Metabolic Rates of Japanese Anchovy (Engraulis japonicus) during Early Development Using a Novel Modified Respirometry Method
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Preparation
2.1.1. Rearing Adult Japanese Anchovies
2.1.2. Rearing Larvae and Juvenile Japanese Anchovies
2.2. Respirometry
2.2.1. Process 1. Selection of the Sample and Acclimation in Respiration Chamber
2.2.2. Process 2. Control of the Water Flow in the Respiration Chamber Using a Peristaltic Pump
2.2.3. Process 3. Determination of Rate of Oxygen Consumption
2.3. Statistics
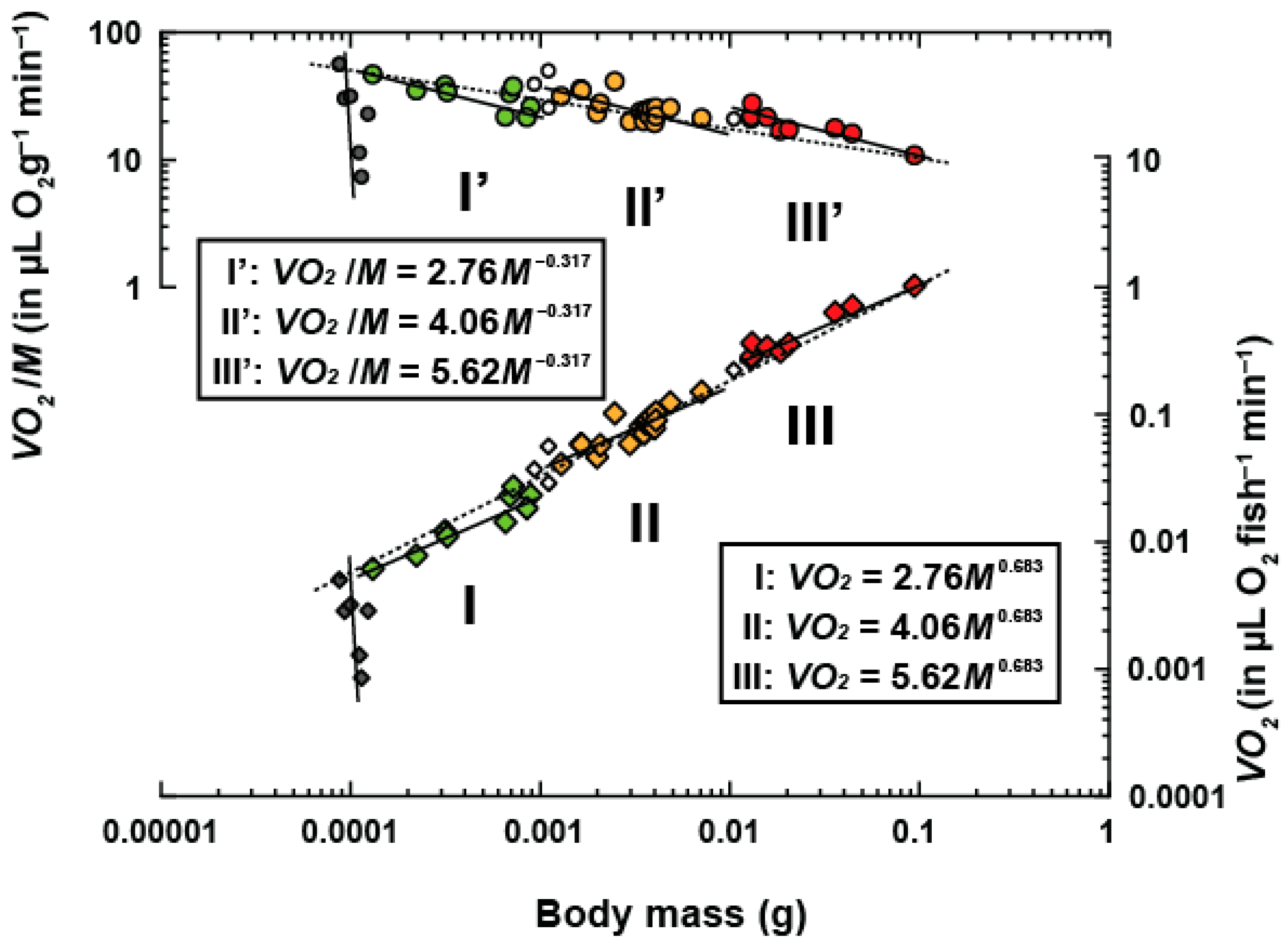
3. Results
4. Discussion
4.1. The Relationship between Metabolic Variation Patterns and Developmental Changes
| Transitional Phase between (Wet Body Weight in g) | Day after Hatching | Morphological and Behavioral Characteristics |
|---|---|---|
| and (approx. 0.001) | 18 |
|
| and (approx. 0.01) | 29 |
4.2. Consideration of Methodological Constraints and Proposals for Further Progress
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozłowski, J.; Teriokhin, A.T. Allocation of energy between growth and reproduction: The Pontryagin Maximum Principle solution for the case of age-and season-dependent mortality. Evol. Ecol. Res. 1999, 1, 423–441. [Google Scholar]
- Czarnołęski, M.; Kozłowski, J.; Stańczykowska, A.; Lewandowski, K. Optimal resource allocation explains growth. Evol. Ecol. Res. 2003, 5, 571–587. [Google Scholar]
- Hou, C.; Zuo, W.; Moses, M.E.; Woodruff, W.H.; Brown, J.H.; West, G.B. Energy uptake and allocation during ontogeny. Science 2008, 322, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Krogh, A. The Respiratory Exchange of Animals and Man; Longmans: London, UK, 1916. [Google Scholar]
- Kleiber, M. Body size and metabolism. Hilgardia 1932, 6, 315–353. [Google Scholar] [CrossRef]
- Brody, S.; Lardy, H.A. Bioenergetics and growth. J. Phys. Chem. 1946, 50, 168–169. [Google Scholar] [CrossRef]
- Zeuthen, E. Oxygen uptake as related to body size in organisms. Q. Rev. Biol. 1953, 28, 1–12. [Google Scholar] [CrossRef]
- Winberg, G. Rate of metabolism and food requirements of fish. Transl. Fish. Res. Board Can. 1956, 194, 1–253. [Google Scholar]
- Hemmingsen, A. Energy metabolism as related to body size and respiratory surfaces, and its evolution. Rep. Steno Meml. Hosp. 1960, 9, 3–110. [Google Scholar]
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Glazier, D.S. The 3/4-power law is not universal: Evolution of isometric, ontogenetic metabolic scaling in pelagic animals. Bioscience 2006, 56, 325–332. [Google Scholar] [CrossRef]
- Schramm, B.W.; Labecka, A.M.; Gudowska, A.; Antoł, A.; Sikorska, A.; Szabla, N.; Bauchinger, U.; Kozlowski, J.; Czarnoleski, M. Concerted evolution of body mass, cell size and metabolic rate among carabid beetles. J. Insect Physiol. 2021, 132, 104272. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K.; Knut, S. Scaling: Why is Animal Size so Important? Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Glazier, D.S. Beyond the ‘3/4-power law’: Variation in the intra-and interspecific scaling of metabolic rate in animals. Biol. Rev. 2005, 80, 611–662. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.E.; Hou, C.; Woodruff, W.H.; West, G.B.; Nekola, J.C.; Zuo, W.; Brown, J.H. Revisiting a model of ontogenetic growth: Estimating model parameters from theory and data. Am. Nat. 2008, 171, 632–645. [Google Scholar] [CrossRef]
- Packard, G.C.; Birchard, G.F. Traditional allometric analysis fails to provide a valid predictive model for mammalian metabolic rates. J. Exp. Biol. 2008, 211, 3581–3587. [Google Scholar] [CrossRef] [PubMed]
- Kolokotrones, T.; Savage, V.; Deeds, E.J.; Fontana, W. Curvature in metabolic scaling. Nature 2010, 464, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Yamaji, K.; Ishida, A.; Prokushkin, S.G.; Masyagina, O.V.; Hagihara, A.; Hoque, A.T.; Suwa, R.; Osawa, A.; Nishizono, T.; et al. Mixed-power scaling of whole-plant respiration from seedlings to giant trees. Proc. Natl. Acad. Sci. USA 2010, 107, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Wieser, W. A distribution must be made between the ontogeny the phylogeny of metabolism in order to understand the mass exponent of energy metabolism. Respir. Physiol. 1984, 55, 1–9. [Google Scholar] [CrossRef]
- Czarnoleski, M.; Kozlowski, J.; Dumiot, G.; Bonnet, J.C.; Mallard, J.; Dupont-Nivet, M. Scaling of metabolism in Helix aspersa snails: Changes through ontogeny and response to selection for increased size. J. Exp. Biol. 2008, 211, 391–400. [Google Scholar] [CrossRef]
- Yagi, M.; Kanda, T.; Takeda, T.; Ishimatsu, A.; Oikawa, S. Ontogenetic phase shifts in metabolism: Links to development and anti-predator adaptation. Proc. Royal Soc. B 2010, 277, 2793–2801. [Google Scholar] [CrossRef]
- Kim, D.I. Ontogeny of the Respiratory Area in Relation to Body Mass with Reference to Resting Metabolism in the Japanese Flounder, Paralichthys olivaceus (Temminck & Schlegel, 1846). Fishes 2022, 7, 39. [Google Scholar]
- Glazier, D.S.; Hirst, A.G.; Atkinson, D. Shape shifting predicts ontogenetic changes in metabolic scaling in diverse aquatic invertebrates. Proc. Royal Soc. B 2015, 282, 20142302. [Google Scholar] [CrossRef]
- Forlenza, A.E.; Galbraith, H.S.; Blakeslee, C.J.; Glazier, D.S. Ontogenetic Changes in Body Shape and the Scaling of Metabolic Rate in the American Eel (Anguilla rostrata). Physiol. Biochem. Zool. 2022, 95, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Yagi, M.; Oikawa, S. Ontogenetic phase shifts in metabolism in a flounder Paralichthys olivaceus. Sci. Rep. 2014, 4, 7135. [Google Scholar] [CrossRef]
- Oikawa, S.; Itazawa, Y. Relationship between metabolic rate in vitro and body mass in a marine teleost, porgy Pagrus major. Fish Physiol. Biochem. 1992, 10, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, S.; Itazawa, Y.; Gotoh, M. Ontogenetic change in the relationship between metabolic rate and body mass in a sea bream Pagrus major (Temminck & Schlegel). J. Fish. Biol. 1991, 38, 483–496. [Google Scholar]
- Moran, D.; Wells, R.M. Ontogenetic scaling of fish metabolism in the mouse-to-elephant mass magnitude range. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 148, 611–620. [Google Scholar] [CrossRef]
- Post, J.R.; Lee, J.A. Metabolic ontogeny of teleost fishes. Can. J. Fish. Aquat. Sci. 1996, 53, 910–923. [Google Scholar] [CrossRef]
- Finn, R.N.; Rønnestad, I.; van der Meeren, T.; Fyhn, H.J. Fuel and metabolic scaling during the early life stages of Atlantic cod Gadus morhua. Mar. Ecol. Prog. Ser. 2002, 243, 217–234. [Google Scholar] [CrossRef]
- Giguère, L.A.; Côté, B.; St-Pierre, J. Metabolic rates scale isometrically in larval fishes. Mar. Ecol. Prog. Ser. 1988, 50, 13–19. [Google Scholar] [CrossRef]
- Warburton, S.J.; Burggren, W.W.; Pelster, B.; Reiber, C.L.; Spicer, J. Comparative Developmental Physiology: Contributions, Tools, and Trends; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Killen, S.S.; Costa, I.; Brown, J.A.; Gamperl, A.K. Little left in the tank: Metabolic scaling in marine teleosts and its implications for aerobic scope. Proc. Royal Soc. B 2007, 274, 431–438. [Google Scholar] [CrossRef]
- Bokma, F. Evidence against universal metabolic allometry. Funct. Ecol. 2004, 18, 184–187. [Google Scholar] [CrossRef]
- Clarke, A.; Johnston, N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- Oikawa, S.; Itazawa, Y. Relative growth of organs and parts of the carp, Cyprinus carpio, with special reference to the metabolism-size relationship. Copeia 1984, 1984, 800–803. [Google Scholar] [CrossRef]
- Oikawa, S.; Hirata, M.; Kita, J.; Itazawa, Y. Ontogeny of respiratory area of a marine teleost, porgy, Pagrus major. Ichthyol. Res. 1999, 46, 233–244. [Google Scholar] [CrossRef]
- Oikawa, S.; Itazawa, Y. Gill and body surface areas of the carp in relation to body mass, with special reference to the metabolism-size relationship. J. Exp. Biol. 1985, 117, 1–14. [Google Scholar] [CrossRef]
- Tan, H.; Hirst, A.G.; Glazier, D.S.; Atkinson, D. Ecological pressures and the contrasting scaling of metabolism and body shape in coexisting taxa: Cephalopods versus teleost fish. Philos. Trans. R. Soc. 2019, 374, 20180543. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.A.; Cowen, R.K. Size, growth, development, and survival of the planktonic larvae of Pomatomus saltatrix (Pisces: Pomatomidae). Ecology 1997, 78, 2415–2431. [Google Scholar] [CrossRef]
- Shannon, C.E. Communication in the presence of noise. Proc. IRE 1949, 37, 10–21. [Google Scholar] [CrossRef]
- Svendsen, M.; Bushnell, P.G.; Christensen, E.; Steffensen, J.F. Sources of variation in oxygen consumption of aquatic animals demonstrated by simulated constant oxygen consumption and respirometers of different sizes. J. Fish. Biol. 2016, 88, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.F. Comparison studies of Winkler vs. oxygen sensor. J. Water Pollut. Control Fed. 1969, 41, 2002–2009. [Google Scholar]
- Fukuhara, O. Development and growth of laboratory reared Engraulis japonica (Houttuyn) larvae. J. Fish. Biol. 1983, 23, 641–652. [Google Scholar] [CrossRef]
- Helm, I.; Jalukse, L.; Vilbaste, M.; Leito, I. Micro-Winkler titration method for dissolved oxygen concentration measurement. Anal. Chim. Acta 2009, 648, 167–173. [Google Scholar] [CrossRef]
- Brownlee, K.A. Statistical Theory and Methodology in Science and Engineering; Wiley: New York, NY, USA, 1965. [Google Scholar]
- Feldman, H.A.; McMahon, T.A. The 3/4 mass exponent for energy metabolism is not a statistical artifact. Respir. Physiol. 1983, 52, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Z.; Usami, S.; Hattori, S.; Honjo, Y.; Hayashi, S. Progress Report of the Cooperative Iwashi Resources Investigations; Tokai Regional Fisheries Research Laboratory: Tokyo, Japan, 1955; pp. 1–84. [Google Scholar]
- Yokota, T. Studies on the Feeding Habit of Fishes; Nankai Regional Fisheries Research Laboratory: Tokyo, Japan, 1961; Volume 14, pp. 1–23. [Google Scholar]
- Funamoto, T.; Aoki, I.; Wada, Y. Reproductive characteristics of Japanese anchovy, Engraulis japonicus, in two bays of Japan. Fish. Res. 2004, 70, 71–81. [Google Scholar] [CrossRef]
- Takahashi, M.; Watanabe, Y.; Kinoshita, T.; Watanabe, C. Growth of larval and early juvenile Japanese anchovy, Engraulis japonicus, in the Kuroshio-Oyashio transition region. Fish. Oceanogr. 2001, 10, 235–247. [Google Scholar] [CrossRef]
- Ohizumi, H.; Kuramochi, T.; Amano, M.; Miyazaki, N. Prey switching of Dall¹s porpoise Phocoenoides dalli with population decline of Japanese pilchard Sardinops melanostictus around Hokkaido, Japan. Mar. Ecol. Prog. Ser. 2000, 200, 265–275. [Google Scholar] [CrossRef]
- Ohata, R.; Masuda, R.; Yamashita, Y. Ontogeny of antipredator performance in hatchery-reared Japanese anchovy Engraulis japonicus larvae exposed to visual or tactile predators in relation to turbidity. J. Fish. Biol. 2011, 79, 2007–2018. [Google Scholar] [CrossRef]
- Yonezaki, S.; Kiyota, M.; Baba, N.; Koido, T.; Takemura, A. Size distribution of the hard remains of prey in the digestive tract of northern fur seal (Callorhinus ursinus) and related biases in diet estimation by scat analysis. Mammal Study 2003, 28, 97–102. [Google Scholar] [CrossRef]
- Harder, W. Anatomy of Fishes; Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1975; pp. 1–612. [Google Scholar]
- Garaventa, F.; Gambardella, C.; Di Fino, A.; Pittore, M.; Faimali, M. Swimming speed alteration of Artemia sp. and Brachionus plicatilis as a sub-lethal behavioural end-point for ecotoxicological surveys. Ecotoxicology 2010, 19, 512–519. [Google Scholar] [CrossRef]
- Hayashi, S. Fishery biology of the Japanese anchovy Engraulis japonica (Houttuyn). Bull. Tokai Reg. Fish. Res. Lab. 1961, 31, 145–268. [Google Scholar]
- Masuda, R. Ontogeny of swimming speed, schooling behaviour and jellyfish avoidance by Japanese anchovy Engraulis japonicus. J. Fish. Biol. 2011, 78, 1323–1335. [Google Scholar] [CrossRef]
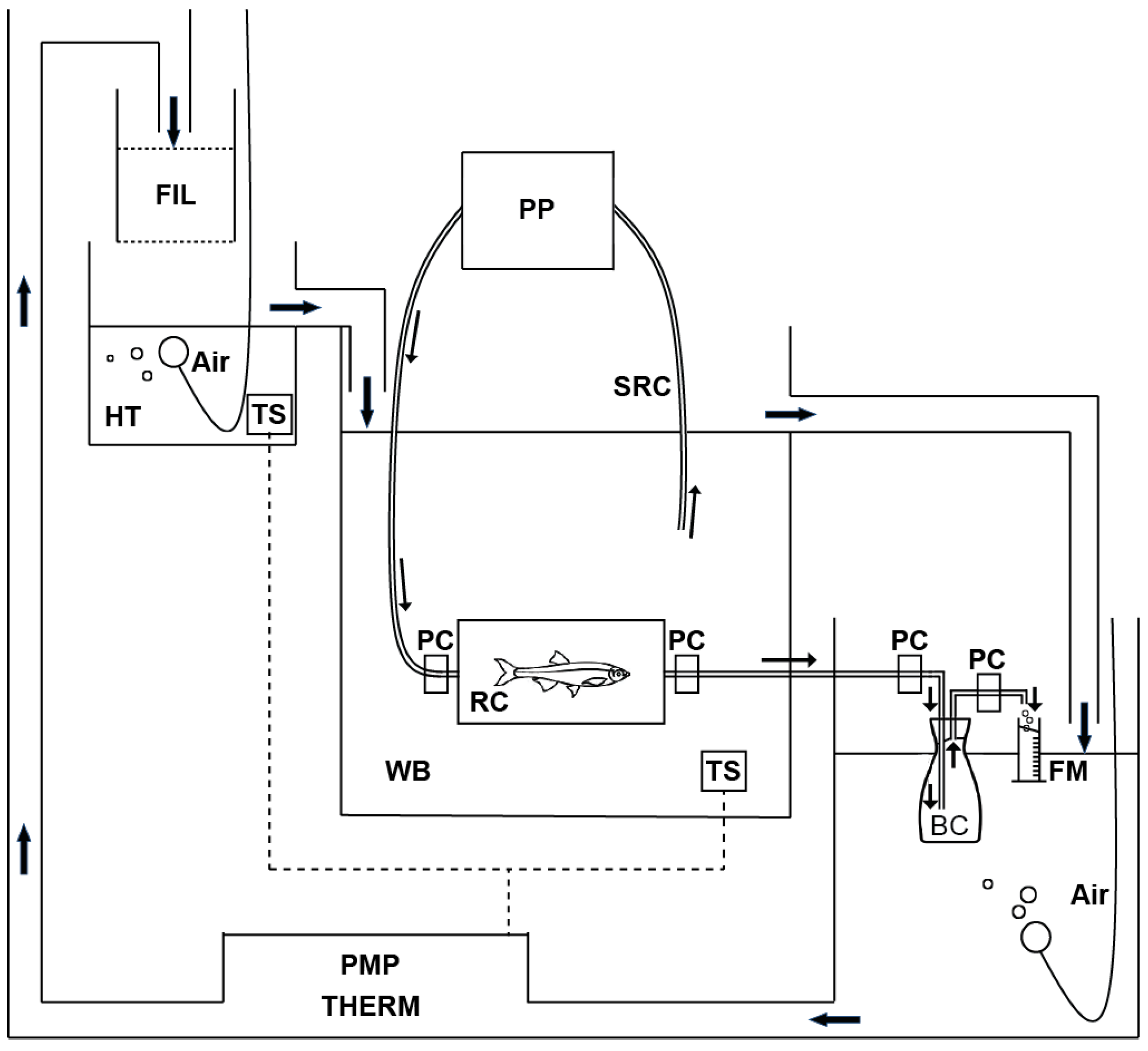
| Range of Body Weight (g) | Volume of Respiration Chamber (mL) | No. of Fish | Respirometry Time (min) | Flow Rate of Water (mL min−1) | |||
|---|---|---|---|---|---|---|---|
| before Measurement | during Measurement | Initial Inflow | Final Outflow | ||||
| 0.0001–0.002 | 36–40 | 41–1 | 60–90 | 120–540 | 2–10 | 4.67 ± 0.11 (23) | 4.33 ± 0.22 (23) |
| 0.003–0.02 | 45–60 | 1 | 90–120 | 90–270 | 12–20 | 4.82 ± 0.10 (20) | 4.14 ± 0.36 (20) |
| 0.03–0.04 | 240–260 | 1 | 180–210 | 200–220 | 23–31 | 4.76 ± 0.03 (2) | 4.20 ± 0.02 (2) |
| 0.05–0.1 | 260–340 | 1 | 210–240 | 180–200 | 31–52 | 4.89 ± 0.10 (2) | 4.33 ± 0.11 (2) |
| Group | N | Range of Body Weight (g) | Scaling Constant | P | R2 | |
|---|---|---|---|---|---|---|
| 1 a | 9 | 0.00013–0.00085 | 3.38 | 0.709 ± 0.100 | 2.29 × 10−2 | 0.878 |
| 2 a | 18 | 0.00129–0.00709 | 4.02 | 0.682 ± 0.094 | 3.90 × 10−3 | 0.765 |
| 3 a | 9 | 0.01295–0.09428 | 5.12 | 0.659 ± 0.064 | 1.12 × 10−3 | 0.938 |
| 1–3 b | 36 | 0.00013–0.09428 | α = 11.22 | = 0.862 ± 0.003 | 1.43 × 10−34 | 1.000 |
| 1–3 c | 36 | 0.00013–0.09428 | 9.79 | 0.839 ± 0.020 | 3.37 × 10−9 | 0.980 |
| Total d | 47 | 0.00009–0.09428 | 14.34 | 0.911 ± 0.030 | 4.93 × 10−3 | 0.953 |
| Term | Sum of Squares | Degrees of Freedom | Mean Square | Mean–Square Ratio | P |
|---|---|---|---|---|---|
| log α | 44.2080678 | 1 | 44.208068 | 8978 | 8.98 × 10−41 |
| µi | 0.00291609 | 1 | 0.002916 | 0.59 | 4.47 × 10−1 |
| ( − )i | 0.05621026 | 1 | 0.056210 | 11.42 | 1.93 × 10−3 |
| xij | 10.7264528 | 1 | 10.726453 | 2178 | 5.24 × 10−31 |
| εij | 0.15756394 | 32 | 0.004924 | ||
| Total (about mean) | 10.9431431 | 35 | |||
| Total (about zero) | 55.1512109 | 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.I. Metabolic Rates of Japanese Anchovy (Engraulis japonicus) during Early Development Using a Novel Modified Respirometry Method. Animals 2023, 13, 1035. https://doi.org/10.3390/ani13061035
Kim DI. Metabolic Rates of Japanese Anchovy (Engraulis japonicus) during Early Development Using a Novel Modified Respirometry Method. Animals. 2023; 13(6):1035. https://doi.org/10.3390/ani13061035
Chicago/Turabian StyleKim, Dong In. 2023. "Metabolic Rates of Japanese Anchovy (Engraulis japonicus) during Early Development Using a Novel Modified Respirometry Method" Animals 13, no. 6: 1035. https://doi.org/10.3390/ani13061035
APA StyleKim, D. I. (2023). Metabolic Rates of Japanese Anchovy (Engraulis japonicus) during Early Development Using a Novel Modified Respirometry Method. Animals, 13(6), 1035. https://doi.org/10.3390/ani13061035





