Improvement of the Seminal Characteristics in Rams Using Agri-Food By-Products Rich in Phytomelatonin
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Determination of Melatonin Content in Vegetables and Residues after In Vitro Digestion
2.2. Analysis of Chemical Composition of the By-Products
2.3. Analysis of In Vitro Digestibility of the By-Products
2.4. Animals and Diets
2.5. Semen Collection and Seminal Plasma Extraction
2.6. Sperm Motility Analysis
2.7. Sperm Morphological Study
2.8. Flow Cytometry Analyses
2.8.1. Evaluation of Sperm Membrane Integrity
2.8.2. Intracellular Content of ROS
2.8.3. Detection of Membrane Phosphatidylserine Translocation
2.9. Melatonin Evaluation in Seminal Plasma
2.10. Antioxidant Enzyme Activity Assays in Seminal Plasma
2.10.1. Glutathione Reductase (GRD, EC.1.6.4.2)
2.10.2. Glutathione Peroxidase (GPx, EC.1.11.1.9)
2.10.3. Catalase (CAT, EC. 1.11.1.6)
2.11. Statistical Analyses
3. Results
3.1. Melatonin Content in Vegetables
3.2. Melatonin Content after In Vitro Digestion and Diet Composition
3.3. Effects of Phytomelatonin-Rich Diets on Seminal Plasma
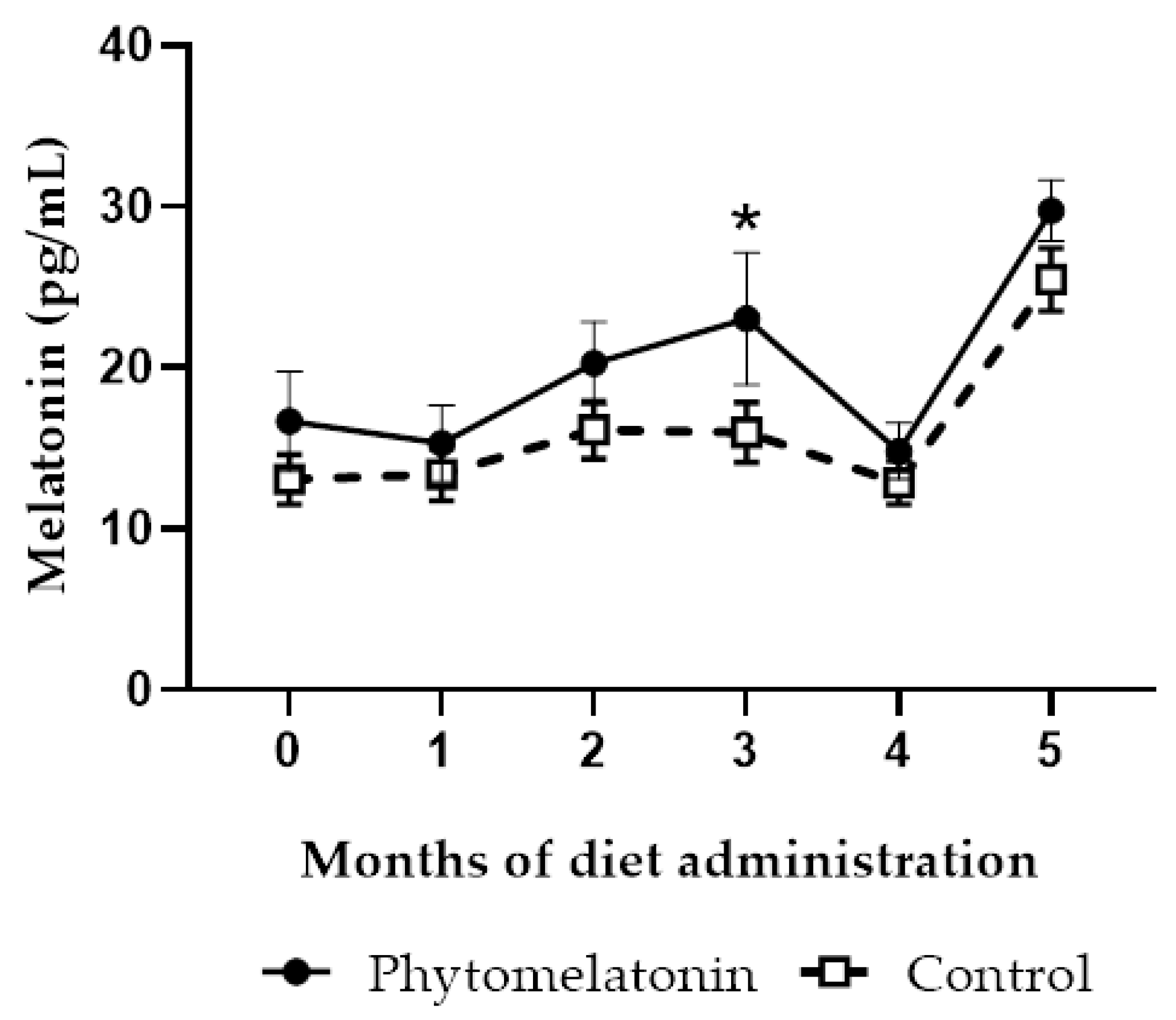
3.3.1. Effect on Melatonin Levels in Seminal Plasma
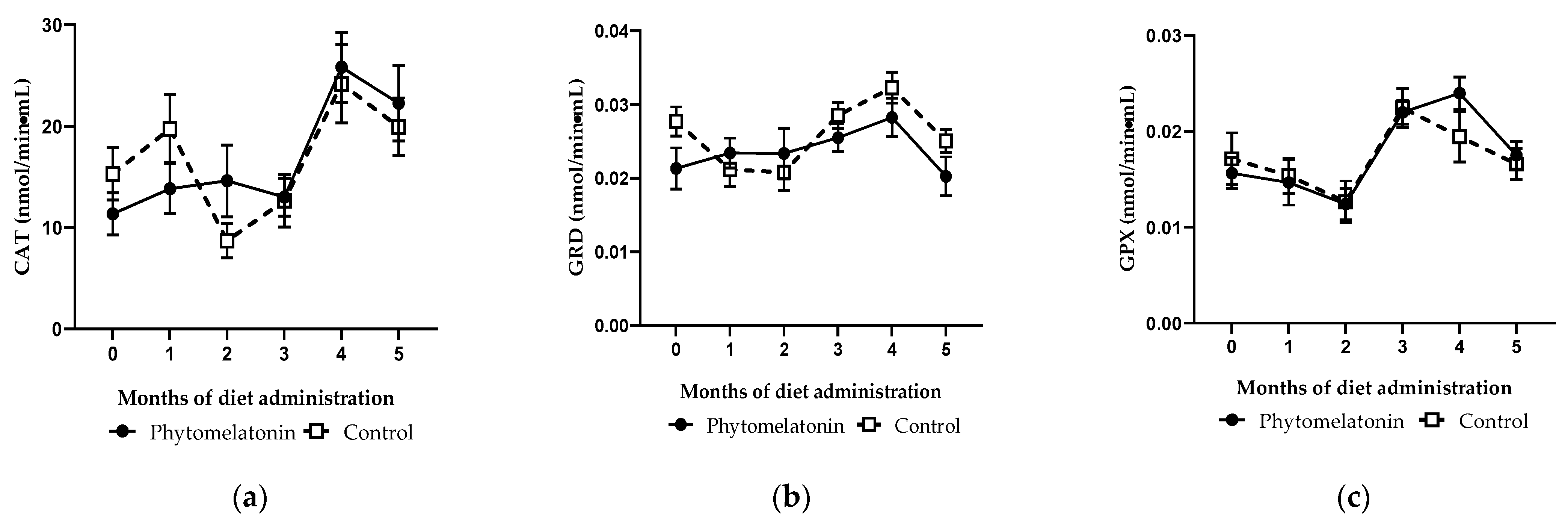
3.3.2. Effect on Antioxidant Enzymes Activity in Seminal Plasma
3.4. Effects of Phytomelatonin-Rich Diets on Sperm Quality
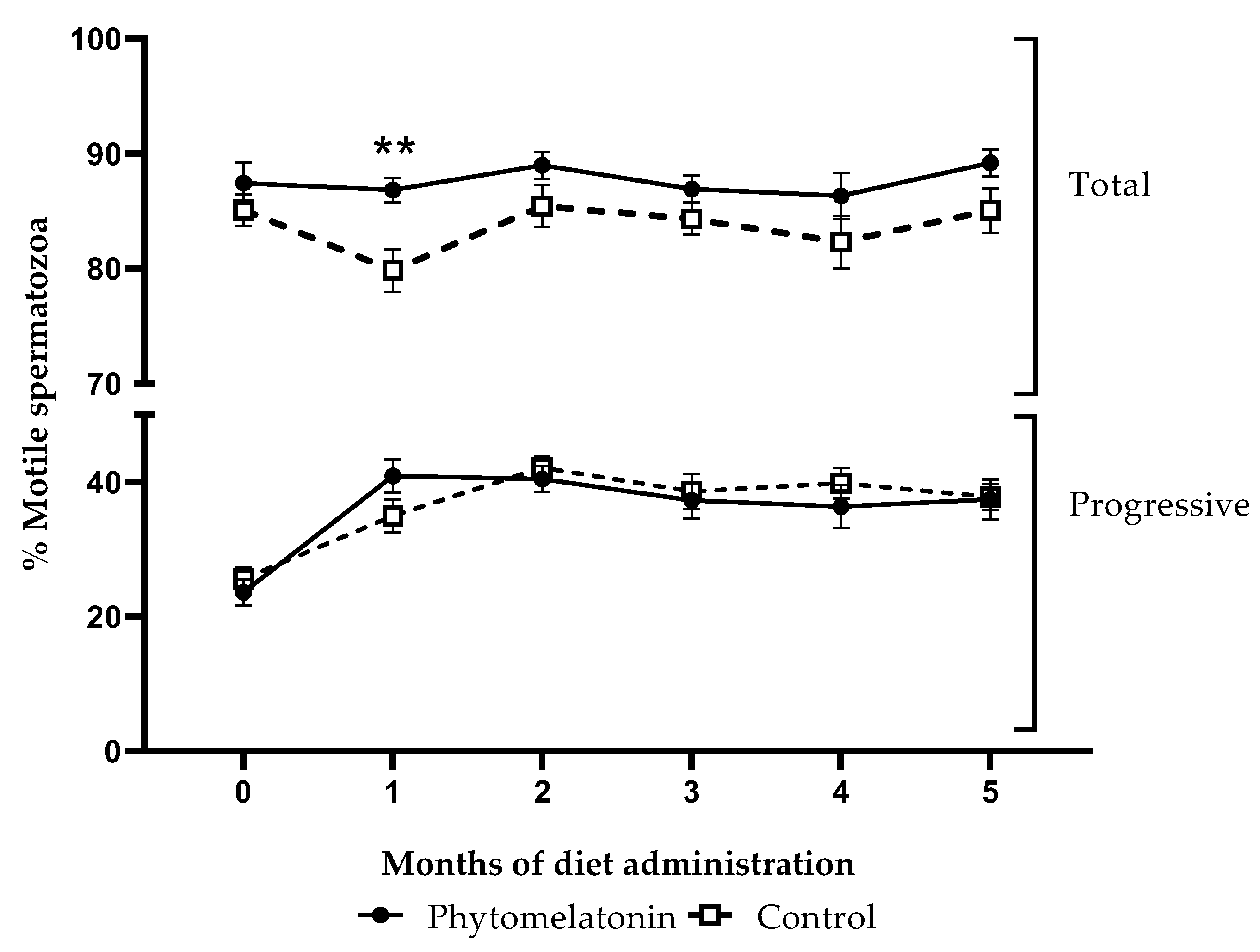
3.4.1. Effect on Sperm Motility
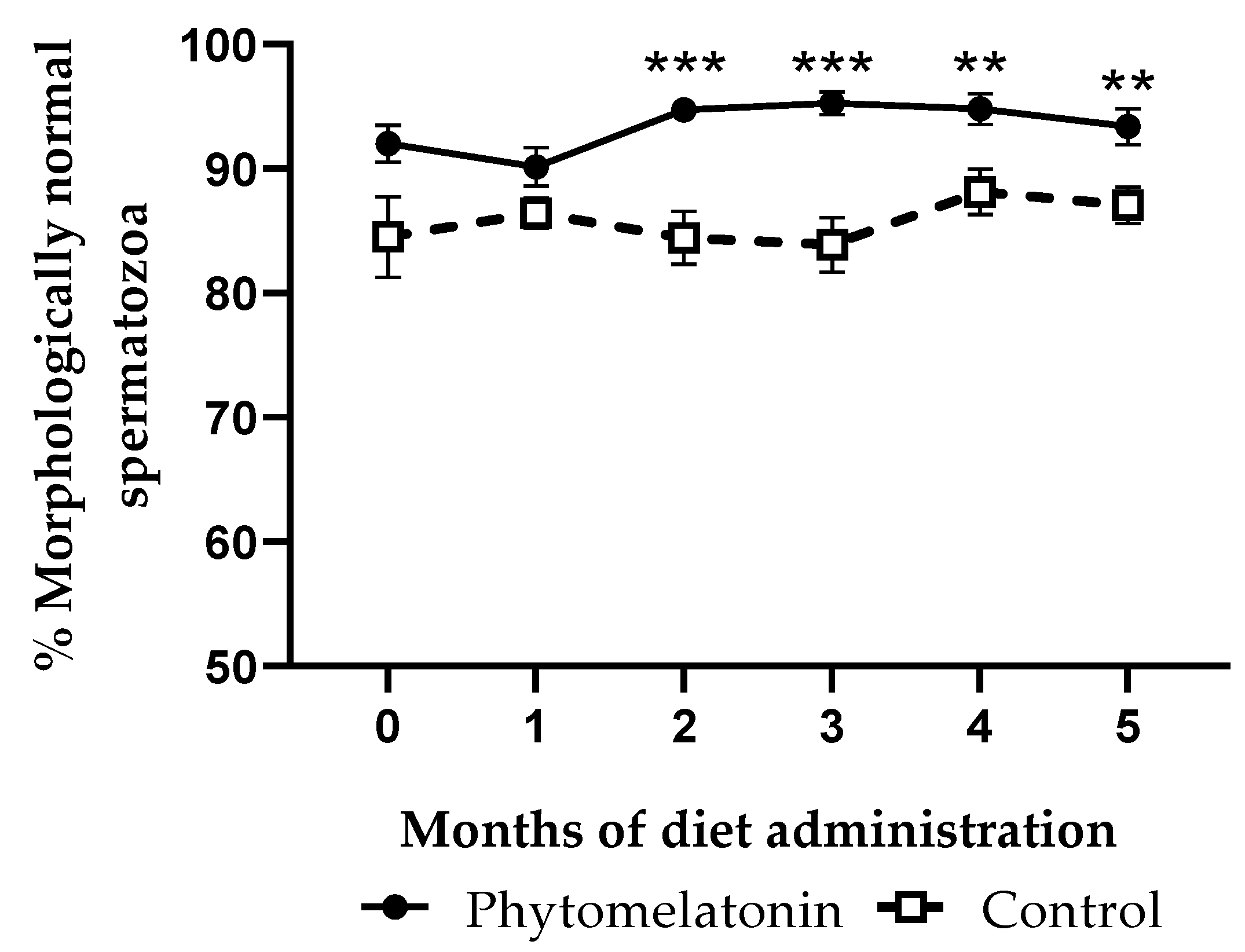
3.4.2. Effect on Sperm Morphology
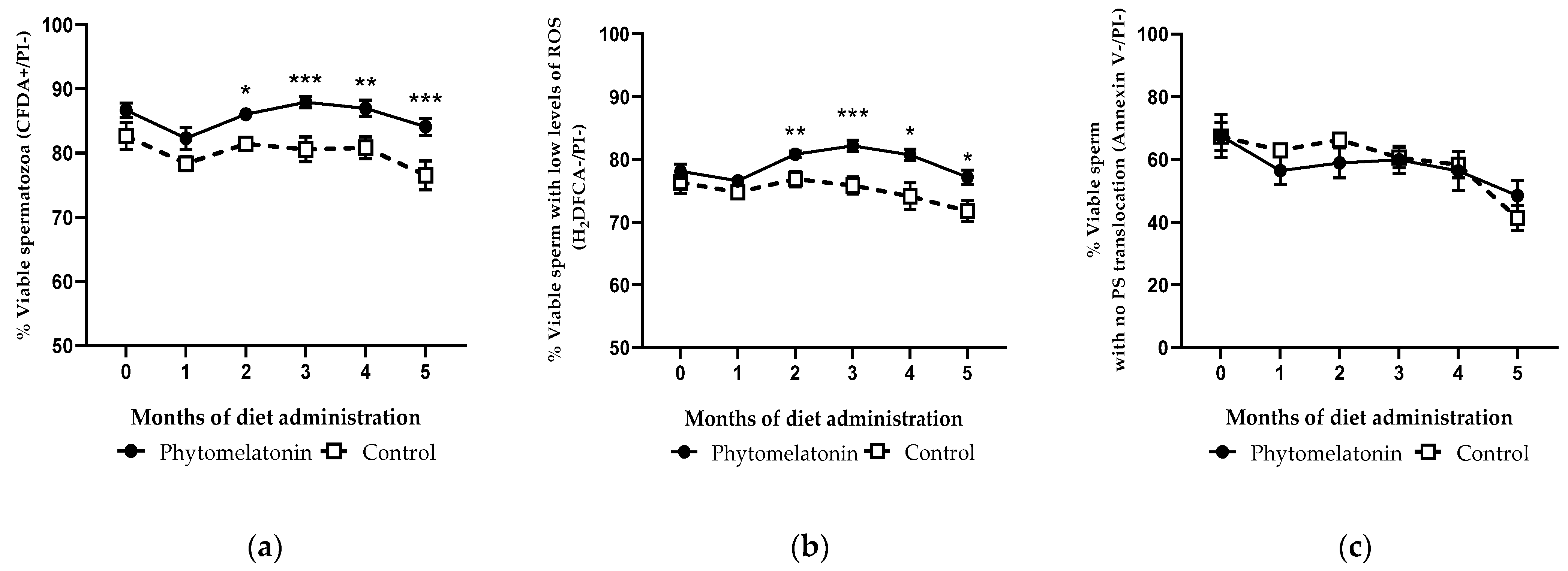
3.4.3. Effect on Sperm Viability, ROS Levels and PS Translocation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernués, A.; Ruiz, R.; Olaizola, A.; Villalba, D.; Casasús, I. Sustainability of Pasture-Based Livestock Farming Systems in the European Mediterranean Context: Synergies and Trade-Offs. Livest. Sci. 2011, 139, 44–57. [Google Scholar] [CrossRef]
- Gandini, G.C.; Oldenbroek, J.K. Strategies for Moving from Conservation to Utilisation. Chapter 2: Utilisation and Conservation of Farm Animal Genetic Resources. Util. Conserv. Farm Anim. Genet. Resour. 2007, 29, 29–54. [Google Scholar] [CrossRef]
- Sierra Alfranca, I. Razas Aragonesas de Ganado; Gobierno de Aragón, Departamento de Agricultura: Zaragoza, Spain, 2002; ISBN 84-7753-972-3. [Google Scholar]
- Rossi, R. The Sheep and Goat Sector in the EU—Main Features, Challenges and Prospects. Available online: https://www.europarl.europa.eu/thinktank/en/document/EPRS_BRI(2017)608663 (accessed on 27 February 2023).
- Chemineau, P.; Guillaume, D.; Migaud, M.; Thiéry, J.C.; Pellicer-Rubio, M.T.; Malpaux, B. Seasonality of Reproduction in Mammals: Intimate Regulatory Mechanisms and Practical Implications. Reprod. Domest. Anim. 2008, 43, 40–47. [Google Scholar] [CrossRef]
- Gonzalez-Arto, M.; Hamilton, T.R.D.S.; Gallego, M.; Gaspar-Torrubia, E.; Aguilar, D.; Serrano-Blesa, E.; Abecia, J.A.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.A.; et al. Evidence of Melatonin Synthesis in the Ram Reproductive Tract. Andrology 2016, 4, 163–171. [Google Scholar] [CrossRef]
- Casao, A.; Cebrián, I.; Asumpção, M.E.; Pérez-Pé, R.; Abecia, J.A.; Forcada, F.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Seasonal Variations of Melatonin in Ram Seminal Plasma Are Correlated to Those of Testosterone and Antioxidant Enzymes. Reprod. Biol. Endocrinol. 2010, 8, 59. [Google Scholar] [CrossRef]
- Casao, A.; Mendoza, N.; Pérez-Pé, R.; Grasa, P.; Abecia, J.A.; Forcada, F.; Cebrián-Pérez, J.A.; Muino-Blanco, T. Melatonin Prevents Capacitation and Apoptotic-like Changes of Ram Spermatozoa and Increases Fertility Rate. J. Pineal Res. 2010, 48, 39–46. [Google Scholar] [CrossRef]
- Gimeno-Martos, S.; Casao, A.; Yeste, M.; Cebrián-Pérez, J.A.; Muiño-Blanco, T.; Pérez-Pé, R. Melatonin Reduces CAMP-Stimulated Capacitation of Ram Spermatozoa. Reprod. Fertil. Dev. 2019, 31, 420–431. [Google Scholar] [CrossRef]
- Miguel-Jiménez, S.; Carvajal-Serna, M.; Calvo, S.; Casao, A.; Cebrián-Pérez, J.Á.; Muiño-Blanco, T.; Pérez-Pe, R. Does Melatonin Exert Its Effect on Ram Sperm Capacitation through Nitric Oxide Synthase Regulation? Int. J. Mol. Sci. 2020, 21, 2093. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Seamark, R.F. Circulating Levels of Melatonin Following Its Oral Administration or Subcutaneous Injection in Sheep and Goats. Aust. J. Biol. Sci. 1980, 33, 349–354. [Google Scholar] [CrossRef]
- Arendt, J.; Symons, A.M.; Laud, C.A.; Pryde, S.J. Melatonin Can Induce Early Onset of the Breeding Season in Ewes. J. Endocrinol. 1983, 97, 395–400. [Google Scholar] [CrossRef]
- Ronayne, E.; Jordan, B.; Quirke, J.F.; Roche, J.F. The Effect of Frequency of Administration of Melatonin on the Time of Onset of the Breeding Season in Anoestrous Ewes. Anim. Reprod. Sci. 1989, 18, 13–24. [Google Scholar] [CrossRef]
- Kusakari, N.; Ohara, M. Effect of Melatonin Feeding on Testicular Development, Reproductive Behaviour and Sperm Production in Suffolk Rams. J. Reprod. Dev. 1993, 39, 357–361. [Google Scholar] [CrossRef]
- Kaya, A.; Baspinar, N.; Yildiz, C.; Kurtoglu, F.; Ataman, M.B.; Haliloglu, S. Influence of Melatonin Implantation on Sperm Quality, Biochemical Composition of the Seminal Plasma and Plasma Testosterone Levels in Rams. Rev. Med. Vet. 2000, 151, 1143–1146. [Google Scholar]
- Casao, A.; Pérez-Pé, R.; Abecia, J.A.; Forcada, F.; Muiño-Blanco, T.; Cebrián-Pérez, J.Á. The Effect of Exogenous Melatonin during the Non-Reproductive Season on the Seminal Plasma Hormonal Profile and the Antioxidant Defence System of Rasa Aragonesa Rams. Anim. Reprod. Sci. 2013, 138, 168–174. [Google Scholar] [CrossRef]
- Egerszegi, I.; Sarlós, P.; Rátky, J.; Solti, L.; Faigl, V.; Kulcsár, M.; Cseh, S. Effect of Melatonin Treatment on Semen Parameters and Endocrine Function in Black Racka Rams out of the Breeding Season. Small Rumin. Res. 2014, 116, 192–198. [Google Scholar] [CrossRef]
- Rekik, M.; Taboubi, R.; Ben Salem, I.; Fehri, Y.; Sakly, C.; Lassoued, N.; Hilali, M.E. Melatonin Administration Enhances the Reproductive Capacity of Young Rams under a Southern Mediterranean Environment. Anim. Sci. J. 2015, 86, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Pool, K.R.; Rickard, J.P.; Pini, T.; de Graaf, S.P. Exogenous Melatonin Advances the Ram Breeding Season and Increases Testicular Function. Sci. Rep. 2020, 10, 9711. [Google Scholar] [CrossRef]
- Žaja, I.; Berta, V.; Valpotić, H.; Samardžija, M.; Milinković-Tur, S.; Vilić, M.; Šuran, J.; Hlede, J.P.; Đuričić, D.; Špoljarić, B.; et al. The Influence of Exogenous Melatonin on Antioxidative Status in Seminal Plasma and Spermatozoa in French Alpine Bucks during the Nonbreeding Season. Domest. Anim. Endocrinol. 2020, 71, 106400. [Google Scholar] [CrossRef]
- Ramadan, T.A.; Kumar, D.; Ghuman, S.S.; Singh, I. Melatonin-Improved Buffalo Semen Quality during Nonbreeding Season under Tropical Condition. Domest. Anim. Endocrinol. 2019, 68, 119–125. [Google Scholar] [CrossRef]
- Samir, H.; Nyametease, P.; Elbadawy, M.; Nagaoka, K.; Sasaki, K.; Watanabe, G. Administration of Melatonin Improves Testicular Blood Flow, Circulating Hormones, and Semen Quality in Shiba Goats. Theriogenology 2020, 146, 111–119. [Google Scholar] [CrossRef]
- Perumal, P.; Chang, S.; Baruah, K.K.; Srivastava, N. Administration of Slow Release Exogenous Melatonin Modulates Oxidative Stress Profiles and in Vitro Fertilizing Ability of the Cryopreserved Mithun (Bos frontalis) Spermatozoa. Theriogenology 2018, 120, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B. Phytomelatonin: Discovery, Content, and Role in Plants. Adv. Bot. 2014, 2014, 815769. [Google Scholar] [CrossRef]
- Manchester, L.C.; Tan, D.X.; Reiter, R.J.; Park, W.; Monis, K.; Qi, W. High Levels of Melatonin in the Seeds of Edible Plants: Possible Function in Germ Tissue Protection. Life Sci. 2000, 67, 3023–3029. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.; Tan, D.; Manchester, L.; Simopoulos, A.; Maldonado, M.; Flores, L.; Terron, M. Melatonin in Edible Plants (Phytomelatonin): Identification, Concentrations, Bioavailability and Proposed Functions. World Rev. Nutr. Diet. 2007, 97, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Reiter, R.J.; Manchester, L.C.; Tan, D.X. Melatonin in Walnuts: Influence on Levels of Melatonin and Total Antioxidant Capacity of Blood. Nutrition 2005, 21, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Sangsopha, J.; Johns, N.P.; Johns, J.; Moongngarm, A. Dietary Sources of Melatonin and Benefits from Production of High Melatonin Pasteurized Milk. J. Food Sci. Technol. 2020, 57, 2026–2037. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Fernández-Gómez, B.; Herrero, M.; Aguilera, Y.; Martín-Cabrejas, M.A.; Uribarri, J.; Del Castillo, M.D. Inhibition of the Maillard Reaction by Phytochemicals Composing an Aqueous Coffee Silverskin Extract via a Mixed Mechanism of Action. Foods 2019, 8, 438. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Mertens, D.R.; Allen, M.; Carmany, J.; Clegg, J.; Davidowicz, A.; Drouches, M.; Frank, K.; Gambin, D.; Garkie, M.; Gildemeister, B.; et al. Gravimetric Determination of Amylase-Treated Neutral Detergent Fiber in Feeds with Refluxing in Beakers or Crucibles: Collaborative Study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar]
- Robertson, J.B.; Van Soest, P.J. The Detergent System of Analysis and Its Application to Human Foods. In The Analysis of Dietary Fiber in Foods; James, W.P., Theander, O., Eds.; Marcel Dekker: New York, NY, USA, 1981; pp. 123–158. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A Simple Gas Production Method Using a Pressure Transducer to Determine the Fermentation Kinetics of Ruminant Feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Mould, F.L.; Kliem, K.E.; Morgan, R.; Mauricio, R.M. In Vitro Microbial Inoculum: A Review of Its Function and Properties. Anim. Feed Sci. Technol. 2005, 123–124, 31–50. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Stern, M.D. A Three-Step in Vitro Procedure for Estimating Intestinal Digestion of Protein in Ruminants. J. Anim. Sci. 1995, 73, 1459–1465. [Google Scholar] [CrossRef]
- Ax, R.L.; Dally, M.; Didion, B.; Lenz, R.; Love, C.; Varner, D.; Hafez, B.; Bellin, M. Semen Evaluation. In Reproduction in Farm Animals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; pp. 363–375. ISBN 978-1-119-26530-6. [Google Scholar]
- Harrison, R.A.; Vickers, S.E. Use of Fluorescent Probes to Assess Membrane Integrity in Mammalian Spermatozoa. J. Reprod. Fertil. 1990, 88, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Bass, D.A.; Parce, J.W.; Dechatelet, L.R.; Szejda, P.; Seeds, M.C.; Thomas, M. Flow Cytometric Studies of Oxidative Product Formation by Neutrophils: A Graded Response to Membrane Stimulation. J. Immunol. 1983, 130, 1910–1917. [Google Scholar] [CrossRef]
- Carter, W.O.; Narayanan, P.K.; Robinson, J.P. Intracellular Hydrogen Peroxide and Superoxide Anion Detection in Endothelial Cells. J. Leukoc. Biol. 1994, 55, 253–258. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Welch, G.R. Determination of Intracellular Reactive Oxygen Species and High Mitochondrial Membrane Potential in Percoll-Treated Viable Boar Sperm Using Fluorescence-Activated Flow Cytometry. J. Anim. Sci. 2006, 84, 2089–2100. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutelingsperger, C. A Novel Assay for Apoptosis. Flow Cytometric Detection of Phosphatidylserine Expression on Early Apoptotic Cells Using Fluorescein Labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Carvajal-Serna, M.; Fatnassi, M.; Torres-Ruda, F.; Cardozo, J.A.; Grajales-Lombana, H.; Hammadi, M.; Abecia, J.A.; Muiño-Blanco, T.; Pérez-Pe, R.; Cebrián-Pérez, J.Á.; et al. Vasectomy and Photoperiodic Regimen Modify the Protein Profile, Hormonal Content and Antioxidant Enzymes Activity of Ram Seminal Plasma. Int. J. Mol. Sci. 2020, 21, 8063. [Google Scholar] [CrossRef]
- Marti, E.; Mara, L.; Marti, J.I.; Muiño-Blanco, T.; Cebrián-Pérez, J.A. Seasonal Variations in Antioxidant Enzyme Activity in Ram Seminal Plasma. Theriogenology 2007, 67, 1446–1454. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitative and Qualitative Characterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Van Tassel, D.L.; Roberts, N.; Lewy, A.; O’Neill, S.D. Melatonin in Plant Organs. J. Pineal Res. 2001, 31, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Casao, A.; Vega, S.; Palacín, I.; Pérez-Pe, R.; Laviña, A.; Quintín, F.J.; Sevilla, E.; Abecia, J.A.; Cebrián-Pérez, J.A.; Forcada, F.; et al. Effects of Melatonin Implants during Non-Breeding Season on Sperm Motility and Reproductive Parameters in Rasa Aragonesa Rams. Reprod. Domest. Anim. 2010, 45, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Succu, S.; Berlinguer, F.; Pasciu, V.; Satta, V.; Leoni, G.G.; Naitana, S. Melatonin Protects Ram Spermatozoa from Cryopreservation Injuries in a Dose-Dependent Manner. J. Pineal Res. 2011, 50, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Hagenaar, K.; Lampiao, F. The in Vitro Effects of Melatonin on Human Sperm Function and Its Scavenging Activities on NO and ROS. Andrologia 2010, 42, 112–116. [Google Scholar] [CrossRef]
- Jang, H.Y.; Kim, Y.H.; Kim, B.W.; Park, I.C.; Cheong, H.T.; Kim, J.T.; Park, C.K.; Kong, H.S.; Lee, H.K.; Yang, B.K. Ameliorative Effects of Melatonin against Hydrogen Peroxide-Induced Oxidative Stress on Boar Sperm Characteristics and Subsequent in Vitro Embryo Development. Reprod. Domest. Anim. 2010, 45, 943–950. [Google Scholar] [CrossRef]
- Balao Da Silva, C.M.; MacÍas-García, B.; Miró-Morán, A.; González-Fernández, L.; Morillo-Rodriguez, A.; Ortega-Ferrusola, C.; Gallardo-Bolaños, J.M.; Stilwell, G.; Tapia, J.A.; Peña, F.J. Melatonin Reduces Lipid Peroxidation and Apoptotic-like Changes in Stallion Spermatozoa. J. Pineal Res. 2011, 51, 172–179. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of Antioxidant Enzymes: A Significant Role for Melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Sharma, P.; McClees, S.F.; Afaq, F. Pomegranate for Prevention and Treatment of Cancer: An Update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant Nutritional Quality of Tomato. Mol. Nutr. Food Res. 2007, 51, 609–617. [Google Scholar] [CrossRef]
- Cotoras, M.; Vivanco, H.; Melo, R.; Aguirre, M.; Silva, E.; Mendoza, L. In Vitro and in Vivo Evaluation of the Antioxidant and Prooxidant Activity of Phenolic Compounds Obtained from Grape (Vitis Vinifera) Pomace. Molecules 2014, 19, 21154–21167. [Google Scholar] [CrossRef] [PubMed]
| Sample | Melatonin (ng/g) |
|---|---|
| Pomegranate pomace | 35.81 ± 0.40 |
| Pomegranate peels | 127.03 ± 8.73 |
| Tomato pomace | 23.76 ± 1.37 |
| Brewer’s spent grains | 3.01 ± 0.15 |
| Malt sprouts | 3.33 ± 0.04 |
| Spent yeast | 5.03 ± 0.11 |
| Grape pulp | 45.94 ± 4.19 |
| Grape seeds | 9.26 ± 0.52 |
| Sunflower meal | 41.55 ± 5.16 |
| Sample | Melatonin Content (ng/g) * | ||
|---|---|---|---|
| After Ruminal Incubation | After HCl-Pepsin Treatment | ||
| Liquid Fraction | Solid Fraction | ||
| Pomegranate pomace | 0.53 ± 0.04 | n.d. | 0.93 ± 0.58 |
| Pomegranate peels | 1.56 ± 0.47 | 0.62 ± 0.14 | n.d. |
| Tomato pomace | 2.69 ± 0.69 | 0.32 ±0.02 | 0.54 ± 0.11 |
| Grape pulp | 1.58 ± 0.19 | 0.34 ± 0.12 | n.d. |
| Sunflower meal | 1.22 ± 0.15 | 0.58 ± 0.06 | 0.32 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peña-Delgado, V.; Carvajal-Serna, M.; Fondevila, M.; Martín-Cabrejas, M.A.; Aguilera, Y.; Álvarez-Rivera, G.; Abecia, J.A.; Casao, A.; Pérez-Pe, R. Improvement of the Seminal Characteristics in Rams Using Agri-Food By-Products Rich in Phytomelatonin. Animals 2023, 13, 905. https://doi.org/10.3390/ani13050905
Peña-Delgado V, Carvajal-Serna M, Fondevila M, Martín-Cabrejas MA, Aguilera Y, Álvarez-Rivera G, Abecia JA, Casao A, Pérez-Pe R. Improvement of the Seminal Characteristics in Rams Using Agri-Food By-Products Rich in Phytomelatonin. Animals. 2023; 13(5):905. https://doi.org/10.3390/ani13050905
Chicago/Turabian StylePeña-Delgado, Victoria, Melissa Carvajal-Serna, Manuel Fondevila, María A. Martín-Cabrejas, Yolanda Aguilera, Gerardo Álvarez-Rivera, José A. Abecia, Adriana Casao, and Rosaura Pérez-Pe. 2023. "Improvement of the Seminal Characteristics in Rams Using Agri-Food By-Products Rich in Phytomelatonin" Animals 13, no. 5: 905. https://doi.org/10.3390/ani13050905
APA StylePeña-Delgado, V., Carvajal-Serna, M., Fondevila, M., Martín-Cabrejas, M. A., Aguilera, Y., Álvarez-Rivera, G., Abecia, J. A., Casao, A., & Pérez-Pe, R. (2023). Improvement of the Seminal Characteristics in Rams Using Agri-Food By-Products Rich in Phytomelatonin. Animals, 13(5), 905. https://doi.org/10.3390/ani13050905








