Feeding Behavior of Finishing Pigs under Diurnal Cyclic Heat Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
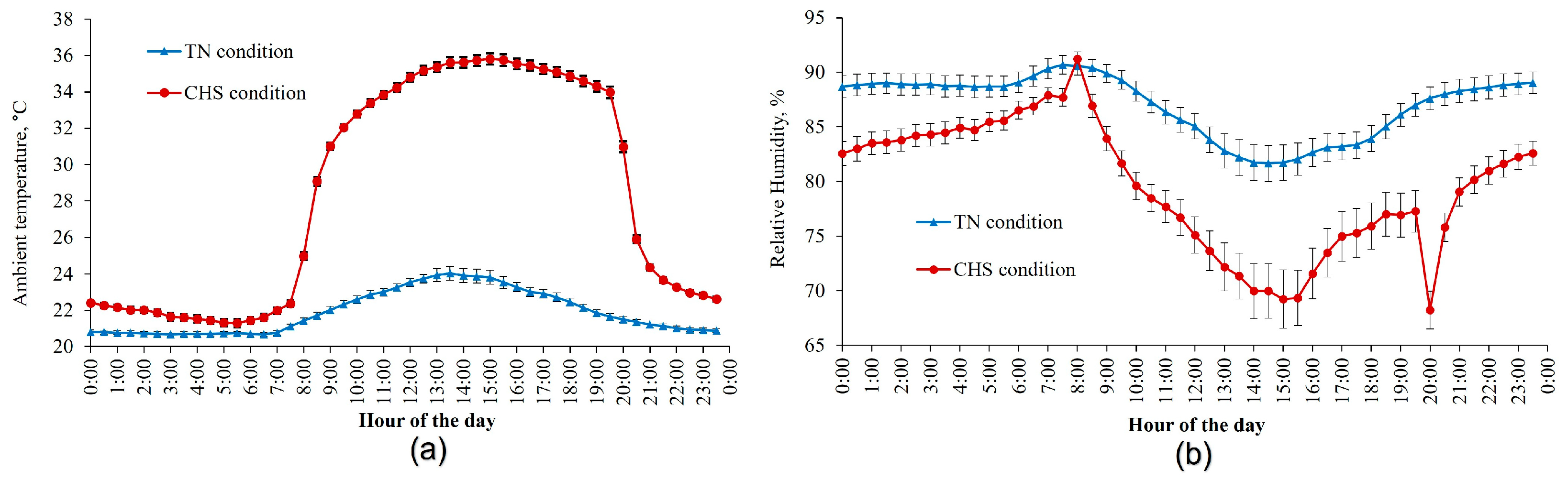
2.1. Data Source
2.2. Data Collection and Management
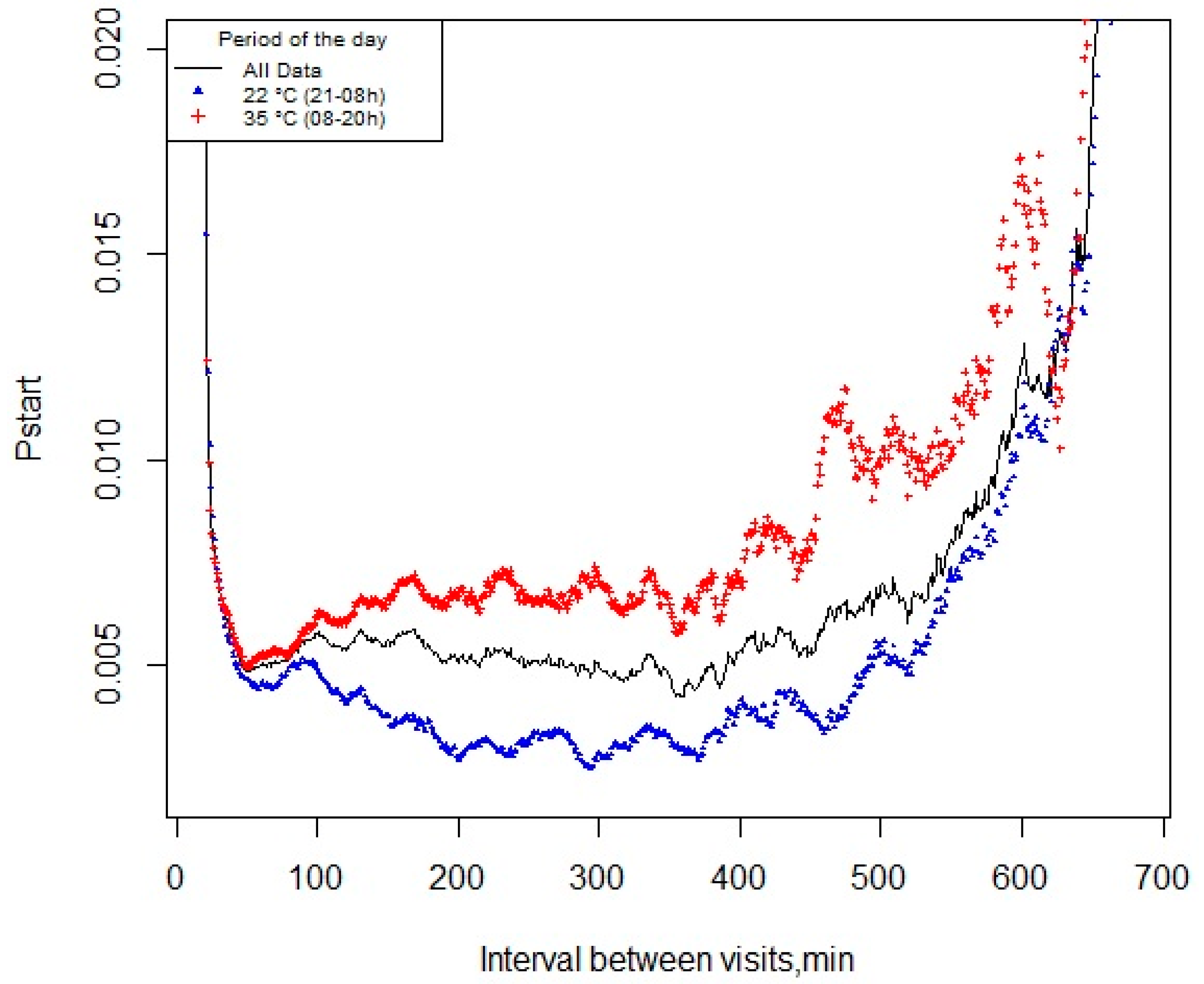
2.3. Definition of the Meal Criterion
2.4. Calculations and Statistical Analysis
3. Results
3.1. General Observations
3.2. pstart and Meal Criterion
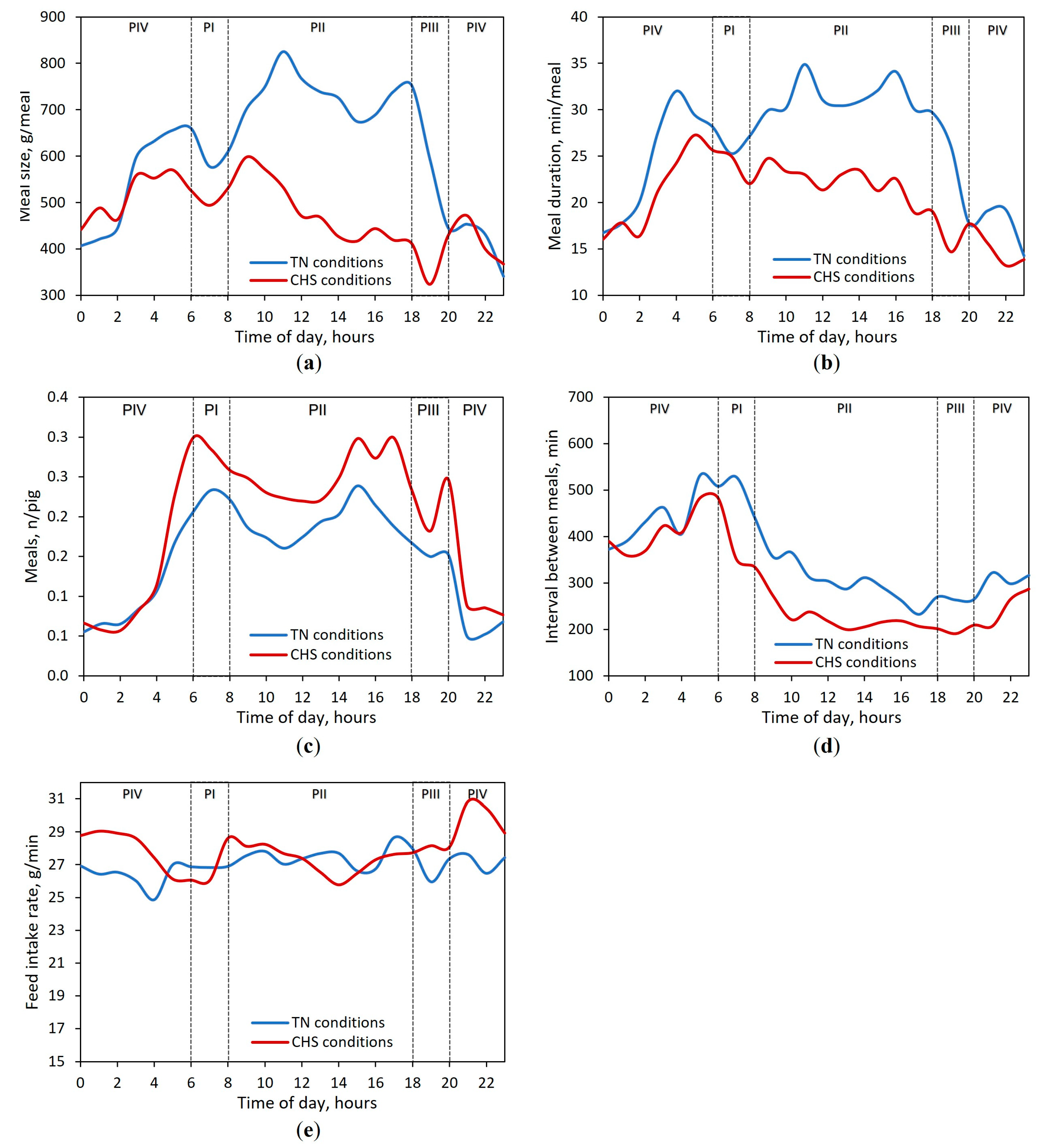
3.3. Feeding Behavior Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Oliveira, M.J.K.; Melo, A.D.B.; Marçal, D.A.; Valini, G.A.C.; Silva, C.A.; Veira, A.M.; Fraga, A.Z.; Arnaut, P.R.; Campos, P.H.R.F.; dos Santos, L.S.; et al. Effects of lowering dietary protein content without or with increased protein-bound and feed-grade amino acids supply on growth performance, body composition, metabolism, and acute-phase protein of finishing pigs under daily cyclic heat stress. J. Anim. Sci. 2022, 101, skac387. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.B.; Ma, X.Y.; Zheng, C.T.; Hu, Y.J.; Yang, X.F.; Gao, K.G.; Wang, L.; Jiang, Z.Y. Effects of high ambient temperature on meat quality, serum hormone concentrations, and gene expression in the longissimus dorsi muscle of finishing pigs. Anim. Prod. Sci. 2016, 57, 1031–1039. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; DeShazer, J.A. Feeding patterns of growing pigs at warm constant and cyclic temperatures. Trans. Am. Soc. Agric. Eng. 1992, 35, 319–323. [Google Scholar] [CrossRef]
- Quiniou, N.; Massabie, P.; Granier, R. Diurnally variation of ambient temperature around 24 or 28 C: Influence on performance and feeding behavior of growing pigs. In Swine Housing, Proceedings of the First International Conference, Des Moines, IA, USA, 9–11 October 2000; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2000; pp. 332–339. [Google Scholar]
- Bus, J.D.; Boumans, I.J.M.M.; Webb, L.E.; Bokkers, E.A.M. The potential of feeding patterns to assess generic welfare in growing-finishing pigs. Appl. Anim. Behav. Sci. 2021, 241, 105383. [Google Scholar] [CrossRef]
- Quiniou, N.; Dubois, S.; Noblet, J. Voluntary feed intake and feeding behaviour of group-housed growing pigs are affected by ambient temperature and body weight. Livest. Prod. Sci. 2000, 63, 245–253. [Google Scholar] [CrossRef]
- Fraga, A.Z.; Hauschild, L.; Campos, P.H.R.F.; Valk, M.; Bello, D.Z.; Kipper, M.; Andretta, I. Genetic selection modulates feeding behavior of group-housed pigs exposed to daily cyclic high ambient temperatures. PLoS ONE 2022, 17, e0258904. [Google Scholar] [CrossRef]
- Adelowo, O.V.; Adebiyi, O.A.; Odu, O. Effects of pen colour and photoperiod interactions on the performance and respiratory rate of finisher gilts. Niger. J. Anim. Prod. 2018, 45, 58–65. [Google Scholar] [CrossRef]
- Mrosovsky, N. Masking: History, definitions, and measurement. Chronobiol. Int. 1999, 16, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Smale, L.; Nunez, A.A. Circadian and photic modulation of daily rhythms in diurnal mammals. Eur. J. Neurosci. 2020, 51, 551–566. [Google Scholar] [CrossRef]
- Cervantes, M.; Cota, M.; Arce, N.; Castillo, G.; Avelar, E.; Espinoza, S.; Morales, A. Effect of heat stress on performance and expression of selected amino acid and glucose transporters, HSP90, leptin and ghrelin in growing pigs. J. Therm. Biol. 2016, 59, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Brown-Brandl, T.M.; Rohrer, G.A.; Eigenberg, R.A. Analysis of feeding behavior of group housed growing-finishing pigs. Comput. Electron. Agric. 2013, 96, 246–252. [Google Scholar] [CrossRef]
- Hessel, E.F.; Van den Weghe HF, A. Individual online-monitoring of feeding frequency and feeding duration of group-housed weaned piglets via high frequent radiofrequency identification (HF RFID). In Proceedings of the 5th European Conference on Precision Livestock Farming, Prague, Czech Republic, 11–14 July 2011; pp. 210–222. [Google Scholar]
- Pomar, J.; López, V.; Pomar, C. Agent-based simulation framework for virtual prototyping of advanced livestock precision feeding systems. Comput. Electron. Agric. 2011, 78, 88–97. [Google Scholar] [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Swine, 12th ed.; National Academy Press: Washington, WA, USA, 2012. [Google Scholar]
- Morales, A.; Cota, S.E.M.; Ibarra, N.O.; Arce, N.; Htoo, J.K.; Cervantes, M. Effect of heat stress on the serum concentrations of free amino acids and some of their metabolites in growing pigs. J. Anim. Sci. 2016, 94, 2835–2842. [Google Scholar] [CrossRef]
- Lan, R.; Kim, I. Effects of feeding diets containing essential oils and betaine to heat-stressed growing-finishing pigs. Arch. Anim. Nutr. 2018, 72, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.A.; Emmans, G.C.; Tolkamp, B.J.; Kyriazakis, I. Analysis of the feeding behavior of pigs using different models. Physiol. Behav. 2000, 68, 395–403. [Google Scholar] [CrossRef]
- Howie, J.A.; Tolkamp, B.J.; Avendano, S.; Kyriazakis, I. A novel flexible method to split feeding behaviour into bouts. Appl. Anim. Behav. Sci. 2009, 116, 101–109. [Google Scholar] [CrossRef]
- Gomes, B.C.; Andretta, I.; Valk, M.; Pomar, C.; Hauschild, L.; Fraga, A.Z.; Kipper, M.; Trevizan, L.; Remus, A. Prandial Correlations and Structure of the Ingestive Behavior of Pigs in Precision Feeding Programs. Animals 2021, 11, 2998. [Google Scholar] [CrossRef]
- Santos, L.S.; Pomar, C.; Campos, P.H.R.F.; da Silva, W.C.; Gobi, J.P.; Veira, A.M.; Fraga, A.Z.; Hauschild, L. Precision feeding strategy for growing pigs under heat stress conditions. J. Anim. Sci. 2018, 96, 4789–4801. [Google Scholar] [CrossRef]
- Andretta, I.; Pomar, C.; Kipper, M.; Hauschild, L.; Rivest, J. Feeding behavior of growing–finishing pigs reared under precision feeding strategies. J. Anim. Sci. 2016, 94, 3042–3050. [Google Scholar] [CrossRef]
- Renaudeau, D.; Giorgi, M.; Silou, F.; Weisbecker, J.L. Effect of breed (lean or fat pigs) and sex on performance and feeding behaviour of group housed growing pigs in a tropical climate. Asian-Australas. J. Anim. Sci. 2006, 19, 593–600. [Google Scholar] [CrossRef]
- Remus, A.; Hauschild, L.; Létourneau-Montminy, M.P.; Andretta, I.; Pomar, C. Feeding behavior of growing and finishing pigs fed different dietary threonine levels in a group-phase feeding and individual precision feeding system. Transl. Anim. Sci. 2020, 4, txaa177. [Google Scholar] [CrossRef]
- Nielsen, B.L. On the interpretation of feeding behaviour measures and the use of feeding rate as an indicator of social constraint. Appl. Anim. Behav. Sci. 1999, 63, 79–91. [Google Scholar] [CrossRef]
- Baumung, R.; Lercher, G.; Willam, A.; Sölkner, J. Feed intake behaviour of different pig breeds during performance testing on station. Arch. Anim. Breed. 2006, 49, 77–88. [Google Scholar] [CrossRef]
- Collin, A.; Van Milgen, J.; Dubois, S.; Noblet, J. Effect of high temperature and feeding level on energy utilization in piglets. J. Anim. Sci. 2001, 79, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Rauw, W.M.; Soler, J.; Tibau, J.; Reixach, J.; Gomez Raya, L. Feeding time and feeding rate and its relationship with feed intake, feed efficiency, growth rate, and rate of fat deposition in growing Duroc barrows. J. Anim. Sci. 2006, 84, 3404–3409. [Google Scholar] [CrossRef] [PubMed]
- Renaudeau, D. Impact of single or repeated short-term heat challenges mimicking summer heat waves on thermoregulatory responses and performances in finishing pigs. Transl. Anim. Sci. 2020, 4, txaa192. [Google Scholar] [CrossRef]
- Kim, B.; Reddy, K.E.; Kim, H.R.; Kim, K.H.; Lee, Y.; Kim, M.; Ji, S.Y.; Lee, S.D.; Jeong, J.Y. Effects of recovery from short-term heat stress exposure on feed intake, plasma amino acid profiles, and metabolites in growing pigs. J. Anim. Sci. Technol. 2021, 63, 531–544. [Google Scholar] [CrossRef]
- Mayorga, E.J.; Kvidera, S.K.; Horst, E.A.; Al-Qaisi, M.; Dickson, M.J.; Seibert, J.T.; Lei, S.; Keating, A.F.; Ross, J.W.; Rhoads, R.P.; et al. Effects of zinc amino acid complex on biomarkers of gut integrity and metabolism during and following heat stress or feed restriction in pigs. J. Anim. Sci. 2018, 96, 4173–4185. [Google Scholar] [CrossRef]
- Quiniou, N.; Noblet, J.; van Milgen, J.; Dubois, S. Modelling heat production and energy balance in group-housed growing pigs exposed to low or high ambient temperatures. Br. J. Nutr. 2001, 85, 97–106. [Google Scholar] [CrossRef]
- Collin, A.; van Milgen, J.; Le Dividich, J. Modeling the effect of high, constant temperature on food intake in young growing pigs. Anim. Sci. 2001, 72, 519–527. [Google Scholar] [CrossRef]
- Challet, E. Interactions between light, mealtime and calorie restriction to control daily timing in mammals. J. Comp. Physiol. B 2010, 180, 631–644. [Google Scholar] [CrossRef] [PubMed]
| Temperature (T) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thermoneutrality | Cyclic Heat Stress | ANOVA p-Value 4 | ||||||||||
| Period of the Day (P) | PI (06–08 h) | PII (08–18 h) | PIII (18–20 h) | PIV (20–06 h) | PI (TN, 06–08 h) | PII (HS, 08–18 h) | PIII (HS, 18–20 h) | PIV (TN, 20–06 h) | P × T | P × EP | T × EP | P × EP × T |
| Feed intake rate, g/min 5 | 27.4 (0.40) | 27.9 (0.32) | 27.4 (0.45) | 27.4 (0.35) | 27.6 b (0.39) | 27.5 b (0.32) | 28.6 a (0.41) | 27.9 ab (0.34) | <0.01 | 0.01 | 0.89 | 0.30 |
| Meal size 6, g/meal | 605 (15) | 751 (12) | 557 (18) | 604 (14) | 516 (15) | 483 (12) | 404 (16) | 510 (13) | <0.01 | <0.01 | 0.83 | <0.01 |
| Meal duration 3,6, min/meal | 25.9 (0.87) | 31.5 (0.59) | 22.4 (1.03) | 26.0 (0.72) | 23.3 (0.83) | 22.0 (0.56) | 16.4 (0.91) | 21.5 (0.66) | <0.01 | 0.02 | 0.50 | <0.01 |
| Interval between meals 3,6, min | 507 (14) | 310 (11) | 300 (15) | 504 (12) | 350 (13) | 224 (11) | 219 (14) | 421 (12) | <0.01 | <0.01 | 0.73 | 0.01 |
| Number of meals, n/pig | 0.23 (0.01) | 0.19 (0.01) | 0.15 (0.01) | 0.09 (0.01) | 0.27 (0.01) | 0.25 (0.01) | 0.21 (0.01) | 0.12 (0.01) | 0.17 | 0.71 | 0.82 | 0.81 |
| Period of the Day (P) | ANOVA | ||||
|---|---|---|---|---|---|
| Experimental Phase (EP) | PI (06–08 h) | PII (08–18 h) | PIII (18–20 h) | PIV (20–06 h) | p-Value 3 |
| Experimental phase 1 (0–20 days) | |||||
| Feed intake rate, g/min | 25.6 b (0.40) | 25.7 b (0.32) | 26.9 a (0.42) | 26.2 ab (0.34) | <0.01 |
| Experimental phase 2 (21–38 days) | |||||
| Feed intake rate, g/min | 29.3 (0.40) | 29.7 (0.32) | 29.3 (0.44) | 29.2 (0.35) | 0.22 |
| Temperature (T) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Thermoneutrality | Cyclic Heat Stress | ANOVA | |||||||
| Period of the Day (P) | PI (06–08 h) | PII (08–18 h) | PIII (18–20 h) | PIV (20–06 h) | PI (TN, 06–08 h) | PII (HS, 08–18 h) | PIII (HS, 18–20 h) | PIV (TN, 20–06 h) | p-Value 4 |
| Experimental phase 1 (0–20 days) | |||||||||
| Meal size, g/meal | 587 b (22) | 686 a (17) | 581 b (24) | 569 b (19) | 484 a (21) | 470 a (17) | 387 b (22) | 460 a (18) | <0.01 |
| Meal duration 3, min/meal | 27.0 b (1.22) | 31.6 a (0.82) | 24.1 b (1.43) | 24.7 b (0.96) | 23.7 ab (1.15) | 23.1 a (0.79) | 16.4 c (1.24) | 20.7 b (0.93) | <0.01 |
| Interval between meals 3, min | 507 a (19) | 289 b (15) | 303 b (21) | 432 a (17) | 381 b (18) | 222 c (15) | 204 c (19) | 393 a (16) | <0.01 |
| Experimental phase 2 (21–38 days) | |||||||||
| Meal size, g/meal | 624 b (22) | 816 a (17) | 534 c (25) | 640 b (20) | 547 a (21) | 496 b (17) | 420 c (23) | 559 a (18) | <0.01 |
| Meal duration, min/meal | 24.7 b (1.25) | 31.5 a (0.84) | 20.7 c (1.50) | 27.2 b (1.07) | 23.0 a (1.19) | 20.8 a (0.80) | 16.5 b (1.33) | 22.3 a (0.95) | <0.01 |
| Interval between meals, min | 507 b (19) | 331 c (15) | 297 c (22) | 576 a (18) | 319 b (19) | 226 c (15) | 234 bc (20) | 448 a (16) | <0.01 |
| Period of the Day (P) | ANOVA p-Value 3 | ||||||
|---|---|---|---|---|---|---|---|
| Temperature (T) | PI (06–08 h) | PII (08–18 h) | PIII (18–20 h) | PIV (20–06 h) | C1 | C2 | C3 |
| Thermoneutrality | |||||||
| Meal size, g/meal | 605 (15) | 751 (12) | 557 (18) | 604 (14) | 0.15 | <0.01 | <0.01 |
| Cyclic heat stress | |||||||
| Meal size, g/meal | 516 (15) | 483 (12) | 404 (16) | 510 (13) | <0.01 | <0.01 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, M.J.K.; Valk, M.; Melo, A.D.B.; Marçal, D.A.; Silva, C.A.; Valini, G.A.d.C.; Arnaut, P.R.; Gonçalves, J.P.R.; Andretta, I.; Hauschild, L. Feeding Behavior of Finishing Pigs under Diurnal Cyclic Heat Stress. Animals 2023, 13, 908. https://doi.org/10.3390/ani13050908
de Oliveira MJK, Valk M, Melo ADB, Marçal DA, Silva CA, Valini GAdC, Arnaut PR, Gonçalves JPR, Andretta I, Hauschild L. Feeding Behavior of Finishing Pigs under Diurnal Cyclic Heat Stress. Animals. 2023; 13(5):908. https://doi.org/10.3390/ani13050908
Chicago/Turabian Stylede Oliveira, Marllon José Karpeggiane, Marcio Valk, Antônio Diego Brandão Melo, Danilo Alves Marçal, Cleslei Alisson Silva, Graziela Alves da Cunha Valini, Pedro Righetti Arnaut, Joseane Penteado Rosa Gonçalves, Ines Andretta, and Luciano Hauschild. 2023. "Feeding Behavior of Finishing Pigs under Diurnal Cyclic Heat Stress" Animals 13, no. 5: 908. https://doi.org/10.3390/ani13050908
APA Stylede Oliveira, M. J. K., Valk, M., Melo, A. D. B., Marçal, D. A., Silva, C. A., Valini, G. A. d. C., Arnaut, P. R., Gonçalves, J. P. R., Andretta, I., & Hauschild, L. (2023). Feeding Behavior of Finishing Pigs under Diurnal Cyclic Heat Stress. Animals, 13(5), 908. https://doi.org/10.3390/ani13050908






