Early Milk Total and Differential Cell Counts as a Diagnostic Tool to Improve Antimicrobial Therapy Protocols
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Herd and Cow Selection
2.2. Sample Collection and Analysis
2.3. Statistical Analysis
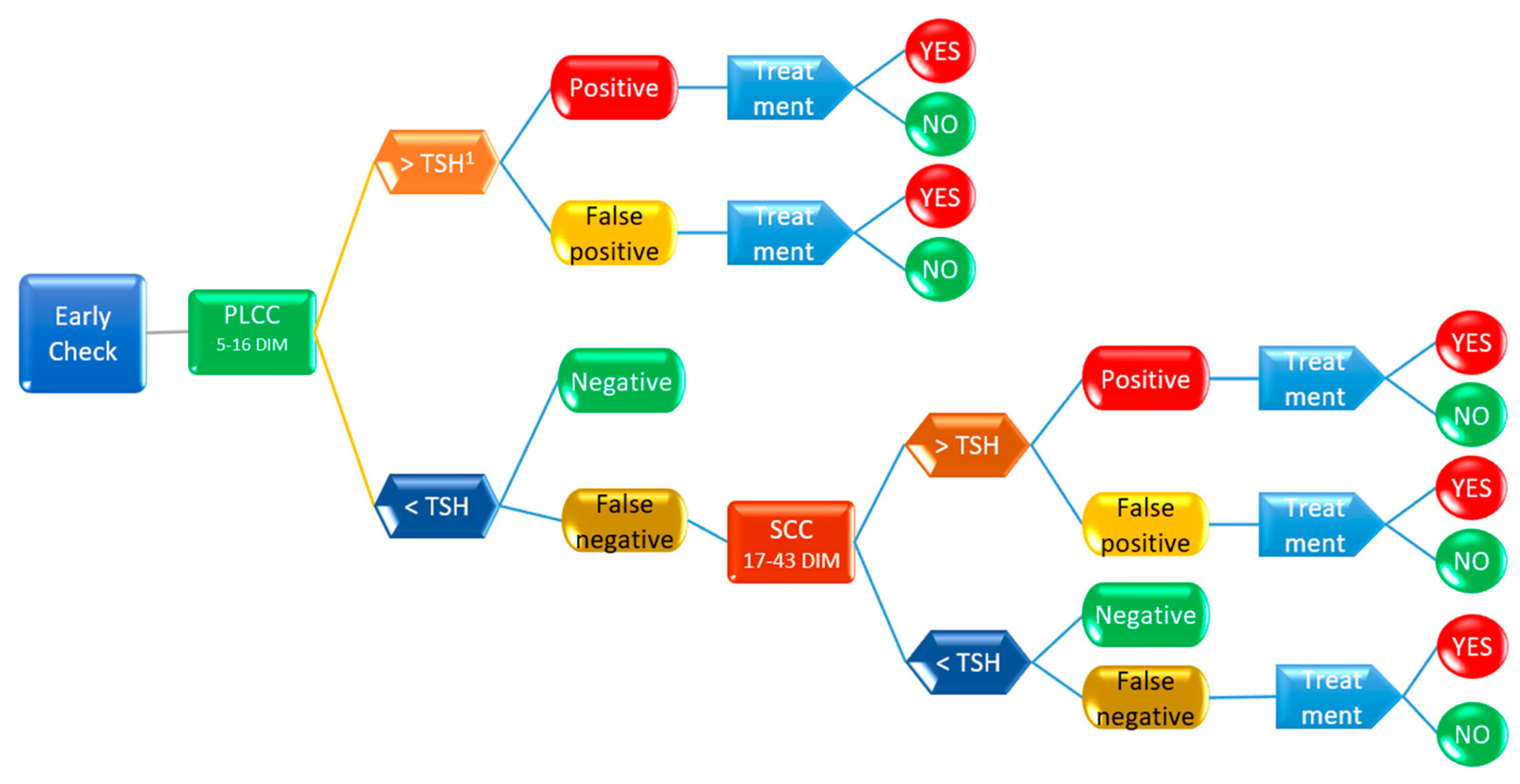
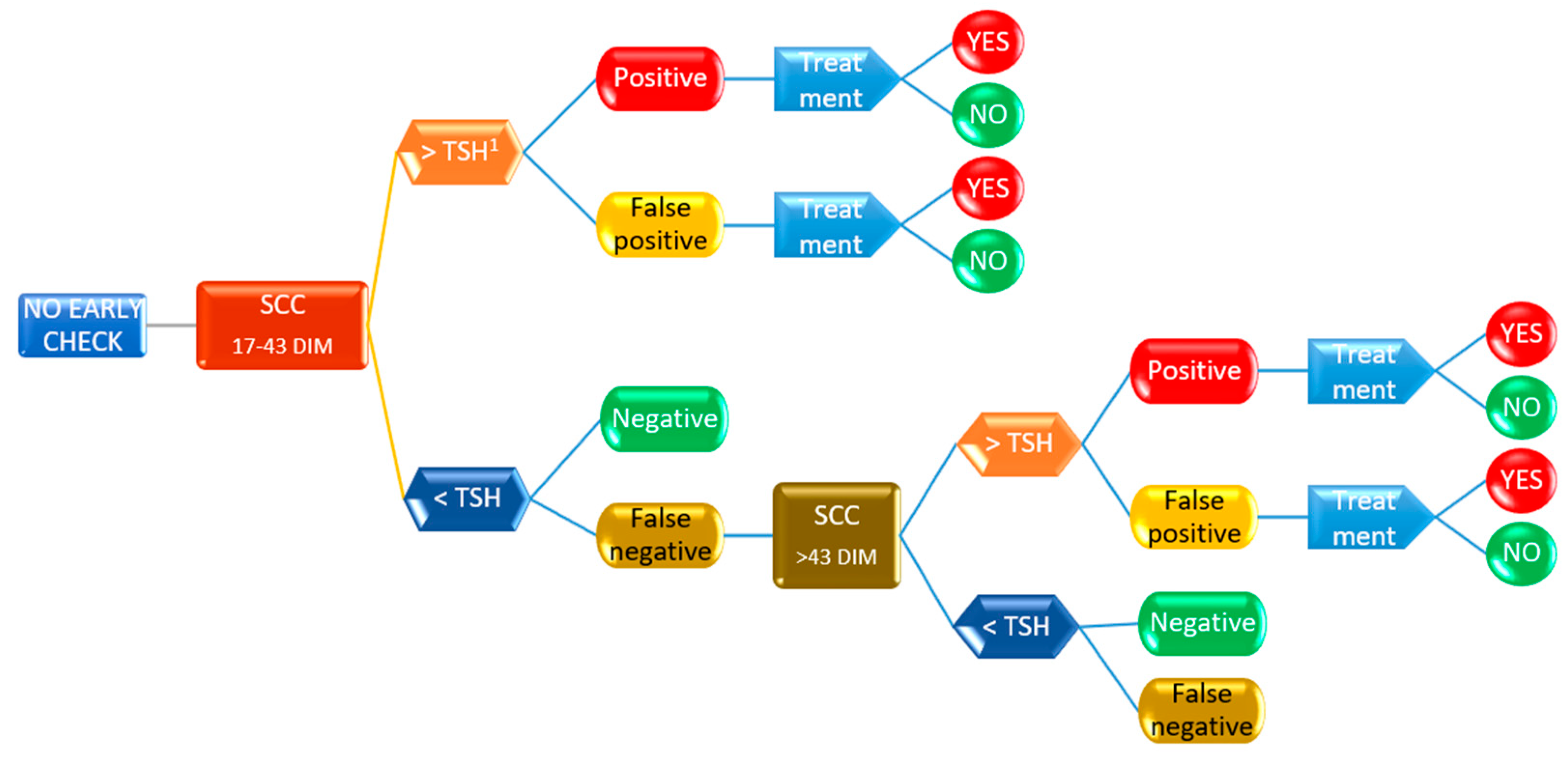
2.4. Decision Tree Analysis
3. Results
3.1. Data Description
3.2. SCC, DSCC and PLCC as Markers for Major Pathogens
3.3. Decsion Tree Model for Antimicrobial Treatment Probability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Summer, A.; Franceschi, P.; Formaggioni, P.; Malacarne, M. Influence of milk somatic cell content on Parmigiano-Reggiano cheese yield. J. Dairy Res. 2015, 82, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Sora, V.M.; Panseri, S.; Nobile, M.; Di Cesare, F.; Meroni, G.; Chiesa, L.M.; Zecconi, A. Milk Quality and Safety in a One Health Perspective: Results of a Prevalence Study on Dairy Herds in Lombardy (Italy). Life 2022, 12, 786. [Google Scholar] [CrossRef]
- Zecconi, A.; Dell’Orco, F.; Rizzi, N.; Vairani, D.; Cipolla, M.; Pozzi, P.; Zanini, L. Cross-sectional study on the prevalence of contagious pathogens in bulk tank milk and their effects on somatic cell counts and milk yield. Ital. J. Anim. Sci. 2019, 19, 66–74. [Google Scholar] [CrossRef]
- Halasa, T.; Huijps, K.; Osteras, O.; Hogeveen, H. Economic effects of bovine mastitis and mastitis management: A review. Vet. Q. 2007, 29, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Steeneveld, W.; Nielen, M.; Zanini, L.; Zecconi, A. Linear Mixed-Effects Model to Quantify the Association between Somatic Cell Count and Milk Production in Italian Dairy Herds. Animals 2023, 13, 80. [Google Scholar] [CrossRef]
- Trevisi, E.; Zecconi, A.; Cogrossi, S.; Razzuoli, E.; Grossi, P.; Amadori, M. Strategies for reduced antibiotic usage in dairy cattle farms. Res. Vet. Sci. 2014, 96, 229–233. [Google Scholar] [CrossRef]
- Kuipers, A.; Koops, W.J.; Wemmenhove, H. Antibiotic use in dairy herds in the Netherlands from 2005 to 2012. J. Dairy Sci. 2016, 99, 1632–1648. [Google Scholar] [CrossRef]
- Chiesa, L.M.; DeCastelli, L.; Nobile, M.; Martucci, F.; Mosconi, G.; Fontana, M.; Castrica, M.; Arioli, F.; Panseri, S. Analysis of antibiotic residues in raw bovine milk and their impact toward food safety and on milk starter cultures in cheese-making process. LWT 2020, 131, 109783. [Google Scholar] [CrossRef]
- Firth, C.L.; Kremer, K.; Werner, T.; Kasbohrer, A. The Effects of Feeding Waste Milk Containing Antimicrobial Residues on Dairy Calf Health. Pathogens 2021, 10, 112. [Google Scholar] [CrossRef]
- McEwen, S.A.; Fedorka-Cray, P.J. Antimicrobial use and resistance in animals. Clin. Infect. Dis. 2002, 34, S93–S106. [Google Scholar] [CrossRef]
- Barkema, H.W.; Schukken, Y.H.; Zadoks, R.N. Invited review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef]
- Barlow, J. Mastitis Therapy and Antimicrobial Susceptibility: A Multispecies Review with a Focus on Antibiotic Treatment of Mastitis in Dairy Cattle. J. Mammary Gland Biol. Neoplasia 2011, 16, 383–407. [Google Scholar] [CrossRef]
- Zecconi, A.; Piccinini, R.; Fox, K.L. Epidemiologic study of intramammary infections with Staphylococcus aureus during a control program in nine commercial dairy herds. J. Am. Vet. Med. Assoc. 2003, 223, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A. Contagious mastitis control. FIL-IDF Bull. 2007, 416, 34–40. [Google Scholar]
- Farre, M.; Zecconi, A.; Kelton, D. Guidelines for Defining Quarter and Udder Health Status and Cured Clinical and Subclinical Mastitis cases; International Dairy Federation: Bruxelles, Belgium, 2022; p. 34. [Google Scholar]
- Zecconi, A.; Meroni, G.; Sora, V.; Mattina, R.; Cipolla, M.; Zanini, L. Total and differential cell counts as a tool to identify intramammary infections in cows after calving. Animals 2021, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Vairani, D.; Cipolla, M.; Rizzi, N.; Zanini, L. Assessment of Subclinical Mastitis Diagnostic Accuracy by Differential Cell Count in Individual Cow Milk. Ital. J. Anim. Sci. 2018, 18, 435–440. [Google Scholar] [CrossRef]
- Burvenich, C.; Merris, V.V.; Mehrzad, J.; Diez-Fraile, A.; Duchateau, L. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 2003, 34, 521–564. [Google Scholar] [CrossRef]
- Zecconi, A.; Cesaris, L.; Liandris, E.; Daprà, V.; Piccinini, R. Role of several Staphylococcus aureus virulence factors on the inflammatory response in bovine mammary gland. Microb. Pathog. 2006, 40, 177–183. [Google Scholar] [CrossRef]
- Archer, N.; Egan, S.; Coffey, T.; Emes, R.; Addis, M.; Ward, P.; Blanchard, A.; Leigh, J. A Paradox in Bacterial Pathogenesis: Activation of the Local Macrophage Inflammasome Is Required for Virulence of Streptococcus uberis. Pathogens 2020, 9, 997. [Google Scholar] [CrossRef] [PubMed]
- Pyorala, S. Indicators of inflammation in the diagnosis of mastitis. Vet. Res. 2003, 34, 565–578. [Google Scholar] [CrossRef]
- Dohoo, I.; Smith, J.; Andersen, S.; Kelton, D.F.; Godden, S.; Mastitis Res Workers, C. Diagnosing intramammary infections: Evaluation of definitions based on a single milk sample. J. Dairy Sci. 2011, 94, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, R.J.; Hutchinson, L.J.; Spencer, S.B. Somatic cell counts in DHI samples. In Proceedings of the Annual Meeting—National Mastitis Council, Inc., Louisville, KY, USA, 19–21 February 1979; p. 32. [Google Scholar]
- Schukken, Y.; Wilson, D.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R. Monitoring udder health and milk quality using somatic cell counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef] [PubMed]
- Zecconi, A.; Zanini, L.; Cipolla, M.; Stefanon, B. Factors Affecting the Patterns of Total Amount and Proportions of Leukocytes in Bovine Milk. Animals 2020, 10, 992. [Google Scholar] [CrossRef] [PubMed]
- Bellazzi, R.; Zupan, B. Predictive data mining in clinical medicine: Current issues and guidelines. Int. J. Med. Inform. 2008, 77, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Damm, M.; Holm, C.; Blaabjerg, M.; Bro, M.N.; Schwarz, D. Differential somatic cell count-A novel method for routine mastitis screening in the frame of Dairy Herd Improvement testing programs. J. Dairy Sci. 2017, 100, 4926–4940. [Google Scholar] [CrossRef]
- N.M.C. Laboratory Handbook on Bovine Mastitis; N.M.C.: New Prague, MN, USA, 2017. [Google Scholar]
- Ferronatto, J.A.; Ferronatto, T.C.; Schneider, M.; Pessoa, L.F.; Blagitz, M.G.; Heinemann, M.B.; Della Libera, A.M.M.P.; Souza, F.N. Diagnosing mastitis in early lactation: Use of Somaticell®, California mastitis test and somatic cell count. Ital. J. Anim. Sci. 2018, 17, 723–729. [Google Scholar] [CrossRef]
- Schewe, R.L.; Kayitsinga, J.; Contreras, G.A.; Odom, C.; Coats, W.A.; Durst, P.; Hovingh, E.P.; Martinez, R.O.; Mobley, R.; Moore, S.; et al. Herd management and social variables associated with bulk tank somatic cell count in dairy herds in the eastern United States. J. Dairy Sci. 2015, 98, 7650–7665. [Google Scholar] [CrossRef]
- Zecconi, A.; Gusmara, C.; Di Giusto, T.; Cipolla, M.; Marconi, P.; Zanini, L. Observational study on application of a selective dry-cow therapy protocol based on individual somatic cell count thresholds. Ital. J. Anim. Sci. 2020, 19, 1341–1348. [Google Scholar] [CrossRef]
- Winder, C.B.; Sargeant, J.M.; Kelton, D.F.; Leblanc, S.J.; Duffield, T.F.; Glanville, J.; Wood, H.; Churchill, K.J.; Dunn, J.; Bergevin, M.D.; et al. Comparative efficacy of blanket versus selective dry-cow therapy: A systematic review and pairwise meta-analysis. Anim. Health Res. Rev. 2019, 20, 217–228. [Google Scholar] [CrossRef]
- Smith, K.L.; Todhunter, D.A.; Schoenberger, P.S. Environmental mastitis: Cause, prevalence, prevention. J. Dairy Sci. 1985, 68, 1531–1553. [Google Scholar] [CrossRef]
- Hoischen-Taubner, S.; Habel, J.; Uhlig, V.; Schwabenbauer, E.M.; Rumphorst, T.; Ebert, L.; Moller, D.; Sundrum, A. The Whole and the Parts-A New Perspective on Production Diseases and Economic Sustainability in Dairy Farming. Sustainability 2021, 13, 9044. [Google Scholar] [CrossRef]
- Zecconi, A.; Piccinini, R.; Fox, K.L. Epidemiological study of non-contagious intramammary infections in nine commercial dairy herds following a Staphylococcus aureus control programme. J. Vet. Med. B 2004, 51, 333–336. [Google Scholar] [CrossRef] [PubMed]

| Lactation Period | N | Days in Milk ± Std.dev (d) | SCC 1 ± Std.dev (Log10/mL) | DSCC 2 ± Std.dev (%) | PLCC 3 ± Std.dev (Log10/mL) |
|---|---|---|---|---|---|
| A (5–16 d) | 995 | 11.0 ± 3.3 | 5.0 ± 0.6 | 63.5 ± 15.9 | 4.8 ± 0.7 |
| B (17–43 d) | 831 | 24.9 ± 7.00 | 4.9 ± 0.7 | 64.0 ± 18.3 | 4.7 ± 0.7 |
| C (44–300 d) | 907 | 167.7 ± 93.2 | 5.1 ± 0.6 | 64.2 ± 17.6 | 4.9 ± 0.7 |
| Lactation Period | Quarter (N) | MajP 1 | Other Pathogens | Negative |
|---|---|---|---|---|
| A (5–16 d) | 2407 | 1.5% | 38.0% | 60.5% |
| B (17–43 d) | 2336 | 1.6% | 28.2% | 70.3% |
| C (44–300 d) | 2001 | 6.8% | 38.1% | 55.2% |
| Lactation Period | Parameter | Thres Hold | Sensitivity | Lower Bound (95%) | Upper Bound (95%) | Specificity | Lower Bound (95%) | Upper Bound (95%) | PPV 4 | NPV 5 | Accuracy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A (5–16 d) | PLCC 1 | 163,500 | 0.700 | 0.544 | 0.819 | 0.779 | 0.752 | 0.804 | 0.117 | 0.984 | 0.776 |
| SCC 2 | 198,000 | 0.725 | 0.570 | 0.839 | 0.756 | 0.728 | 0.782 | 0.111 | 0.985 | 0.755 | |
| DSCC 3 | 75.2% | 0.575 | 0.422 | 0.715 | 0.742 | 0.714 | 0.769 | 0.086 | 0.977 | 0.736 | |
| B (17–43 d) | PLCC | 53,000 | 0.686 | 0.519 | 0.815 | 0.613 | 0.579 | 0.646 | 0.072 | 0.978 | 0.616 |
| SCC | 40,000 | 0.886 | 0.733 | 0.959 | 0.418 | 0.385 | 0.453 | 0.063 | 0.988 | 0.438 | |
| DSCC | 68.7% | 0.686 | 0.519 | 0.815 | 0.563 | 0.528 | 0.597 | 0.065 | 0.976 | 0.568 | |
| C (44–300 d) | PLCC | 84,000 | 0.821 | 0.749 | 0.876 | 0.610 | 0.575 | 0.644 | 0.278 | 0.949 | 0.643 |
| SCC | 140,000 | 0.800 | 0.725 | 0.858 | 0.635 | 0.600 | 0.668 | 0.286 | 0.946 | 0.660 | |
| DSCC | 74.4% | 0.586 | 0.503 | 0.664 | 0.729 | 0.696 | 0.759 | 0.283 | 0.906 | 0.707 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zecconi, A.; Zaghen, F.; Meroni, G.; Sora, V.; Martino, P.A.; Laterza, G.; Zanini, L. Early Milk Total and Differential Cell Counts as a Diagnostic Tool to Improve Antimicrobial Therapy Protocols. Animals 2023, 13, 1143. https://doi.org/10.3390/ani13071143
Zecconi A, Zaghen F, Meroni G, Sora V, Martino PA, Laterza G, Zanini L. Early Milk Total and Differential Cell Counts as a Diagnostic Tool to Improve Antimicrobial Therapy Protocols. Animals. 2023; 13(7):1143. https://doi.org/10.3390/ani13071143
Chicago/Turabian StyleZecconi, Alfonso, Francesca Zaghen, Gabriele Meroni, Valerio Sora, Piera Anna Martino, Giulia Laterza, and Lucio Zanini. 2023. "Early Milk Total and Differential Cell Counts as a Diagnostic Tool to Improve Antimicrobial Therapy Protocols" Animals 13, no. 7: 1143. https://doi.org/10.3390/ani13071143
APA StyleZecconi, A., Zaghen, F., Meroni, G., Sora, V., Martino, P. A., Laterza, G., & Zanini, L. (2023). Early Milk Total and Differential Cell Counts as a Diagnostic Tool to Improve Antimicrobial Therapy Protocols. Animals, 13(7), 1143. https://doi.org/10.3390/ani13071143






