Evaluation of Substance P as a New Stress Parameter in Horses in a Stress Model Involving Four Different Stress Levels
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sampling Methods
2.3. Experimental Procedure
2.4. Ridden Horse Analysis
2.5. Analysis of Cortisol and Substance P
2.6. Establishing Reference Values
2.7. Statistical Analysis
3. Results
3.1. Establishing Reference Values of Substance P
3.2. Four Different Stress Levels—Correlation with Stress Parameters
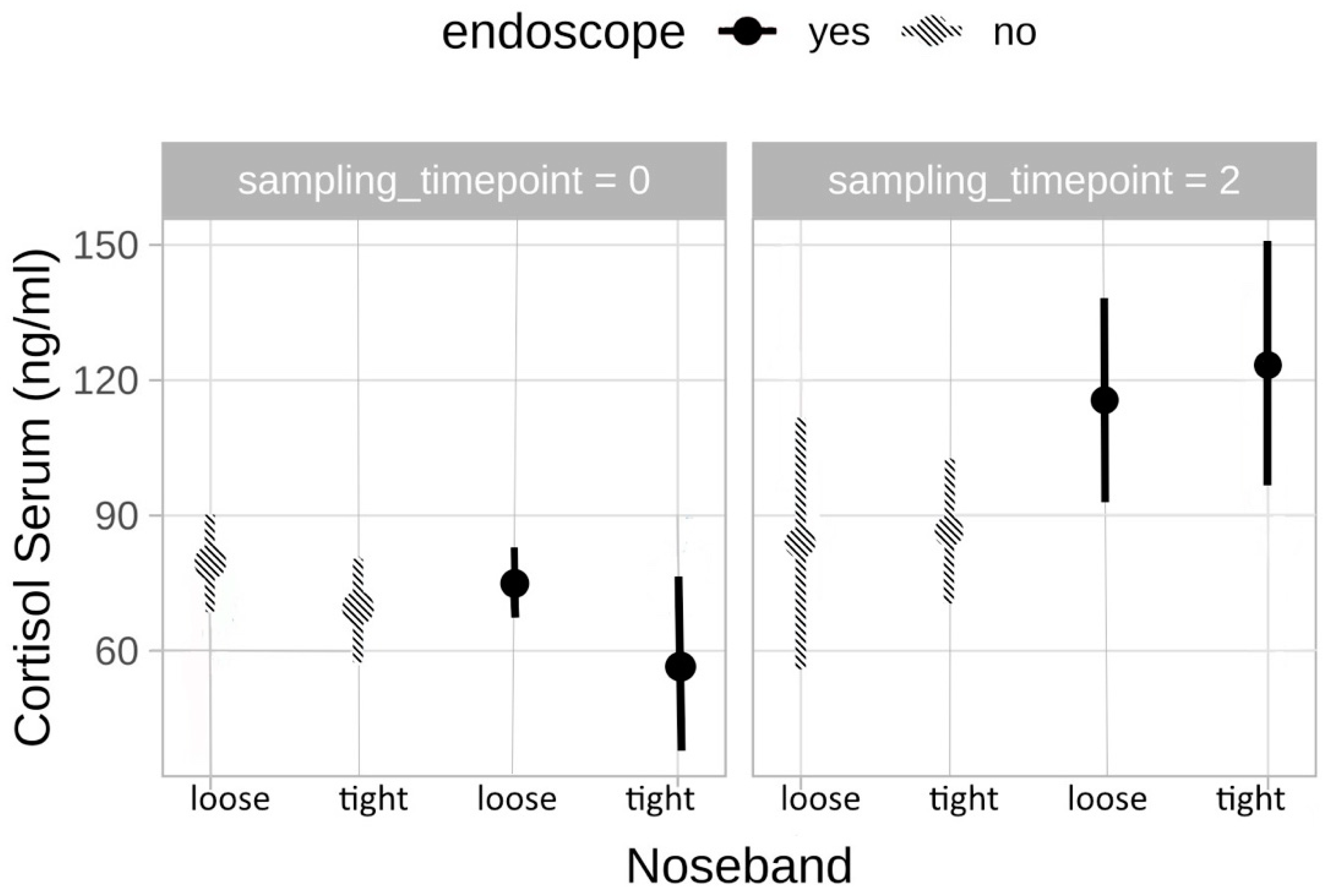
3.2.1. Cortisol
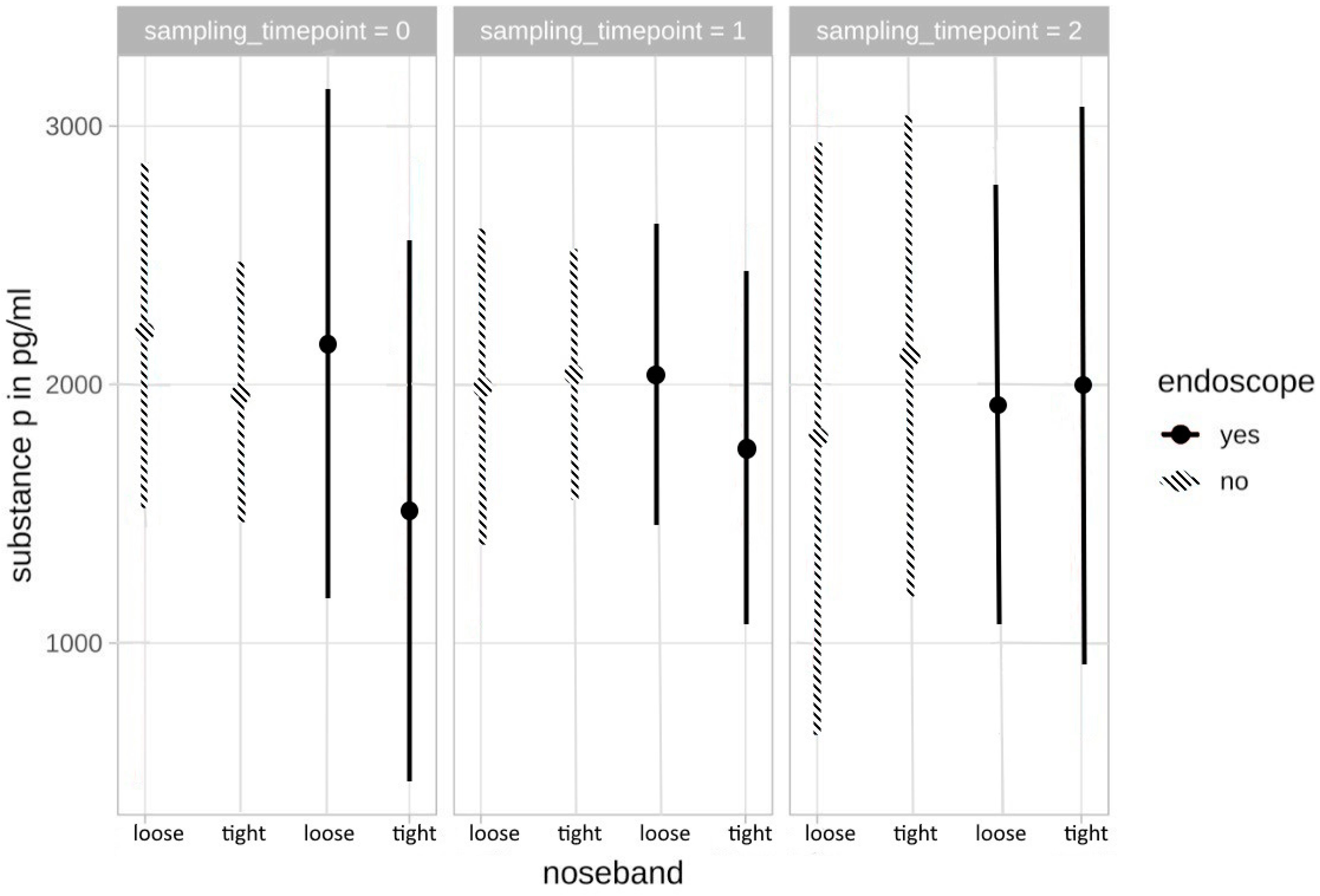
3.2.2. Substance P
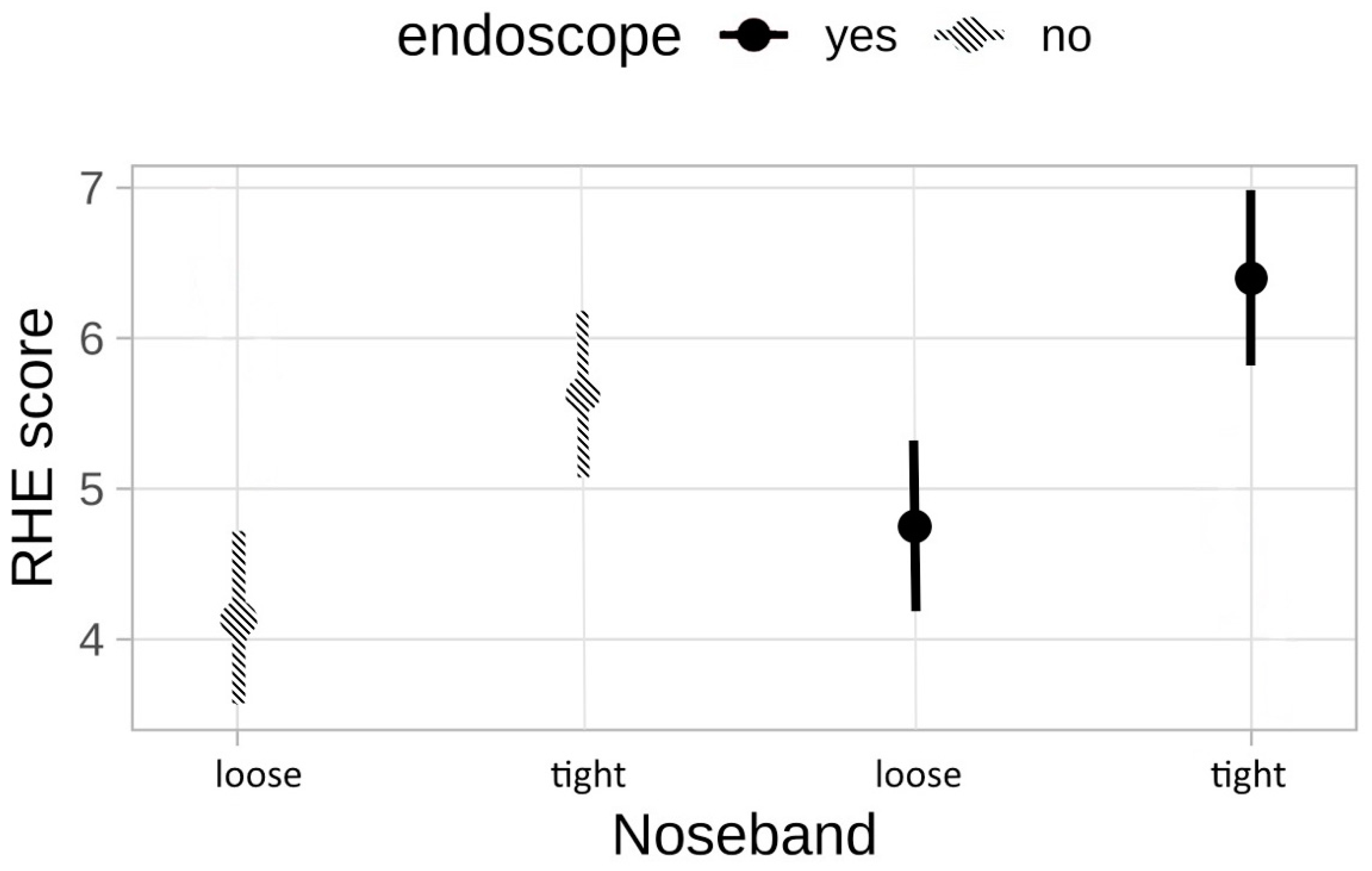
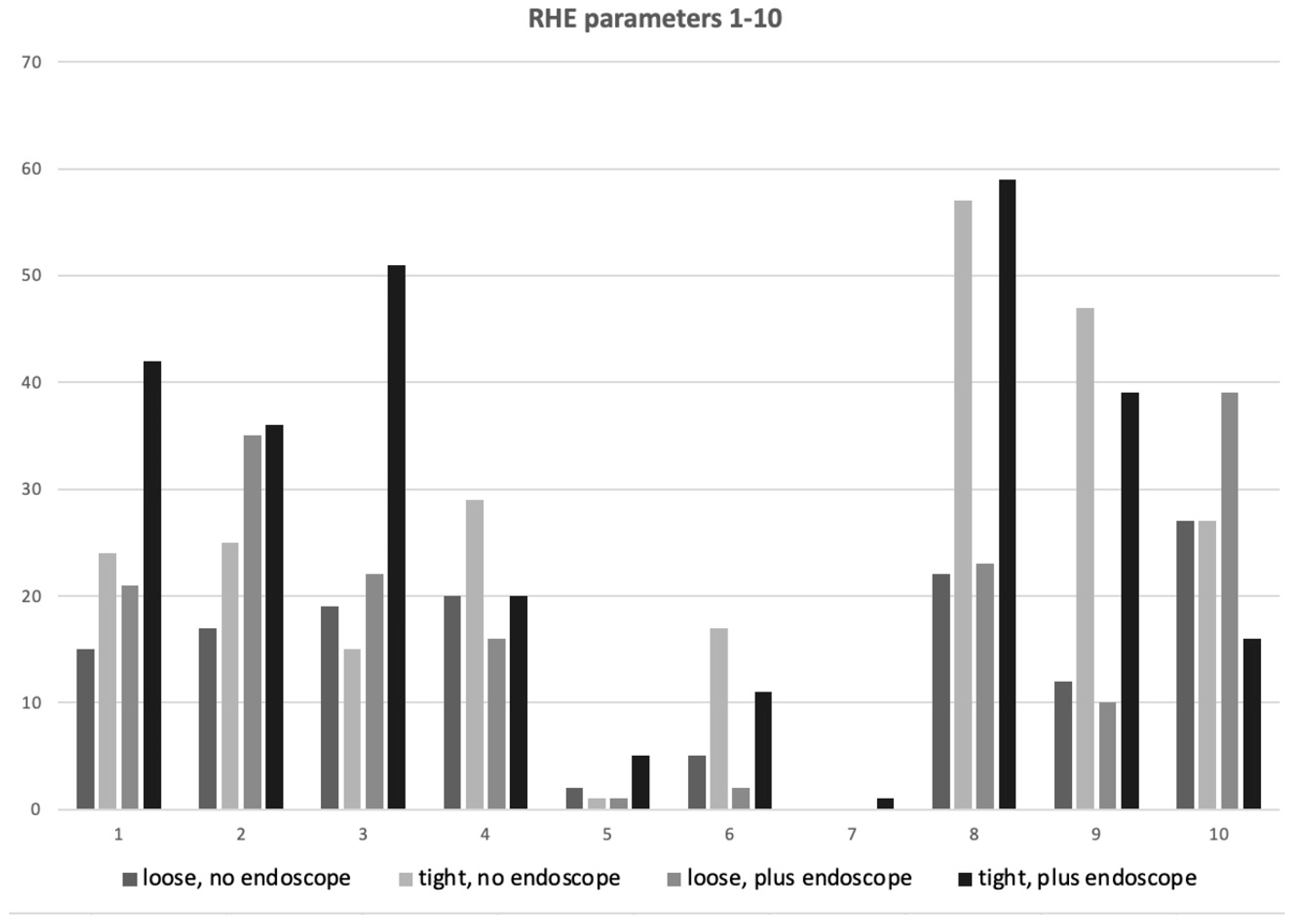
3.3. Ridden Horse Ethogram (RHE)
3.4. Correlation between Stress Parameters and Ridden Horse Ethogram (RHE)
3.4.1. Cortisol
3.4.2. Substance P
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heuschmann, G. Tug of War: Classical Versus ‘Modern’ Dressage. In Why Classic Training Works and How Incorrect ‘Modern’ Riding Negatively Affects Horses’ Health; Trafalgar Square Books: North Pomfret, VT, USA, 2018; p. 144. [Google Scholar]
- Weller, D.; Franklin, S.; Shea, G.; White, P.; Fenner, K.; Wilson, B.; Wilkins, C.; McGreevy, P. The Reported Use of Nosebands in Racing and Equestrian Pursuits. Animals 2020, 10, 776. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Manrique, L.; León-Pérez, K.; Zamora-Sánchez, E.; Davies, S.; Ober, C.; Wilson, B.; McGreevy, P. Prevalence and Distribution of Lesions in the Nasal Bones and Mandibles of a Sample of 144 Riding Horses. Animals 2020, 10, 1661. [Google Scholar] [CrossRef] [PubMed]
- Doherty, O.; Casey, V.; McGreevy, P.; Arkins, S. Noseband Use in Equestrian Sports—An International Study. PLoS ONE 2017, 12, e0169060. [Google Scholar] [CrossRef] [PubMed]
- Merkies, K.; Copelin, C.; Small, N.; Young, J. Noseband Fit: Measurements and Perceptions of Canadian Equestrians. Animals 2022, 12, 2685. [Google Scholar] [CrossRef] [PubMed]
- Fenner, K.; Yoon, S.; White, P.; Starling, M.; McGreevy, P. The Effect of Noseband Tightening on Horses’ Behavior, Eye Temperature, and Cardiac Responses. PLoS ONE 2016, 11, e0154179. [Google Scholar] [CrossRef]
- Uldahl, M.; Clayton, H.M. Lesions associated with the use of bits, nosebands, spurs and whips in Danish competition horses. Equine Veter. J. 2018, 51, 154–162. [Google Scholar] [CrossRef]
- Duke, D. The Effects of a Variation in Noseband Tightness on the Rein Tension of the Ridden Horse. Ph.D. Thesis, University of Plymouth, Plymouth, UK, 2017. [Google Scholar]
- McGreevy, P.; Warren-Smith, A.; Guisard, Y. The effect of double bridles and jaw-clamping crank nosebands on temperature of eyes and facial skin of horses. J. Veter. Behav. 2012, 7, 142–148. [Google Scholar] [CrossRef]
- Sloet van Oldruitenborgh-Oosterbaan, M.S.; Blok, M.B.; Begeman, L.; Kamphuis, M.C.D.; Lameris, M.C.; Spierenburg, A.J.; Lashley, M.J.J.O. Workload and stress in horses: Comparison in horses ridden deep and round (‘rollkur’) with a draw rein and horses ridden in a natural frame with only light rein contact. Tijdschr Diergeneeskd 2006, 131, 152–157. [Google Scholar] [PubMed]
- McGreevy, P.D. The advent of equitation science. Veter. J. 2007, 174, 492–500. [Google Scholar] [CrossRef]
- McGreevy, P. The fine line between pressure and pain: Ask the horse. Veter. J. 2011, 188, 250–251. [Google Scholar] [CrossRef]
- Heleski, C.; McGreevy, P.; Kaiser, L.; Lavagnino, M.; Tans, E.; Bello, N.; Clayton, H. Effects on behaviour and rein tension on horses ridden with or without martingales and rein inserts. Veter. J. 2009, 181, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.; Berger, J.; Ellis, A.D.; Mullard, J. Development of an ethogram for a pain scoring system in ridden horses and its application to determine the presence of musculoskeletal pain. J. Veter. Behav. 2018, 23, 47–57. [Google Scholar] [CrossRef]
- Dyson, S.; Ellis, A. Application of a Ridden Horse Pain Ethogram to horses competing at 5-star three-day-events: Comparison with performance. Equine Vet. Educ. 2022, 34, 306–315. [Google Scholar] [CrossRef]
- Hellhammer, D.H.; Wüst, S.; Kudielka, B.M. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 2009, 34, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Ehlert, U. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology 2012, 37, 1111–1134. [Google Scholar] [CrossRef] [PubMed]
- Ebner, K.; Singewald, N. The role of substance P in stress and anxiety responses. Amino Acids 2006, 31, 251–272. [Google Scholar] [CrossRef]
- Nieber, K.; Oehme, P. Substance P—A neuropeptide transmitter. Z. Gesamte Inn. Med. 1982, 37, 577–582. [Google Scholar]
- McLean, S.; Ganong, A.H.; Seeger, T.F.; Bryce, D.K.; Pratt, K.G.; Reynolds, L.S.; Siok, C.J.; Lowe, J.A.; Heym, J. Activity and Distribution of Binding Sites in Brain of a Nonpeptide Substance P (NK 1) Receptor Antagonist. Science 1991, 251, 437–439. [Google Scholar] [CrossRef]
- Ebner, K.; Rupniak, N.M.; Saria, A.; Singewald, N. Substance P in the medial amygdala: Emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc. Natl. Acad. Sci. USA 2004, 101, 4280–4285. [Google Scholar] [CrossRef]
- Herbert, M.K.; Holzer, P. Why are substance P(NK1)-receptor antagonists ineffective in pain treatment? Anaesthesist 2002, 51, 308–319. [Google Scholar] [CrossRef]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Ebner, K.; Muigg, P.; Singewald, G.; Singewald, N. Substance P in stress and anxiety: NK-1 receptor antagonism interacts with key brain areas of the stress circuitry. Ann. N. Y. Acad. Sci. 2008, 1144, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Oehme, P.; Hecht, K.; Faulhaber, H.D.; Roske, I.; Rathsack, R. Relationship of substance P to catecholamines, stress, and hypertension. J. Cardiovasc. Pharmacol. 1987, 10 (Suppl. 12), S109–S111. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, K.; Siddiq, A.; Baig, S.G.; Zehra, S. Substance P: A neuropeptide involved in the psychopathology of anxiety disorders. Neuropeptides 2019, 79, 101993. [Google Scholar] [CrossRef]
- Jessop, D.S.; Renshaw, D.; Larsen, P.J.; Chowdrey, H.S.; Harbuz, M.S. Substance P Is Involved in Terminating the Hypothalamo-Pituitary-Adrenal Axis Response to Acute Stress Through Centrally Located Neurokinin-1 Receptors. Stress 2000, 3, 209–220. [Google Scholar] [CrossRef]
- Mashaghi, A.; Marmalidou, A.; Tehrani, M.; Grace, P.M.; Pothoulakis, C.; Dana, R. Neuropeptide Substance P and the Immune Response. Cell Mol. Life Sci. 2016, 73, 4249–4264. [Google Scholar] [CrossRef]
- Rosenkranz, M.A. Substance P at the nexus of mind and body in chronic inflammation and affective disorders. Psychol. Bull. 2007, 133, 1007–1037. [Google Scholar] [CrossRef]
- Redkiewicz, P. The Regenerative Potential of Substance, P. Int. J. Mol. Sci. 2022, 23, 750. [Google Scholar] [CrossRef]
- Feickert, M.; Burckhardt, B.B. Substance P in cardiovascular diseases—A bioanalytical review. Clin. Chim. Acta 2019, 495, 501–506. [Google Scholar] [CrossRef]
- Tschoner, T.; Feist, M. Substance P concentrations in the blood plasma and serum of adult cattle and calves during different painful procedures and conditions—A systematic review. BMC Veter. Res. 2022, 18, 232. [Google Scholar] [CrossRef]
- Barragan, A.A.; Piñeiro, J.M.; Schuenemann, G.M.; Rajala-Schultz, P.J.; Sanders, D.E.; Lakritz, J.; Bas, S. Assessment of daily activity patterns and biomarkers of pain, inflammation, and stress in lactating dairy cows diagnosed with clinical metritis. J. Dairy Sci. 2018, 101, 8248–8258. [Google Scholar] [CrossRef]
- Bustamante, H.A.; Rodríguez, A.R.; Herzberg, D.E.; Werner, M.P. Stress and pain response after oligofructose induced-lameness in dairy heifers. J. Veter. Sci. 2015, 16, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, J.F.; Lubbers, B.V.; Toerber, S.E.; Gehring, R.; Thomson, D.U.; White, B.J.; Apley, M.D. Plasma concentrations of substance P and cortisol in beef calves after castration or simulated castration. Am. J. Veter. Res. 2008, 69, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Gregg, T.R.; Siegel, A. Brain structures and neurotransmitters regulating aggression in cats: Implications for human aggression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 91–140. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, M.S.; Brandão, M.L. Effects of microinjections of the neuropeptide substance P in the dorsal periaqueductal gray on the behaviour of rats in the plus-maze test. Physiol. Behav. 1996, 60, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Malone, E.D.; Kannan, M.S.; Brown, D.R. Evaluation of substance P as a neurotransmitter in equine jejunum. Am. J. Veter. Res. 2000, 61, 1178–1184. [Google Scholar] [CrossRef]
- Caron, J.P.; Bowker, R.M.; Abhold, R.H.; Toppin, D.S.; Sonea, I.M.; Vex, K.B. Substance P in the synovial membrane and fluid of the equine middle carpal joint. Equine Veter. J. 1992, 24, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Kirker-Head, C.A.; Chandna, V.K.; Agarwal, R.K.; Morris, E.A.; Tidwell, A.; O’Callaghan, M.W.; Rand, W.; Kumar, M.S.A. Concentrations of substance P and prostaglandin E2 in synovial fluid of normal and abnormal joints of horses. Am. J. Veter. Res. 2000, 61, 714–718. [Google Scholar] [CrossRef]
- Everett, J.B.; Schumacher, J.; Doherty, T.J.; Black, R.A.; Amelse, L.L.; Krawzel, P.; Coetzee, J.F.; Whitlock, B.K.; Krawczel, P. Effects of stacked wedge pads and chains applied to the forefeet of Tennessee Walking Horses for a five-day period on behavioral and biochemical indicators of pain, stress, and inflammation. Am. J. Veter. Res. 2018, 79, 21–32. [Google Scholar] [CrossRef]
- Davison, J.; Lumsden, J.; Boston, R.; Ahern, B. Overground endoscopy in 311 Thoroughbred racehorses: Findings and correlation to resting laryngeal function. Aust. Veter. J. 2017, 95, 338–342. [Google Scholar] [CrossRef]
- Ladewig, J.; McLean, A.N.; Wilkins, C.L.; Fenner, K.; Christensen, J.W.; McGreevy, P.D. A review of The Ridden Horse pain Ethogram and its potential to improve ridden horse welfare. J. Vet. Behav. 2022, 54, 54–61. [Google Scholar] [CrossRef]
- Mosher, R.A.; Coetzee, J.F.; Allen, P.S.; Havel, J.A.; Griffith, G.R.; Wang, C. Effects of sample handling methods on substance P concentrations and immunoreactivity in bovine blood samples. Am. J. Veter. Res. 2014, 75, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Bennett-Wimbush, K.; Suagee-Bedore, J.; Amstutz, M.; Duthie, M. Effects of Overcheck Use on Stress Parameters and Welfare Implications in Driving Horses. J. Appl. Anim. Welf. Sci. 2019, 23, 83–94. [Google Scholar] [CrossRef]
- Kędzierski, W.; Cywińska, A.; Strzelec, K.; Kowalik, S. Changes in salivary and plasma cortisol levels in Purebred Arabian horses during race training session. Anim. Sci. J. 2013, 85, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Eugene, A.R. A Model-Based Meta-Analysis Evaluating Gender Differences on Blood Flow Responses to Brachial Artery Infusions of Acetylcholine, Albuterol, ATP, Bradykinin, Estradiol, Glyceryl Trinitrate, L-NMMA, Nevibolol, Norepinephrine, Sodium Nitroprusside, Substance, P., and Verapamil. MEDtube Sci. 2016, 4, 16–28. [Google Scholar]
- Pittenger, S.T.; Swalve, N.; Chou, S.; Smith, M.D.; Hoonakker, A.J.; Pudiak, C.M.; Fleckenstein, A.E.; Hanson, G.R.; Bevins, R.A. Sex differences in neurotensin and substance P following nicotine self-administration in rats. Synapse 2016, 70, 336–346. [Google Scholar] [CrossRef]
- Janzekovic, M.; Prisenk, J.; Katalinic, B. Assessment of Stress on Horses through the Salivary Cortisol Concentration. DAAAM Int. Sci. Book 2017, 16, 55–62. [Google Scholar] [CrossRef]
- Oswald, L.M.; Zandi, P.; Nestadt, G.; Potash, J.B.; Kalaydjian, A.E.; Wand, G.S. Relationship between Cortisol Responses to Stress and Personality. Neuropsychopharmacology 2006, 31, 1583–1591. [Google Scholar] [CrossRef]
- Whitlock, B.; Coffman, E.; Coetzee, J.; Daniel, J. Electroejaculation increased vocalization and plasma concentrations of cortisol and progesterone, but not substance P., in beef bulls. Theriogenology 2012, 78, 737–746. [Google Scholar] [CrossRef]
- Schedlowski, M.; Fluge, T.; Richter, S.; Tewes, U.; Schmidt, R.E.; Wagner, T.O. Beta-endorphin, but not substance-P, is increased by acute stress in humans. Psychoneuroendocrinology 1995, 20, 103–110. [Google Scholar] [CrossRef]
- Dyson, S.; Pollard, D. Application of a Ridden Horse Pain Ethogram and Its Relationship with Gait in a Convenience Sample of 60 Riding Horses. Animals 2020, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Rosén, A.; Brodin, K.; Eneroth, P.; Brodin, E. Short-term restraint stress and s.c. saline injection alter the tissue levels of substance P and cholecystokinin in the peri-aqueductal grey and limbic regions of rat brain. Acta Physiol. Scand. 1992, 146, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Remröd, C.; Lonne-Rahm, S.; Nordlind, K. Study of substance P and its receptor neurokinin-1 in psoriasis and their relation to chronic stress and pruritus. Arch. Dermatol. Res. 2007, 299, 85–91. [Google Scholar] [CrossRef] [PubMed]

| Horse No. | Mean | SD | IQR | Median |
|---|---|---|---|---|
| 1 (n = 4) | 2492.1 | 513.4 | 776.5 | 2533.4 |
| 2 (n = 4) | 2712.6 | 432.9 | 571.9 | 2812.1 |
| 3 (n = 4) | 1232.1 | 550.6 | 774.5 | 1177.2 |
| 4 (n = 4) | 1138.9 | 173.9 | 238.2 | 1103.5 |
| 5 (n = 4) | 1269.7 | 123.7 | 137.3 | 1232.2 |
| 6 (n = 4) | 2263.7 | 81.0 | 96.8 | 2279.3 |
| 7 (n = 4) | 1046.8 | 86.6 | 101.9 | 1062.2 |
| 8 (n = 4) | 843.8 | 74.5 | 95.9 | 852.1 |
| 9 (n = 4) | 4786.4 | 470.1 | 620.1 | 4722.5 |
| 10 (n = 4) | 1942.5 | 367.0 | 427.6 | 1948.4 |
| 11 (n = 4) | 2378.9 | 588.2 | 630.6 | 2178.3 |
| 12 (n = 4) | 1398.4 | 158.3 | 171.5 | 1416.8 |
| 13 (n = 4) | 6132.6 | 1920.4 | 1235.7 | 6528.9 |
| 14 (n = 4) | 4409.5 | 1860.0 | 2403.1 | 3973.8 |
| 15 (n = 4) | 1990.6 | 484.7 | 456.0 | 2101.4 |
| 16 (n = 4) | 4817.9 | 964.6 | 1408.9 | 4671.5 |
| Mean | SD | IQR | Median | |
|---|---|---|---|---|
| (n = 72) | 2472 | 1643 | 1764 | 2007 |
| RHE Score | RHE Description | Loose Noseband, No Endoscope | Tight Noseband, No Endoscope | Loose Noseband Plus Endoscope | Tight Noseband Plus Endoscope |
|---|---|---|---|---|---|
| 1 | Repeated changes of head position (up/down). | 38% (6/16) | 50% (8/16) | 44% (7/16) | 88% (14/16) |
| 2 | Head tilted/tilting repeatedly | 44% (7/16) | 44% (7/16) | 50% (8/16) | 63% (10/16) |
| 3 | Head in front of vertical (>30°) for >10 s | 31% (5/16) | 44% (7/16) | 44% (7/16) | 75% (12/16) |
| 4 | Head behind vertical (>10°) for >10 s | 50% (8/16) | 56% (9/16) | 44% (7/16) | 56% (9/16) |
| 5 | Head position changes regularly, tossed or twisted. | 6% (1/16) | 6% (1/16) | 6% (1/16) | 6% (1/16) |
| 6 | Ears rotated back behind vertical or flat >5 s. | 19% (3/16) | 38% (6/16) | 13% (2/16) | 31% (5/16) |
| 7 | Eyelids closed or half closed for 2–5 s. | 0% (0/16) | 0% (0/16) | 0% (0/16) | 6% (1/16) |
| 8 | Sclera exposed repeatedly | 75% (12/16) | 81% (13/16) | 69% (11/16) | 88% (14/16) |
| 9 | Intense stare for >5 s | 50% (8/16) | 81% (13/16) | 44% (7/16) | 75% (12/16) |
| 10 | Mouth opening, shutting repeatedly for >10 s | 69% (11/16) | 38% (6/16) | 69% (11/16) | 50% (8/16) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scholler, D.; Zablotski, Y.; May, A. Evaluation of Substance P as a New Stress Parameter in Horses in a Stress Model Involving Four Different Stress Levels. Animals 2023, 13, 1142. https://doi.org/10.3390/ani13071142
Scholler D, Zablotski Y, May A. Evaluation of Substance P as a New Stress Parameter in Horses in a Stress Model Involving Four Different Stress Levels. Animals. 2023; 13(7):1142. https://doi.org/10.3390/ani13071142
Chicago/Turabian StyleScholler, Dominik, Yury Zablotski, and Anna May. 2023. "Evaluation of Substance P as a New Stress Parameter in Horses in a Stress Model Involving Four Different Stress Levels" Animals 13, no. 7: 1142. https://doi.org/10.3390/ani13071142
APA StyleScholler, D., Zablotski, Y., & May, A. (2023). Evaluation of Substance P as a New Stress Parameter in Horses in a Stress Model Involving Four Different Stress Levels. Animals, 13(7), 1142. https://doi.org/10.3390/ani13071142





