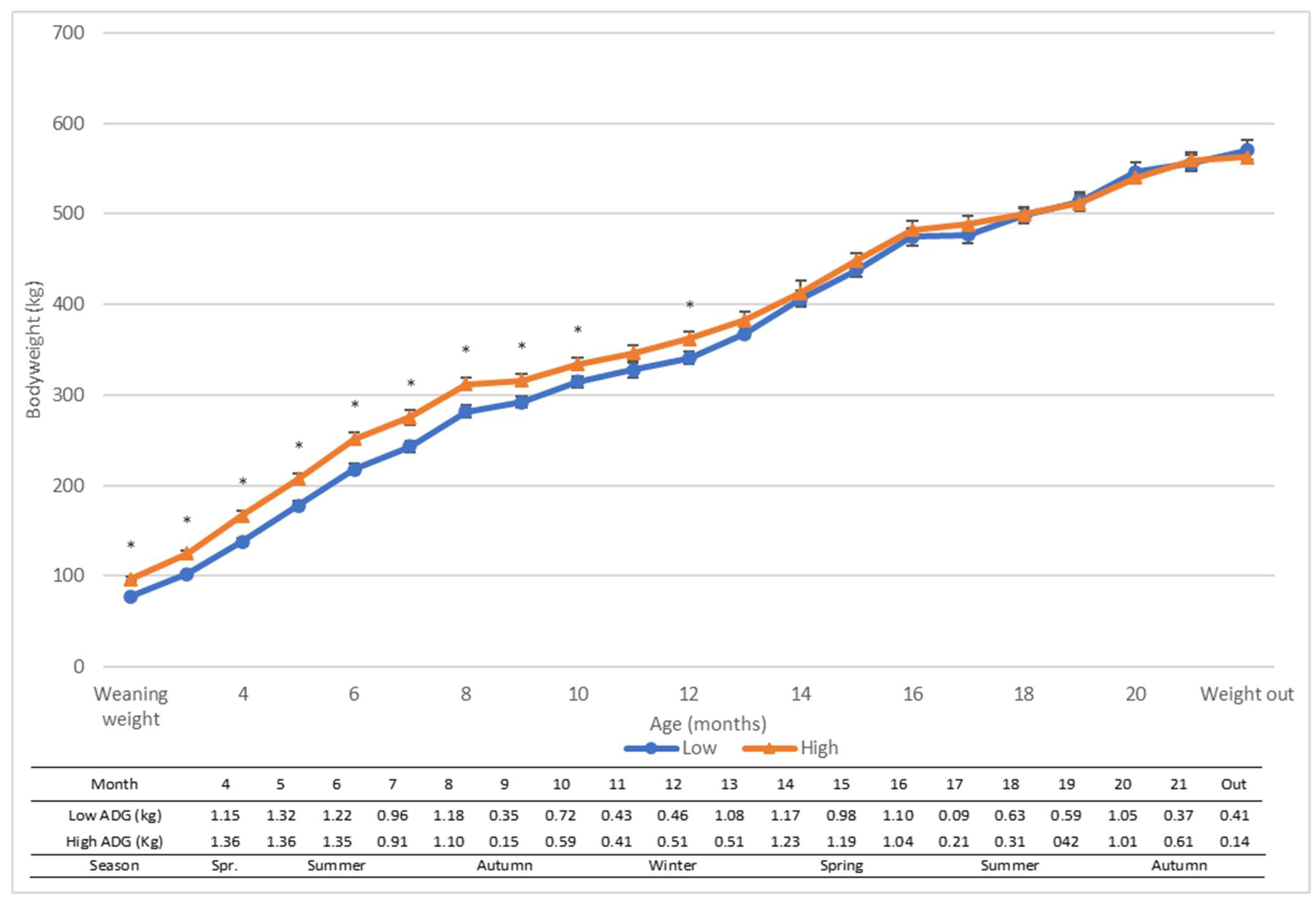
3. Results
4. Discussion
4.1. Growth Rates
4.2. Immune Biomarkers
4.3. Metabolic Biomarkers
4.4. Delayed-Type Hypersensitivity Skinfold Testing
4.5. Reproductive Performance
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dairy Australia. A Guide to Growing More Productive Heifers, 1st ed.; D. A. Limited: Melbourne, Australia, 2020; pp. 1–13. [Google Scholar]
- Moran, J. Rearing Young Stock on Tropical Dairy Farms in Asia, 1st ed.; CSIRO Publishing: Clayton, Australia, 2012; pp. 1–297. [Google Scholar]
- Cabrera, V.E. Invited review: Helping dairy farmers to improve economic performance utilizing data-driving decision support tools. Anim. Int. J. Anim. Biosci. 2018, 12, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Bach, A. Associations between several aspects of heifer development and dairy cow survivability to second lactation. J. Dairy Sci. 2011, 94, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, I.; Friggens, N.C.; Ouweltjes, W.; Scott, H.; Aernouts, B.; Statham, J. Productive life span and resilience rank can be predicted from on-farm first-parity sensor time series but not using a common equation across farms. J. Dairy Sci. 2020, 103, 7155–7171. [Google Scholar] [CrossRef] [PubMed]
- Ouweltjes, W.; Spoelstra, M.; Ducro, B.; de Haas, Y.; Kamphuis, C. A data-driven prediction of lifetime resilience of dairy cows using commercial sensor data collected during first lactation. J. Dairy Sci. 2021, 104, 11759–11769. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.P. Major Advances in Our Understanding of Nutritional Influences on Bovine Health. J. Dairy Sci. 2006, 89, 1292–1301. [Google Scholar] [CrossRef]
- Lucas, A. Programming by Early Nutrition: An Experimental Approach. J. Nutr. 1998, 128, 401–406. [Google Scholar] [CrossRef]
- Herzog, A.; Winckler, C.; Zollitsch, W. In pursuit of sustainability in dairy farming: A review of interdependent effects of animal welfare improvement and environmental impact mitigation. Agric. Ecosyst. Environ. 2018, 267, 174–187. [Google Scholar] [CrossRef]
- Soberon, F.; Van Amburgh, M.E. LACTATION BIOLOGY SYMPOSIUM: The effect of nutrient intake from milk or milk replacer of preweaned dairy calves on lactation milk yield as adults: A meta-analysis of current data1. J. Anim. Sci. 2013, 91, 706–712. [Google Scholar] [CrossRef]
- Soberon, F.; Raffrenato, E.; Everett, R.W.; Van Amburgh, M.E. Preweaning milk replacer intake and effects on long-term productivity of dairy calves. J. Dairy Sci. 2012, 95, 783–793. [Google Scholar] [CrossRef]
- Moallem, U.; Werner, D.; Lehrer, H.; Zachut, M.; Livshitz, L.; Yakoby, S.; Shamay, A. Long-term effects of ad libitum whole milk prior to weaning and prepubertal protein supplementation on skeletal growth rate and first-lactation milk production. J. Dairy Sci. 2010, 93, 2639–2650. [Google Scholar] [CrossRef]
- Olin, A.; Henckel, E.; Chen, Y.; Lakshmikanth, T.; Pou, C.; Mikes, J.; Gustafsson, A.; Bernhardsson, A.K.; Zhang, C.; Bohlin, K.; et al. Stereotypic Immune System Development in Newborn Children. Cell 2018, 174, 1277–1292.e1214. [Google Scholar] [CrossRef]
- Van Eetvelde, M.; Opsomer, G. Innovative look at dairy heifer rearing: Effect of prenatal and post-natal environment on later performance. Reprod. Domest. Anim. 2017, 52, 30–36. [Google Scholar] [CrossRef]
- Kenéz, Á.; Koch, C.; Korst, M.; Kesser, J.; Eder, K.; Sauerwein, H.; Huber, K. Different milk feeding intensities during the first 4 weeks of rearing dairy calves: Part 3: Plasma metabolomics analysis reveals long-term metabolic imprinting in Holstein heifers. J. Dairy Sci. 2018, 101, 8446–8460. [Google Scholar] [CrossRef]
- Kesser, J.; Korst, M.; Koch, C.; Romberg, F.J.; Rehage, J.; Müller, U.; Schmicke, M.; Eder, K.; Hammon, H.M.; Sadri, H.; et al. Different milk feeding intensities during the first 4 weeks of rearing dairy calves: Part 2: Effects on the metabolic and endocrine status during calfhood and around the first lactation. J. Dairy Sci. 2017, 100, 3109–3125. [Google Scholar] [CrossRef]
- Rosadiuk, J.P.; Bruinjé, T.C.; Moslemipur, F.; Fischer-Tlustos, A.J.; Renaud, D.L.; Ambrose, D.J.; Steele, M.A. Differing planes of pre- and postweaning phase nutrition in Holstein heifers: I. Effects on feed intake, growth efficiency, and metabolic and development indicators. J. Dairy Sci. 2021, 104, 1136–1152. [Google Scholar] [CrossRef]
- Ockenden, E.M.; Russo, V.M.; Leury, B.J.; Giri, K.; Wales, W.J. Preweaning Nutrition and Its Effects on the Growth, Immune Competence and Metabolic Characteristics of the Dairy Calf. Animals 2023, 13, 829. [Google Scholar] [CrossRef]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Fisher, A.D. Assessing adaptive immune response phenotypes in Australian Holstein-Friesian heifers in a pasture-based production system1. J. Anim. Sci. 2015, 93, 3713–3721. [Google Scholar] [CrossRef]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Fisher, A.D. An assessment of immune and stress responsiveness in Holstein-Friesian cows selected for high and low feed conversion efficiency. Anim. Prod. Sci. 2017, 57, 244–251. [Google Scholar] [CrossRef]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Stevenson, M.A.; Fisher, A.D. Associations between immune competence, stress responsiveness, and production in Holstein-Friesian and Holstein-Friesian × Jersey heifers reared in a pasture-based production system in Australia. J. Dairy Sci. 2019, 102, 3282–3294. [Google Scholar] [CrossRef]
- Smith, R.J. Logarithmic transformation bias in allometry. Am. J. Phys. Anthropol. 1993, 90, 215–228. [Google Scholar] [CrossRef]
- Korst, M.; Koch, C.; Kesser, J.; Müller, U.; Romberg, F.J.; Rehage, J.; Eder, K.; Sauerwein, H. Different milk feeding intensities during the first 4 weeks of rearing in dairy calves: Part 1: Effects on performance and production from birth over the first lactation. J. Dairy Sci. 2017, 100, 3096–3108. [Google Scholar] [CrossRef] [PubMed]
- Kiezebrink, D.J.; Edwards, A.M.; Wright, T.C.; Cant, J.P.; Osborne, V.R. Effect of enhanced whole-milk feeding in calves on subsequent first-lactation performance. J. Dairy Sci. 2015, 98, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Wicks, H.C.; Fallon, R.J.; Twigge, J.; Dawson, L.E.; Wylie, A.R.; Carson, A.F. Effects of feeding level and protein content of milk replacer on the performance of dairy herd replacements. Anim. Int. J. Anim. Biosci. 2009, 3, 1570–1579. [Google Scholar] [CrossRef]
- Davis Rincker, L.E.; VandeHaar, M.J.; Wolf, C.A.; Liesman, J.S.; Chapin, L.T.; Weber Nielsen, M.S. Effect of intensified feeding of heifer calves on growth, pubertal age, calving age, milk yield, and economics. J. Dairy Sci. 2011, 94, 3554–3567. [Google Scholar] [CrossRef] [PubMed]
- Ryan, W.; Williams, I.; Moir, R. Compensatory growth in sheep and cattle. 1. Growth pattern and feed intake. Aust. J. Agric. Res. 1993, 44, 1609–1621. [Google Scholar] [CrossRef]
- Shamay, A.; Werner, D.; Moallem, U.; Barash, H.; Bruckental, I. Effect of Nursing Management and Skeletal Size at Weaning on Puberty, Skeletal Growth Rate, and Milk Production During First Lactation of Dairy Heifers. J. Dairy Sci. 2005, 88, 1460–1469. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.A.; Sanz, A.; Tamanini, C.; Casasús, I. Metabolic, endocrine, and reproductive responses of beef heifers submitted to different growth strategies during the lactation and rearing periods1. J. Anim. Sci. 2015, 93, 3871–3885. [Google Scholar] [CrossRef]
- De Paula, C.; Rennó, L.N.; Ferreira, M.F.D.L.; Da Silva, Á.E.M.; Moreira, S.S.; De Freitas Assis, G.J.; Detmann, E.; De Campos Valadares Filho, S.; Fonseca Paulino, M.; Dos Santos, G.M. Effect of pre- and post-weaning supplementation on performance, nutritional, and metabolic characteristics in Nellore heifers under grazing. Anim. Prod. Sci. 2022, 62, 1706–1719. [Google Scholar] [CrossRef]
- Foote, M.R.; Nonnecke, B.J.; Beitz, D.C.; Waters, W.R. High Growth Rate Fails to Enhance Adaptive Immune Responses of Neonatal Calves and Is Associated with Reduced Lymphocyte Viability1. J. Dairy Sci. 2007, 90, 404–417. [Google Scholar] [CrossRef]
| Time | Biomarker | Low | High | SED | p-Value |
|---|---|---|---|---|---|
| Pre-vaccination | |||||
| WCC 1 | 7.6 | 8.8 | 0.94 | 0.207 | |
| Neutrophils 1 | 2.5 | 3.6 | 0.87 | 0.246 | |
| Lymphocytes 1 | 4.4 | 4.6 | 0.48 | 0.767 | |
| Monocytes 1 | 0.4 | 0.4 | 0.10 | 0.754 | |
| Eosinophils 1 | 0.2 | 0.3 | 0.09 | 0.482 | |
| Basophils 1 | 0.0 | 0.0 | - | - | |
| IBR Titre (S/N%) | 37.6 | 24.4 | 14.65 | 0.381 | |
| BHB 2 | 0.29 | 0.23 | 0.039 | 0.163 | |
| NEFA 2 | 0.36 | 0.35 | 0.037 | 0.734 | |
| Glucose 2 | 4.62 | 4.61 | 0.186 | 0.968 | |
| Insulin (µIU/mL) | 12.32 | 11.18 | - | 0.762 | |
| Logₑ Insulin | 2.27 | 2.17 | 0.316 | ||
| 10 days post- vaccination | |||||
| WCC 1 | 8.8 | 10.9 | 0.94 | 0.038 * | |
| Neutrophils 1 | 4.1 | 5.9 | 0.91 | 0.057 | |
| Lymphocytes 1 | 3.7 | 3.5 | 0.49 | 0.660 | |
| Monocytes 1 | 0.6 | 0.8 | 0.15 | 0.210 | |
| Eosinophils 1 | 0.3 | 0.6 | 0.18 | 0.130 | |
| Basophils 1 | 0.0 | 0.0 | 0.02 | 0.654 | |
| IBR Titre (S/N%) | 5.2 | 6.4 | 2.49 | 0.651 | |
| BHB 2 | 0.32 | 0.31 | 0.045 | 0.890 | |
| NEFA 2 | 0.29 | 0.26 | 0.028 | 0.274 | |
| Glucose 2 | 4.98 | 4.81 | 0155 | 0.296 | |
| Insulin (µIU/mL) | 18.11 | 13.22 | - | 0.269 | |
| Logₑ Insulin | 2.71 | 2.40 | 0.274 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ockenden, E.M.; Russo, V.M.; Leury, B.J.; Giri, K.; Wales, W.J. The Preservation of the Effects of Preweaning Nutrition on Growth, Immune Competence and Metabolic Characteristics of the Developing Heifer. Animals 2023, 13, 1309. https://doi.org/10.3390/ani13081309
Ockenden EM, Russo VM, Leury BJ, Giri K, Wales WJ. The Preservation of the Effects of Preweaning Nutrition on Growth, Immune Competence and Metabolic Characteristics of the Developing Heifer. Animals. 2023; 13(8):1309. https://doi.org/10.3390/ani13081309
Chicago/Turabian StyleOckenden, Emma M., Victoria M. Russo, Brian J. Leury, Khageswor Giri, and William J. Wales. 2023. "The Preservation of the Effects of Preweaning Nutrition on Growth, Immune Competence and Metabolic Characteristics of the Developing Heifer" Animals 13, no. 8: 1309. https://doi.org/10.3390/ani13081309
APA StyleOckenden, E. M., Russo, V. M., Leury, B. J., Giri, K., & Wales, W. J. (2023). The Preservation of the Effects of Preweaning Nutrition on Growth, Immune Competence and Metabolic Characteristics of the Developing Heifer. Animals, 13(8), 1309. https://doi.org/10.3390/ani13081309






