Oral Palatability and Owners’ Perception of the Effect of Increasing Amounts of Spirulina (Arthrospira platensis) in the Diet of a Cohort of Healthy Dogs and Cats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Intervention Protocol
2.3. Palatability Test
2.4. Impact of Spirulina Intake on Pet’s Health
2.5. Chemical Analysis
2.5.1. Proximate Analysis
2.5.2. Fatty Acids Profile
2.5.3. Amino Acids Profile
2.5.4. Mineral Analysis
2.5.5. Vitamin Quantification
2.5.6. Pigments Quantification
2.6. Statistical Analysis
3. Results
3.1. Pet Owners’ Data
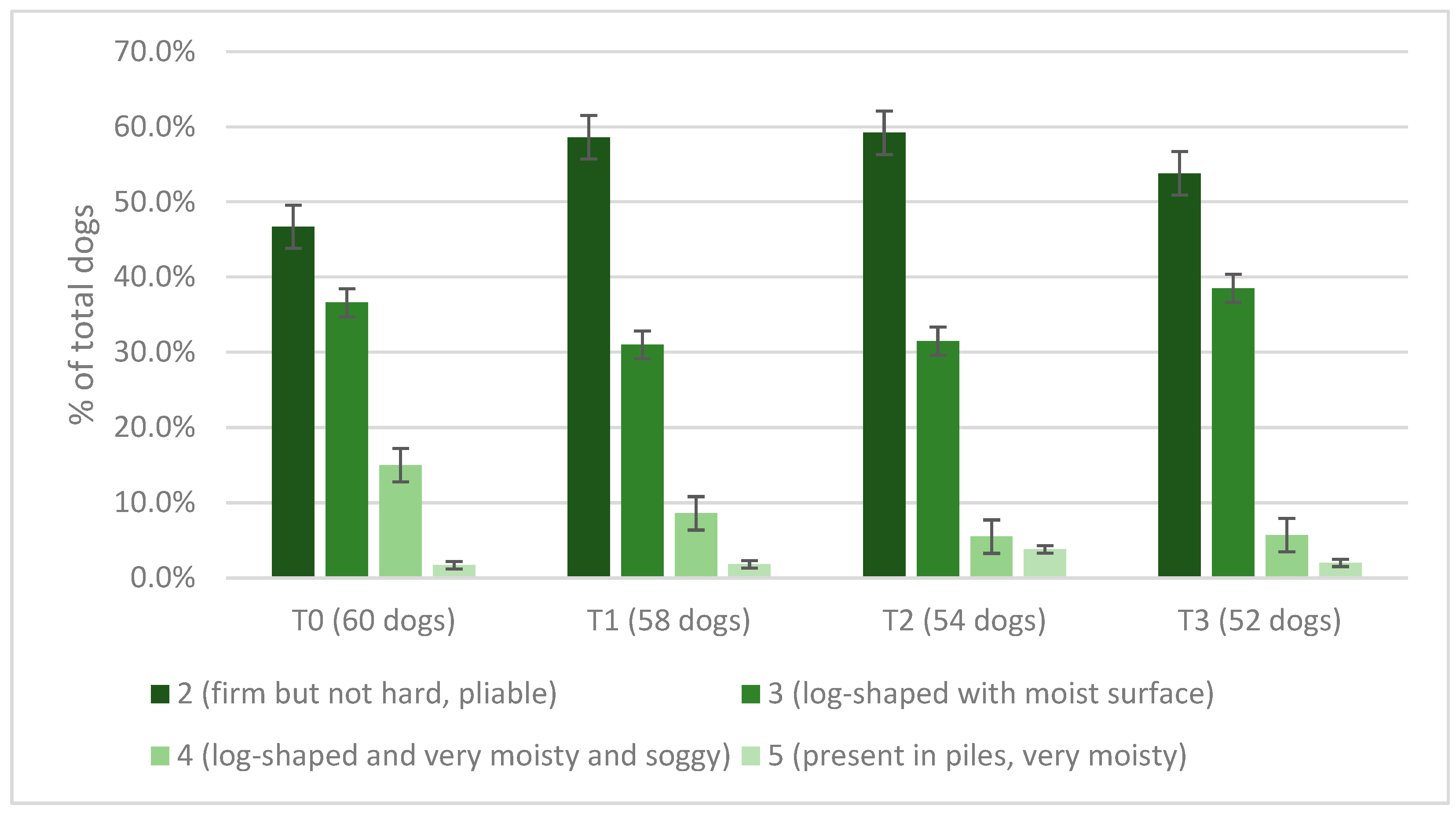
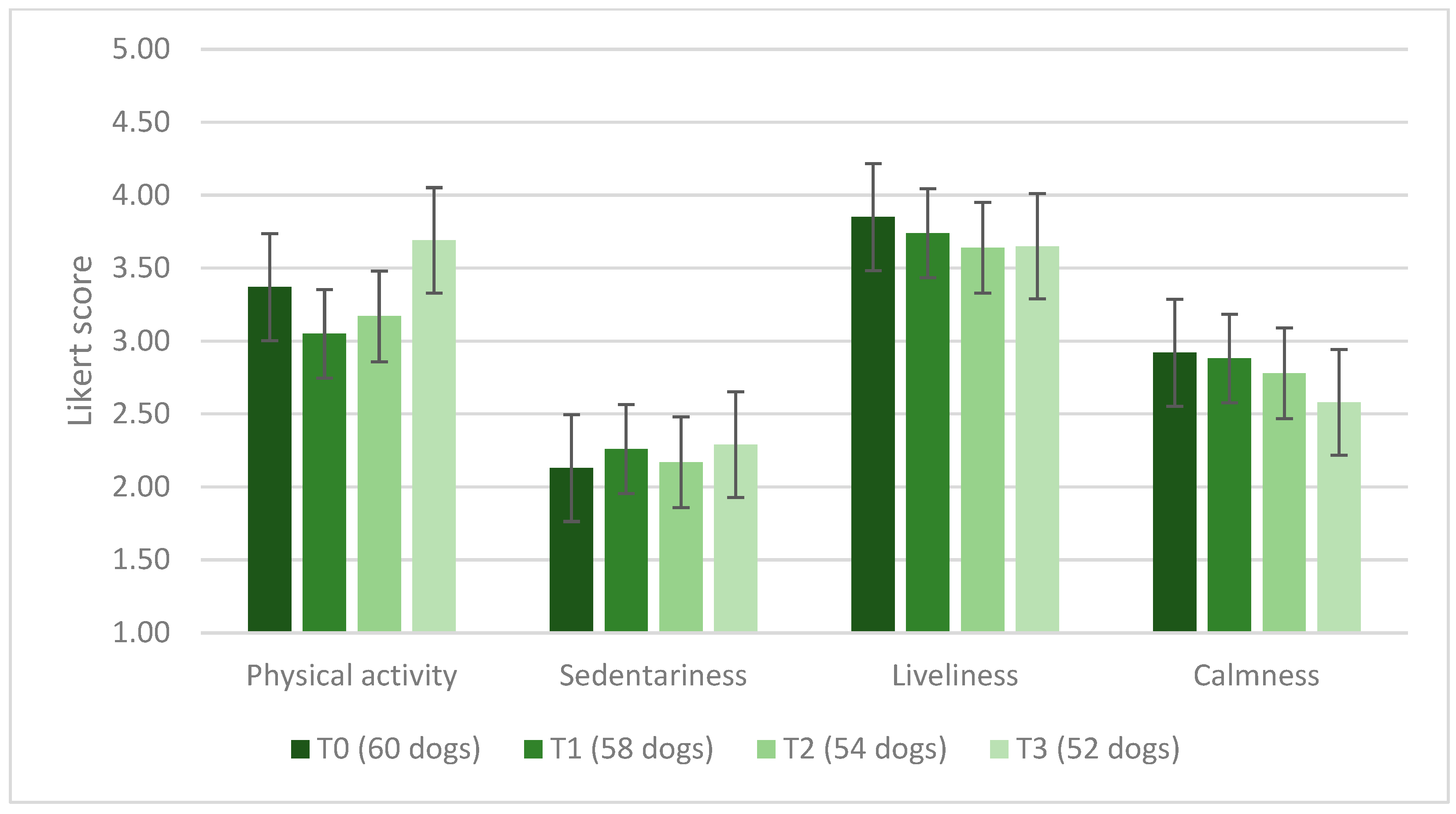
3.2. Canine Population
3.3. Feline Population
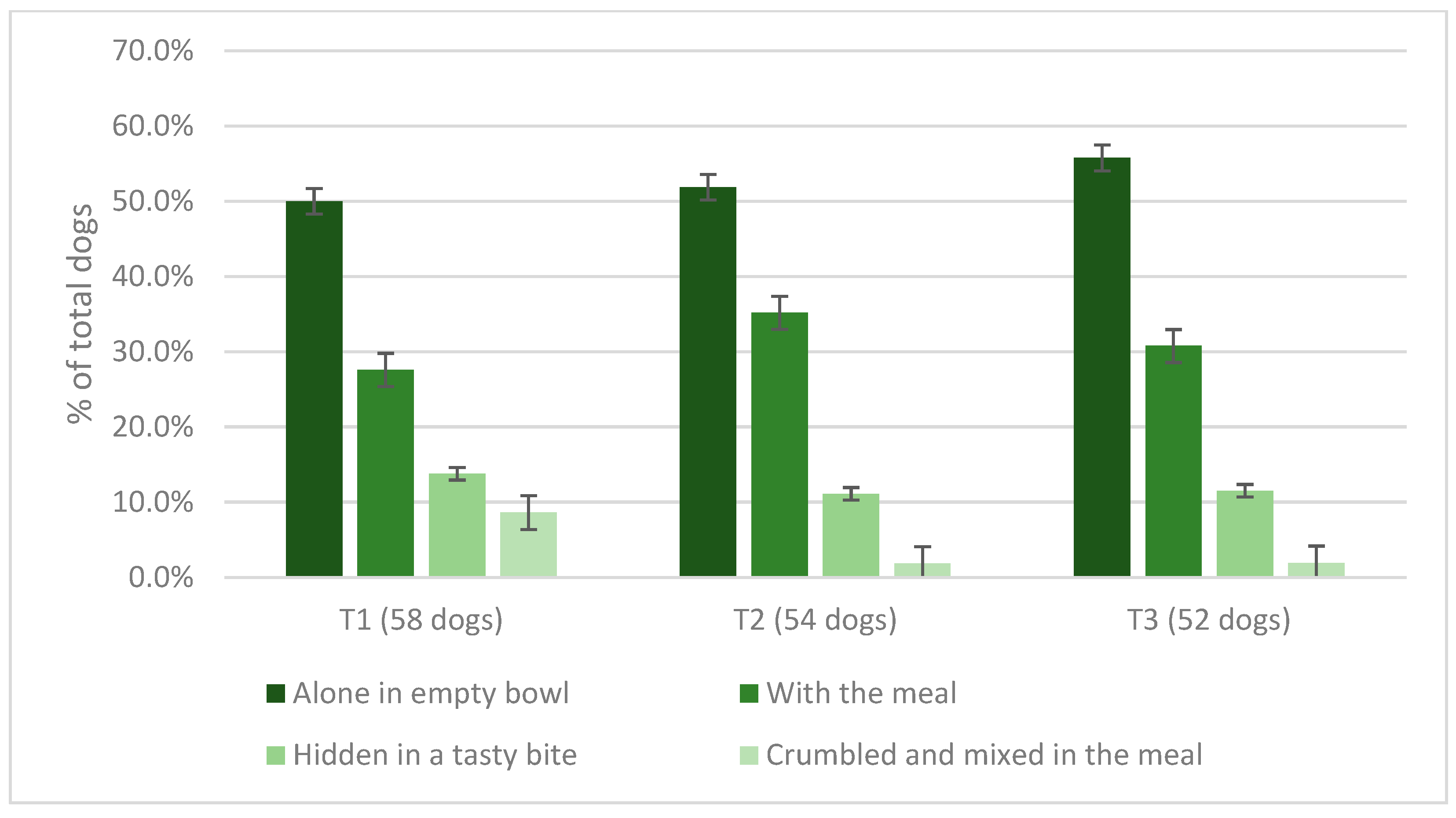
3.4. Dogs’ Spirulina Palatability Test and Owners’ Assessment
3.5. Dog Owners’ Opinion on the Spirulina Supplementation
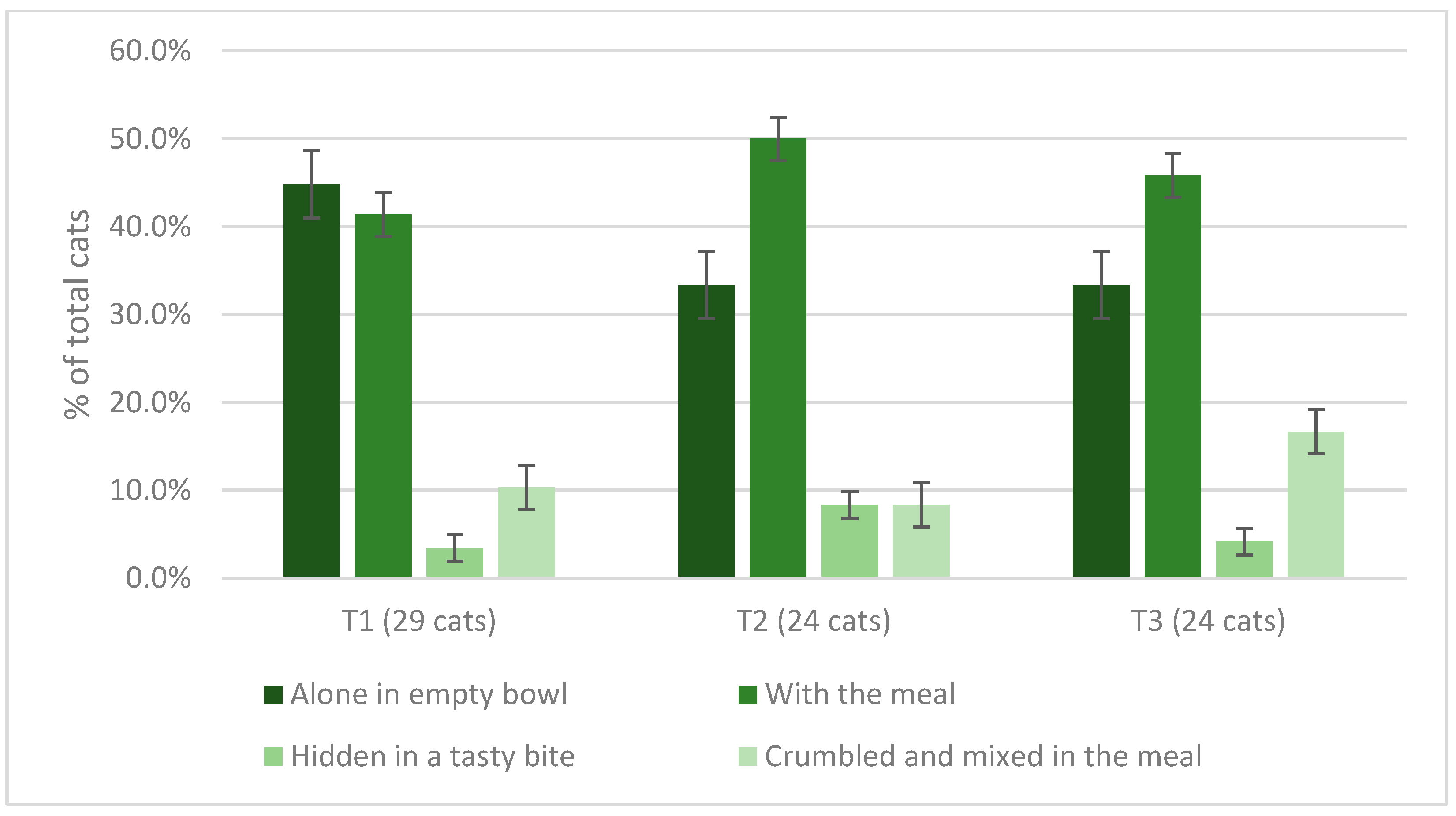
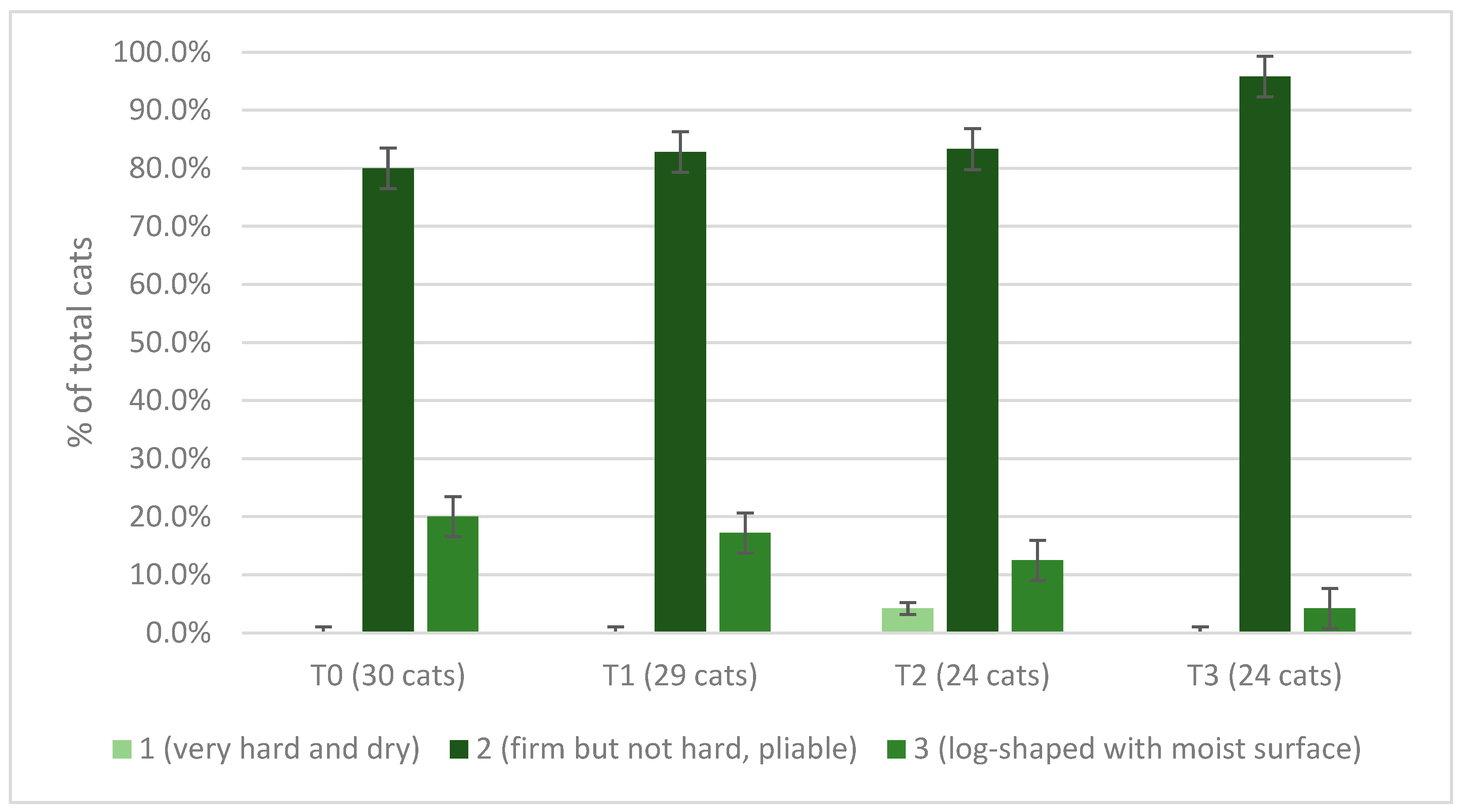
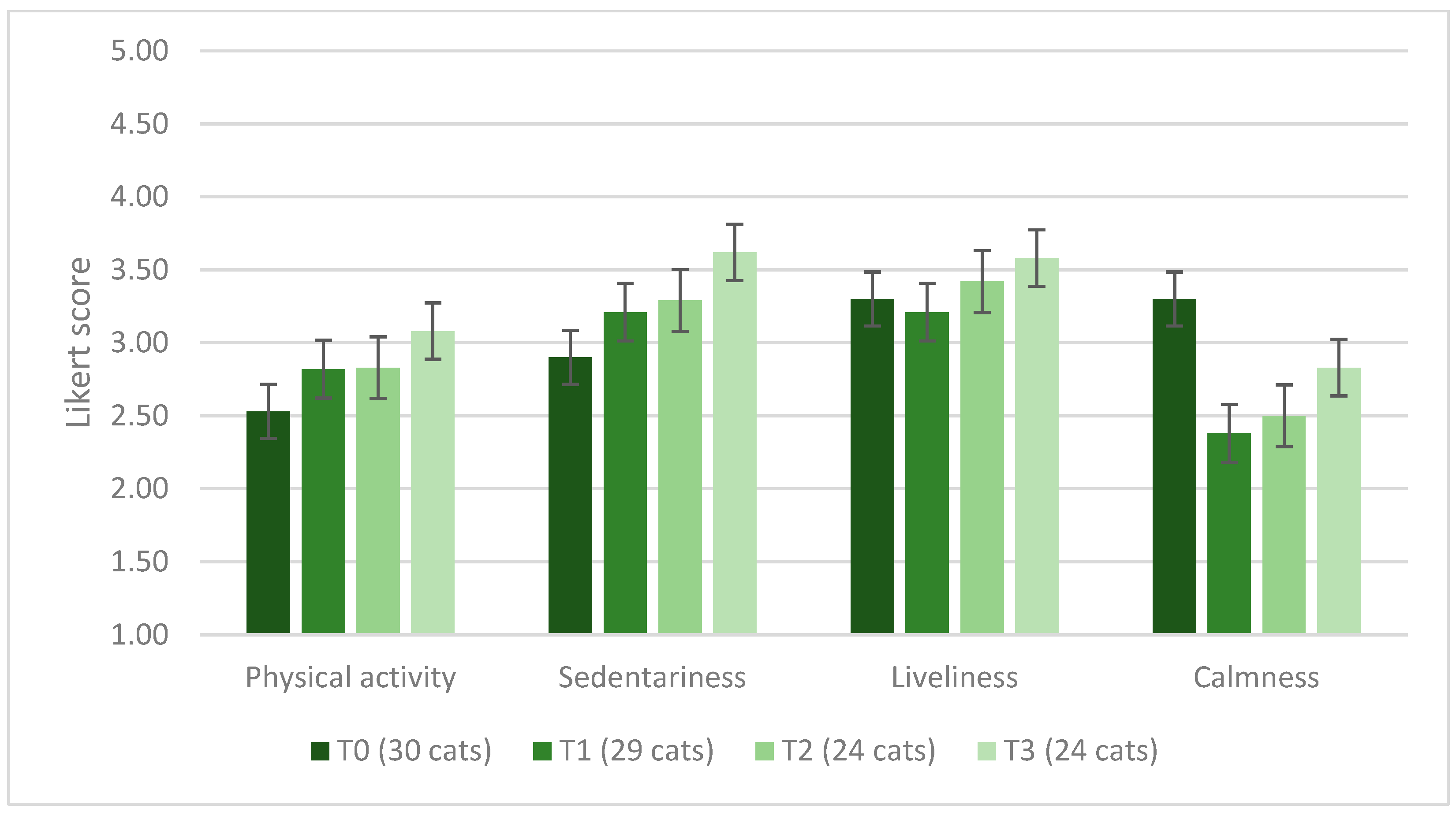
3.6. Cats’ Spirulina Palatability Test and Owners’ Assessment
3.7. Cat Owners’ Opinion on the Spirulina Supplementation
4. Discussion
4.1. Dogs’ Spirulina Palatability Test and Owners’ Assessment
4.1.1. Palatability Test
4.1.2. Gastrointestinal Signs
4.1.3. Effects on Coat
4.1.4. Effects on Dog Behavior
4.2. Cat Spirulina Palatability Test and Owner Assessment
4.2.1. Palatability Test
4.2.2. Gastrointestinal Signs
4.2.3. Effects on Coat
4.2.4. Effects on Cat Behavior
4.3. Owners’ Opinions on the Spirulina Supplementation
4.4. Limitations of this Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in Clinical Practice: Evidence-Based Human Applications. Evid.-Based Complement. Altern. Med. 2010, 2011, enen058. [Google Scholar] [CrossRef]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina–From growth to nutritional product: A review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Chapter 4—Nutraceuticals From Algae and Cyanobacteria. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Nertherland, 2017; pp. 65–89. ISBN 978-0-444-63784-0. [Google Scholar]
- Cabrita, A.R.J.; Guilherme-Fernandes, J.; Valente, I.M.; Almeida, A.; Lima, S.A.C.; Fonseca, A.J.M.; Maia, M.R.G. Nutritional Composition and Untargeted Metabolomics Reveal the Potential of Tetradesmus obliquus, Chlorella vulgaris and Nannochloropsis oceanica as Valuable Nutrient Sources for Dogs. Animals 2022, 12, 2643. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional Evaluation of Australian Microalgae as Potential Human Health Supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef] [PubMed]
- Grosshagauer, S.; Kraemer, K.; Somoza, V. The True Value of Spirulina. J. Agric. Food Chem. 2020, 68, 4109–4115. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.C.; Phuc, A.P.; Dubacq, J.P. Nutritional Value of the Alga Spirulina. Plants Hum. Nutr. 1995, 77, 32–46. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Dogs and Cats; National Academies Press: Washington, DC, USA, 2006; ISBN 978-0-309-08628-8. [Google Scholar]
- Mühling, M.; Belay, A.; Whitton, B.A. Variation in fatty acid composition of Arthrospira (Spirulina) strains. J. Appl. Phycol. 2005, 17, 137–146. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Faghfouri, A.H.; Radkhah, N.; Foroumandi, E.; Khorshidi, M.; Rasouli, A.; Ebrahimi Mamaghani, M. Spirulina supplementation and anthropometric indices: A systematic review and meta-analysis of controlled clinical trials. Phytother. Res. 2021, 35, 577–586. [Google Scholar] [CrossRef]
- Joventino, I.P.; Alves, H.G.R.; Neves, L.C.; Pinheiro-Joventino, F.; Leal, L.K.A.M.; Neves, S.A.; Ferreira, F.V.; Brito, G.A.C.; Viana, G.B. The Microalga Spirulina platensis Presents Anti-inflammatory Action as well as Hypoglycemic and Hypolipidemic Properties in Diabetic Rats. J. Complement. Integr. Med. 2012, 9, 17. [Google Scholar] [CrossRef]
- Wan, X.Z.; Li, T.T.; Zhong, R.T.; Chen, H.B.; Xia, X.; Gao, L.Y.; Zhao, C. Anti-diabetic activity of PUFAs-rich extracts of Chlorella pyrenoidosa and Spirulina platensis in rats. Food Chem. Toxicol. 2019, 128, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Capelli, B.; Cysewski, G.R. Potential health benefits of spirulina microalgae. Nutrafoods 2010, 9, 19–26. [Google Scholar] [CrossRef]
- Petit, L.; Vernès, L.; Cadoret, J.-P. Docking and in silico toxicity assessment of Arthrospira compounds as potential antiviral agents against SARS-CoV-2. J. Appl. Phycol. 2021, 33, 1579–1602. [Google Scholar] [CrossRef] [PubMed]
- de la Jara, A.; Ruano-Rodriguez, C.; Polifrone, M.; Assunçao, P.; Brito-Casillas, Y.; Wägner, A.M.; Serra-Majem, L. Impact of dietary Arthrospira (Spirulina) biomass consumption on human health: Main health targets and systematic review. J. Appl. Phycol. 2018, 30, 2403–2423. [Google Scholar] [CrossRef]
- Serban, M.-C.; Sahebkar, A.; Dragan, S.; Stoichescu-Hogea, G.; Ursoniu, S.; Andrica, F.; Banach, M. A systematic review and meta-analysis of the impact of Spirulina supplementation on plasma lipid concentrations. Clin. Nutr. 2016, 35, 842–851. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Bhat, A.G.; OKeefe, J. Effects of spirulina on weight loss and blood lipids: A review. Open Heart 2020, 7, e001003. [Google Scholar] [CrossRef]
- Gad, A.S.; Khadrawy, Y.A.; El-Nekeety, A.A.; Mohamed, S.R.; Hassan, N.S.; Abdel-Wahhab, M.A. Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition 2011, 27, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, A.; Yildirim, A.; Simsek, N.; Kalkan, Y.; Celebi, F. Spirulina platensis protects against gentamicin-induced nephrotoxicity in rats. Phytother. Res. 2008, 22, 1506–1510. [Google Scholar] [CrossRef]
- Rojas-Franco, P.; Franco-Colín, M.; Camargo, M.E.M.; Carmona, M.M.E.; Ortíz-Butrón, M.d.R.E.; Blas-Valdivia, V.; Cano-Europa, E. Phycobiliproteins and phycocyanin of Arthrospira maxima (Spirulina) reduce apoptosis promoters and glomerular dysfunction in mercury-related acute kidney injury. Toxicol. Res. Appl. 2018, 2, 2397847318805070. [Google Scholar] [CrossRef]
- Khan, Z.; Bhadouria, P.; Bisen, P.S. Nutritional and Therapeutic Potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef]
- Deng, R.; Chow, T.-J. Hypolipidemic, Antioxidant, and Antiinflammatory Activities of Microalgae Spirulina. Cardiovasc. Ther. 2010, 28, e33–e45. [Google Scholar] [CrossRef]
- Sorrenti, V.; Castagna, D.A.; Fortinguerra, S.; Buriani, A.; Scapagnini, G.; Willcox, D.C. Spirulina Microalgae and Brain Health: A Scoping Review of Experimental and Clinical Evidence. Mar. Drugs 2021, 19, 293. [Google Scholar] [CrossRef] [PubMed]
- Finamore, A.; Palmery, M.; Bensehaila, S.; Peluso, I. Antioxidant, immunomodulating, and microbial-modulating activities of the sustainable and ecofriendly spirulina. Oxidative Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Qureshi, M.A.; Ali, R.A. Spirulina Platensis Exposure Enhances Macrophage Phagocytic Function in Cats. Immunopharmacol. Immunotoxicol. 1996, 18, 457–463. [Google Scholar] [CrossRef]
- Satyaraj, E.; Reynolds, A.; Engler, R.; Labuda, J.; Sun, P. Supplementation of Diets With Spirulina Influences Immune and Gut Function in Dogs. Front. Nutr. 2021, 8, 667072. [Google Scholar] [CrossRef]
- Delsante, C.; Pinna, C.; Sportelli, F.; Dalmonte, T.; Stefanelli, C.; Vecchiato, C.G.; Biagi, G. Assessment of the Effects of Edible Microalgae in a Canine Gut Model. Animals 2022, 12, 2100. [Google Scholar] [CrossRef]
- Berk, B.A.; Packer, R.M.-A.; Fritz, J.; Volk, H.A. Oral Palatability Testing of a Medium-Chain Triglyceride Oil Supplement (MCT) in a Cohort of Healthy Dogs in a Non-Clinical Setting. Animals 2022, 12, 1639. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Veterinary Use. Guideline on the Demonstration of Palatability of Veterinary Medicinal Products; European Medicines Agency: Amsterdam, The Netherlands, 2012.
- EC/152/2009; Commission Regulation (EC) No 152/2009 of 27 January 2009, Laying Down the Methods of Sampling and Analysis for the Official Control of Feed. European Commission: Brussels, Belgium, 2009.
- European Pharmacopoeia; Council of Europe: Strasbourg, France, 2005.
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analysis Chemists: Arlington, VA, USA, 2000. [Google Scholar]
- Giménez, E.C.; Martin, F.; Collaborators. Vitamin B12 (cyanocobalamin) in Infant Formula Adult/Pediatric Nutritional Formula by Liquid Chromatography with Ultraviolet Detection: Collaborative Study, Final Action 2014.02. J. AOAC Int. 2018, 101, 1112–1118. [Google Scholar] [CrossRef]
- Brause, A.R.; Woollard, D.C.; Indyk, H.E.; Collaborators. Determination of Total Vitamin C in Fruit Juices and Related Products by Liquid Chromatography: Interlaboratory Study. J. AOAC Int. 2003, 86, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Moran, R. Formulae for Determination of Chlorophyllous Pigments Extracted with N,N-Dimethylformamide 1. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]
- Chamovitz, D.; Sandmann, G.; Hirschberg, J. Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J. Biol. Chem. 1993, 268, 17348–17353. [Google Scholar] [CrossRef]
- Bennet, A.; Bogorad, L. Complementary chromatic adaptation in cyanobacteria. J. Bacteriol. 1973, 130, 82–91. [Google Scholar]
- Chamorro, G.; Salazar, M.; Favila, L.; Bourges, H. Pharmacology and toxicology of Spirulina alga. Rev. Investig. Clin. Organo Del Hosp. Enferm. Nutr. 1996, 48, 389–399. [Google Scholar]
- Chamorro, G.; Salazar, M.; Araújo, K.G.d.L.; dos Santos, C.P.; Ceballos, G.; Castillo, L.F. Update on the pharmacology of Spirulina (Arthrospira), an unconventional food. Arch Lat. Nutr 2002, 52, 232–240. [Google Scholar]
- Salazar, M.; Martínez, E.; Madrigal, E.; Ruiz, L.E.; Chamorro, G.A. Subchronic toxicity study in mice fed Spirulina maxima. J. Ethnopharmacol. 1998, 62, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Naidu, K.A.; Sarada, R.; Manoj, G.; Khan, M.Y.; Swamy, M.M.; Viswanatha, S.; Srinivas, L. Toxicity assessment of phycocyanin-A blue colorant from blue green alga Spirulina platensis. Food Biotechnol. 1999, 13, 51–66. [Google Scholar] [CrossRef]
- Marles, R.J.; Barrett, M.L.; Barnes, J.; Chavez, M.L.; Gardiner, P.; Ko, R.; Mahady, G.B.; Dog, T.L.; Sarma, N.D.; Giancaspro, G.I.; et al. United States Pharmacopeia Safety Evaluation of Spirulina. Crit. Rev. Food Sci. Nutr. 2011, 51, 593–604. [Google Scholar] [CrossRef]
- Le, T.-M.; Knulst, A.C.; Röckmann, H. Anaphylaxis to Spirulina confirmed by skin prick test with ingredients of Spirulina tablets. Food Chem. Toxicol. 2014, 74, 309–310. [Google Scholar] [CrossRef]
- Mazokopakis, E.E.; Karefilakis, C.M.; Tsartsalis, A.N.; Milkas, A.N.; Ganotakis, E.S. Acute rhabdomyolysis caused by Spirulina (Arthrospira platensis). Phytomedicine 2008, 15, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, M.; Yamamoto, M.; Tanaka, Y.; Kaito, M.; Adachi, Y. Spirulina-Associated Hepatotoxicity. Off. J. Am. Coll. Gastroenterol. ACG 2002, 97, 3212. [Google Scholar] [CrossRef]
- Ciferri, O. Spirulina, the edible microorganism. Microbiol. Rev. 1983, 47, 551–578. [Google Scholar] [CrossRef] [PubMed]
- Gershwin, M.E.; Belay, A. Spirulina in Human Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Cheung, M.Y.; Liang, S.; Lee, J. Toxin-producing cyanobacteria in freshwater: A review of the problems, impact on drinking water safety, and efforts for protecting public health. J. Microbiol. 2013, 51, 1–10. [Google Scholar] [CrossRef]
- Bautista, A.C.; Moore, C.E.; Lin, Y.; Cline, M.G.; Benitah, N.; Puschner, B. Hepatopathy following consumption of a commercially available blue-green algae dietary supplement in a dog. BMC Vet. Res. 2015, 11, 136. [Google Scholar] [CrossRef]
- Seager, C.; Wroe, Y.; Baron, M.; Farrell, M.; Hadwin, C. Methodologies used to test the palatability of nutraceuticals. Vet. Nurse 2015, 6, 4–11. [Google Scholar] [CrossRef]
- Vrenna, M.; Peruccio, P.P.; Liu, X.; Zhong, F.; Sun, Y. Microalgae as Future Superfoods: Fostering Adoption through Practice-Based Design Research. Sustainability 2021, 13, 2848. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Kashani, A.; Malau-Aduli, A.E.O. Growth and body conformation responses of genetically divergent Australian sheep to Spirulina (Arthrospira platensis) supplementation. Am. J. Exp. Agric. 2012, 2, 160–173. [Google Scholar] [CrossRef]
- Lamminen, M.; Halmemies-Beauchet-Filleau, A.; Kokkonen, T.; Vanhatalo, A.; Jaakkola, S. The effect of partial substitution of rapeseed meal and faba beans by Spirulina platensis microalgae on milk production, nitrogen utilization, and amino acid metabolism of lactating dairy cows. J. Dairy Sci. 2019, 102, 7102–7117. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A.; Sartori, A.; Bohatir, P.; Rémignon, H.; Ricci, R. Effect of dietary supplementation of Spirulina (Arthrospira platensis) and Thyme (Thymus vulgaris) on growth performance, apparent digestibility and health status of companion dwarf rabbits. Livest. Sci. 2013, 152, 182–191. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Pakpour, S.; Wang, S.; Pan, Z.; Liu, J.; Wei, Q.; She, J.; Cang, H.; Zhang, R.X. Dose Effects of Orally Administered Spirulina Suspension on Colonic Microbiota in Healthy Mice. Front. Cell. Infect. Microbiol. 2019, 9, 243. [Google Scholar] [CrossRef]
- Gunes, S.; Tamburaci, S.; Dalay, M.C.; Deliloglu Gurhan, I. In vitro evaluation of Spirulina platensis extract incorporated skin cream with its wound healing and antioxidant activities. Pharm. Biol. 2017, 55, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.N.; Leite, M.G.A.; Maia Campos, P.M.B.G. Development of hair care formulations containing Spirulina platensis and Ascophyllum nodosum extracts. Int J. Phytocos Nat. Ingred. 2019, 6, 13. [Google Scholar] [CrossRef]
- Fernández, E.; Martínez-Teipel, B.; Armengol, R.; Barba, C.; Coderch, L. Efficacy of antioxidants in human hair. J. Photochem. Photobiol. B Biol. 2012, 117, 146–156. [Google Scholar] [CrossRef]
- Zanzottera, F.; Bizzaro, G.; Michelotti, A.; Nobile, V. Efficacy of a Nutritional Supplement, Standardized in Fatty Acids and Phytosterols, on Hair Loss and Hair Health in both Women and Men. J. Cosmo Trichol 2017, 3, 3. [Google Scholar] [CrossRef]
- Kalafati, M.; Jamurtas, T.; Nikolaidis, M.; Paschalis, V.; Theodorou, A.; Sakellariou, G.; Koutedakis, Y.; Kouretas, D. Ergogenic and Antioxidant Effects of Spirulina Supplementation in Humans. Med. Sci. Sport. Exerc. 2010, 42, 142–151. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, H.; Ding, X.; Liu, S.; Zou, Y. Analysis of the anti-fatigue activity of polysaccharides from Spirulina platensis: Role of central 5-hydroxytryptamine mechanisms. Food Funct. 2020, 11, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Lia Longodor, A.; Coroian, A.; Balta, I.; Taulescu, M.; Toma, C.; Sevastre, B.; Marchiș, Z.; Andronie, L.; Pop, I.; Matei, F.; et al. Protective Effects of Dietary Supplement Spirulina (Spirulina platensis) against Toxically Impacts of Monosodium Glutamate in Blood and Behavior of Swiss mouse. Separations 2021, 8, 218. [Google Scholar] [CrossRef]
- Sinha, S.; Patro, N.; Patro, I.K. Amelioration of neurobehavioral and cognitive abilities of F1 progeny following dietary supplementation with Spirulina to protein malnourished mothers. Brain Behav. Immun. 2020, 85, 69–87. [Google Scholar] [CrossRef]
- Moradi, S.; Zobeiri, M.; Feizi, A.; Clark, C.C.; Entezari, M.H. The effects of spirulina (Arthrospira platensis) supplementation on anthropometric indices, blood pressure, sleep quality, mental health, fatigue status and quality of life in patients with ulcerative colitis: A randomised, double-blinded, placebo-controlled trial. Int. J. Clin. Pract. 2021, 75, e14472. [Google Scholar]
- Tynes, V.V.; Landsberg, G.M. Nutritional Management of Behavior and Brain Disorders in Dogs and Cats. Vet. Clin. Small Anim. Pract. 2021, 51, 711–727. [Google Scholar] [CrossRef]
- Orlando, J.M. Behavioral Nutraceuticals and Diets. Vet. Clin. Small Anim. Pract. 2018, 48, 473–495. [Google Scholar] [CrossRef]
- Zoran, D.L.; Buffington, C.A.T. Effects of nutrition choices and lifestyle changes on the well-being of cats, a carnivore that has moved indoors. J. Am. Vet. Med. Assoc. 2011, 239, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, Y.J.; Ryu, H.K.; Kim, M.H.; Chung, H.W.; Kim, W.Y. A Randomized Double-Blind, Placebo-Controlled Study to Establish the Effects of Spirulina in Elderly Koreans. ANM 2008, 52, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Leung, P.S.; Fischer, L.; German, B.; Yang, C.-Y.; Kenny, T.P.; Cysewski, G.R.; Gershwin, M.E. The effects of Spirulina on anemia and immune function in senior citizens. Cell Mol. Immunol. 2011, 8, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Ziaei, R.; Foshati, S.; Mohammadi, H.; Nachvak, S.M.; Rouhani, M.H. Effects of Spirulina supplementation on obesity: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2019, 47, 102211. [Google Scholar] [CrossRef]
- Hamedifard, Z.; Milajerdi, A.; Reiner, Ž.; Taghizadeh, M.; Kolahdooz, F.; Asemi, Z. The effects of spirulina on glycemic control and serum lipoproteins in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 2609–2621. [Google Scholar] [CrossRef]
- Beynen, A.C. Microalgae in petfood. Creat. Companion 2019, 40, 7. [Google Scholar]
| Animals | T1 (Days 1–14) | T2 (Days 15–28) | T3 (Days 29–42) |
|---|---|---|---|
| Small Dogs | 64.9 ± 27.9 | 129.7 ± 55.8 | 194.6 ± 83.7 |
| Medium Dogs | 49.3 ± 15.0 | 98.6 ± 29.9 | 147.9 ± 44.9 |
| Large Dogs | 38.4 ± 5.0 | 76.8 ± 10.0 | 115.2 ± 15.1 |
| Cats | 83.7 ± 23.5 | 167.3 ± 47.1 | 251.0 ± 70.6 |
| Nutrients | % DM 1 |
|---|---|
| Crude Protein | 60.44 |
| Crude Fat | 6.75 |
| Crude Fiber | 0.00 |
| Ash | 11.48 |
| C14:0 | 0.03 |
| C14:1 | 0.07 |
| C15:0 | 0.03 |
| C16:0 | 2.68 |
| C16:1 | 0.27 |
| C17:0 | 0.01 |
| C17:1 | 0.02 |
| C18:0 | 0.08 |
| C18:1 Ω9 | 0.26 |
| C18:2 Ω6 | 1.59 |
| C18:3 Ω6 | 0.83 |
| C18:3 Ω3 | 0.01 |
| C20:0 | 0.01 |
| C20:1 Ω9 | 0.01 |
| C20:2 | 0.01 |
| C20:3 Ω6 | 0.02 |
| C20:5 Ω3 | 0.00 |
| C22:6 Ω3 | 0.00 |
| Histidine | 1.27 |
| Arginine | 4.26 |
| Serina | 3.23 |
| Glycine | 2.87 |
| Asparagine | 5.55 |
| Glutamine | 9.93 |
| Threonine | 3.23 |
| Alanine | 4.58 |
| Proline | 2.37 |
| Lysine | 3.19 |
| Methionine | 0.50 |
| Tyrosine | 2.33 |
| Valine | 3.63 |
| Cysteine | 0.61 |
| Isoleucine | 3.18 |
| Leucine | 5.87 |
| Phenylalanine | 2.99 |
| Taurine | 0.07 |
| Tryptophan | 1.44 |
| Calcium, Ca | 0.46 |
| Iron, Fe | 0.05 |
| Potassium, K | 2.47 |
| Magnesium, Mg | 0.43 |
| Sodium, Na | 1.46 |
| Phosphorus, P | 1.35 |
| Vitamin B12 (cyanocobalamin) | 13.1 ± 2.6 2 |
| Vitamin C | <20 3 |
| Vitamin E | 31.1 ± 7.8 3 |
| Chlorophyll a | 1.6 ± 0.3 4 |
| Carotenoids | 0.3 ± 0.1 4 |
| Allophycocyanin | 21.9 ± 2.3 4 |
| Phycocyanin | 50.9 ± 3.4 4 |
| Dog Owners, n (%) | Cat Owners, n (%) | ||
|---|---|---|---|
| Gender | Male | 19 (31.7%) | 4 (13.3%) |
| Female | 41 (68.3%) | 26 (86.7%) | |
| Age | 18–34 | 25 (41.6%) | 17 (56.7%) |
| 35–49 | 17 (28.3%) | 10 (33.3%) | |
| 50–64 | 15 (25.0%) | 2 (6.7%) | |
| ≥65 | 3 (5.0%) | 1 (3.3%) |
| Dogs, n (%) | ||
|---|---|---|
| Gender | Male | 29 (48.3%) |
| Female | 31 (51.7%) | |
| Neutering Status | Yes No | 30 (50.0%) 30 (50.0%) |
| Body Condition (According to Owner) | Underweight Ideal weight | 3 (5.0%) 46 (76.7%) |
| Overweight | 11 (18.3%) |
| Cats, n (%) | ||
|---|---|---|
| Gender | Male | 17 (56.7%) |
| Female | 13 (43.3%) | |
| Neutering Status | Yes No | 28 (93.3%) 2 (6.7%) |
| Body Condition (According to Owner) | Underweight Ideal weight | 1 (3.3%) 17 (56.7%) |
| Overweight | 12 (40.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanutti, D.; Tonin, G.; Morelli, G.; Zampieri, R.M.; La Rocca, N.; Ricci, R. Oral Palatability and Owners’ Perception of the Effect of Increasing Amounts of Spirulina (Arthrospira platensis) in the Diet of a Cohort of Healthy Dogs and Cats. Animals 2023, 13, 1275. https://doi.org/10.3390/ani13081275
Stefanutti D, Tonin G, Morelli G, Zampieri RM, La Rocca N, Ricci R. Oral Palatability and Owners’ Perception of the Effect of Increasing Amounts of Spirulina (Arthrospira platensis) in the Diet of a Cohort of Healthy Dogs and Cats. Animals. 2023; 13(8):1275. https://doi.org/10.3390/ani13081275
Chicago/Turabian StyleStefanutti, Davide, Gloria Tonin, Giada Morelli, Raffaella Margherita Zampieri, Nicoletta La Rocca, and Rebecca Ricci. 2023. "Oral Palatability and Owners’ Perception of the Effect of Increasing Amounts of Spirulina (Arthrospira platensis) in the Diet of a Cohort of Healthy Dogs and Cats" Animals 13, no. 8: 1275. https://doi.org/10.3390/ani13081275
APA StyleStefanutti, D., Tonin, G., Morelli, G., Zampieri, R. M., La Rocca, N., & Ricci, R. (2023). Oral Palatability and Owners’ Perception of the Effect of Increasing Amounts of Spirulina (Arthrospira platensis) in the Diet of a Cohort of Healthy Dogs and Cats. Animals, 13(8), 1275. https://doi.org/10.3390/ani13081275








