Predation Rate on Olive Riley Sea Turtle (Lepidochelys olivacea) Nests with Solitary Nesting Activity from 2008 to 2021 at Corozalito, Costa Rica
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
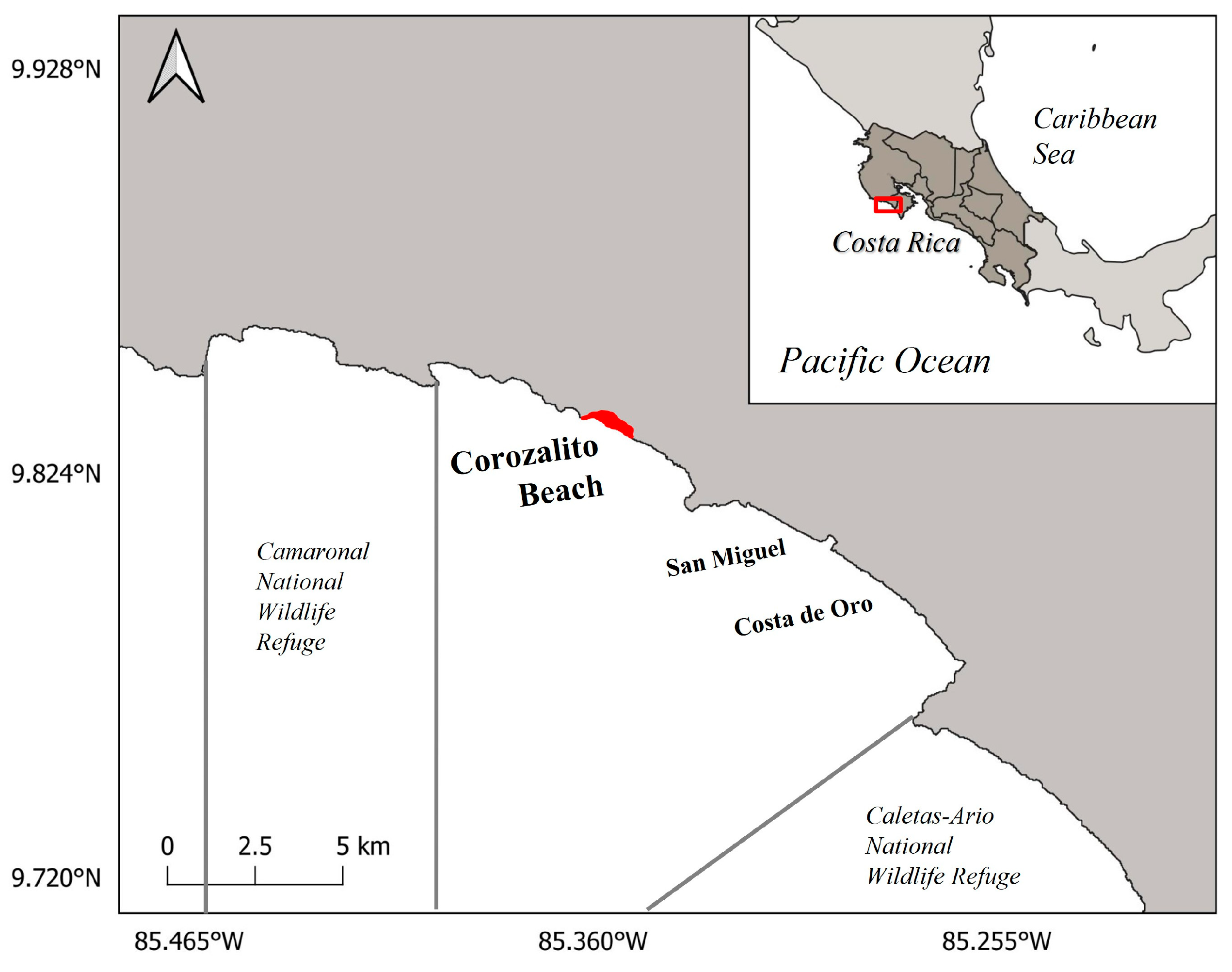
2.1. Study Site
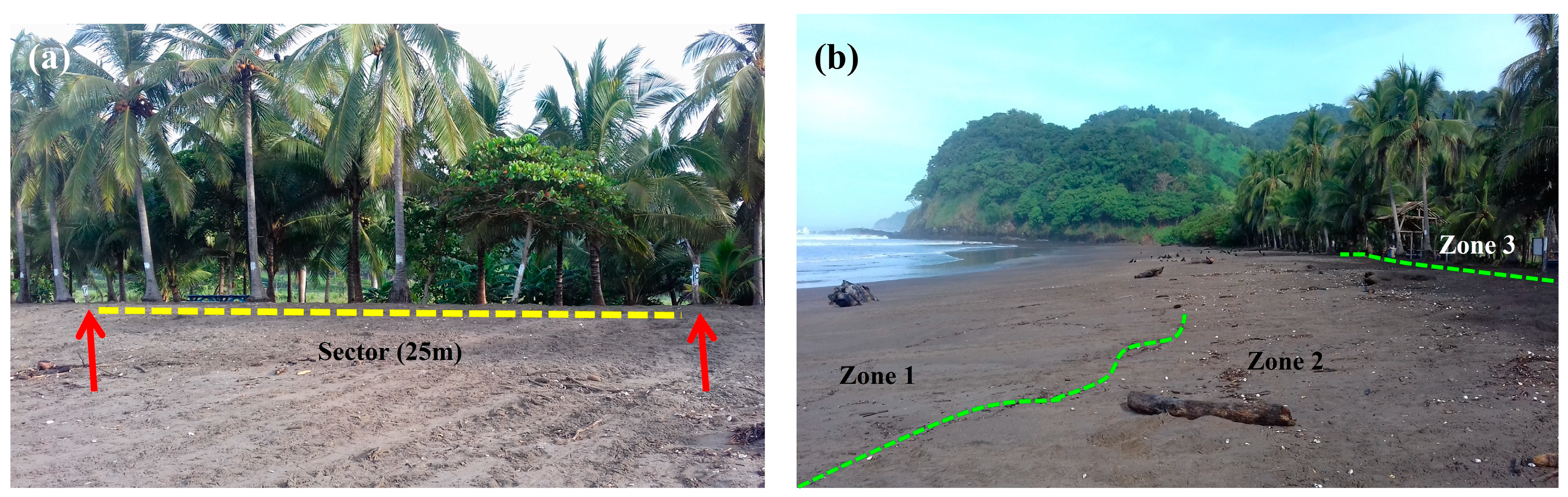
2.2. Data Collection and Analysis
3. Results
3.1. Partial and Complete Predation of Nests
3.2. Distribution of Predation Events
3.3. Nest Predators
4. Discussion
4.1. Predation Rates
4.2. Spatial Distribution of Predation Events
4.3. Predator Identification
4.4. Conservation Efforts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowen, B.W.; Clark, A.M.; Abreu-Grobois, F.A. Global phylogeography of ridley sea turtles (Lepidochelys spp.) as inferred from mitochondrial DNA sequences. Genetica 1998, 101, 179–189. [Google Scholar] [CrossRef]
- Abreu-Grobois, A.; Plotkin, P. Lepidochelys olivacea. IUCN Red List of Threatened Species. 2008. Available online: https://www.iucnredlist.org/species/11534/3292503 (accessed on 17 May 2021).
- Cornelius, S.; Robinson, D. Abundance, Distribution and Movements of Olive Ridley Sea Turtles in Costa Rica, II; Report N° 14-16-0002-81-225; U.S. Fish and Wildlife Service, World Wildlife Fund: Washington, DC, USA, 1982; pp. 1–101.
- Pritchard, P.C.H. Evolution, phylogeny and current status. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 1–28. [Google Scholar]
- Marcovaldi, M.A. Status and distribution of the Olive Ridley Turtle, Lepidochelys olivacea, in the Western Atlantic Ocean. In Proceedings of the Marine Turtle Conservation in the Wider Caribbean Region—A Dialogue for Effective Regional Management, Santo Domingo, Dominican Republic, 16–18 November 1999; Eckert, K., Abreu-Grobois, A., Eds.; pp. 52–56. [Google Scholar]
- Bernardo, J.; Plotkin, P.T. An evolutionary perspective on the arribada phenomenon and reproductive behavioral polymorphism of olive ridley sea turtles (Lepidochelys olivacea). In Biology and Conservation of Ridley Sea Turtles; Plotkin, P.T., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 2007; pp. 59–87. [Google Scholar]
- Eguchi, T.; Gerrodette, T.; Pitman, R.L.; Seminoff, J.A.; Dutton, P.H. At-sea density and abundance estimates of the Olive Ridley sea turtle Lepidochelys olivacea in the eastern tropical Pacific. Endanger. Species Res. 2007, 3, 191–203. [Google Scholar] [CrossRef]
- Shanker, K.; Pandav, B.; Choudhury, B.C. An assessment of the olive ridley turtle (Lepidochelys olivacea) nesting population in Orissa, India. Biol. Conserv. 2004, 115, 149–160. [Google Scholar] [CrossRef]
- Fonseca, L.G.; Valverde, R.A. Reporte Final de la Anidación de Tortuga Lora (Lepidochely solivacea), Playa Nancite, Parque Nacional Santa Rosa, Costa Rica (Temporada 2009-2010); Ministerio de Ambiente, Energía y Telecomunicaciones: San José, Costa Rica, 2010; pp. 1–26.
- Ocana, M.; Harfush-Melendez, M.; Heppell, S.S. Mass nesting of olive ridley sea turtles Lepidochelys olivacea at La Escobilla, Mexico: Linking nest density and rates of destruction. Endanger. Species Res. 2012, 16, 45–54. [Google Scholar] [CrossRef]
- Valverde, R.A.; Orrego, C.M.; Tordoir, M.T.; Gómez, F.M.; Solís, D.S.; Hernández, R.A.; Gómez, G.B.; Brenes, L.S.; Baltodano, J.P.; Fonseca, L.G.; et al. Olive Ridley Mass Nesting Ecology and Egg Harvest at Ostional Beach, Costa Rica. Chelonian Conserv. Biol. 2012, 11, 1–11. [Google Scholar] [CrossRef]
- Rojas-Cañizales, D.; Mejías-Balsalobre, C.; Espinoza-Rodríguez, N.; Bézy, V.S.; Naranjo, I.; Arauz, R.; Valverde, R.A. Corozalito: A nascent arribada nesting beach in Costa Rica. Mar. Biol. 2022, 169, 1–12. [Google Scholar] [CrossRef] [PubMed]
- CITES. Convención Sobre el Comercio Internacional de Especies Amenazadas de Fauna y Flora Silvestres. Apéndice I, II y III. Ginebra, Suiza. 2018. Available online: www.cites.org (accessed on 11 September 2022).
- Ley de Conservación de Vida Silvestre No. 7317, No. Gaceta: 235 del 07/12/1992. Asamblea Legislativa, Sistema Costarricense de Información Jurídica: San José, Costa Rica. Available online: http://www.pgrweb.go.cr/scij/Busqueda/Normativa/Normas/nrm_texto_completo.aspx?param1=NRTC&nValor1=1&nValor2=12648&nValor3=92418 (accessed on 14 October 2022).
- Ministerio de Ambiente y Energía (MINAE)-Costa Rica. Estrategia Nacional para la Conservación y Protección de las Tortugas Marinas, 1st ed.; Ministerio de Ambiente y Energía (MINAE): San José, Costa Rica, 2018.
- Listas de Especies Nacionales (Resolución No. 092-2017), Costa Rica. Available online: https://www.conagebio.go.cr/Conagebio/public/documentos/legislacion/Directrices/Resolucion92.pdf (accessed on 14 October 2022).
- Richard, J.D.; Hughes, D.A. Some observations of sea turtle nesting activity in Costa Rica. Mar. Biol. 1972, 16, 297–309. [Google Scholar] [CrossRef]
- Tripathy, B. Distribution and dynamics of reproductive patch in olive ridley sea turtles (Lepicochelys olivacea) off Rushikulya, Orissa coast of India. Indian J. Geo-Mar. Sci. 2013, 42, 343–348. [Google Scholar]
- Richardson, J.I. Priorities for Studies of Reproduction and Nest Biology. In Research and Management Techniques for the Conservation of Sea Turtles; Eckert, K.L., Bjorndal, K.A., Abreu-Grobois, F.A., Donnelly, M., Eds.; IUCN/SSC Marine Turtle Specialist Group: Marin County, CA, USA, 1999; pp. 9–11. [Google Scholar]
- Fowler, L. Hatching success and nest predation in the Green Sea Turtle, Chelonia mydas, at Tortuguero, Costa Rica. Ecology 1979, 60, 946–955. [Google Scholar] [CrossRef]
- Reavis, J.L.; Rojas-Cañizales, D.; Mejías-Balsalobre, C.; Naranjo, I.; Arauz, R.; Senko, J. Dynamics of human take and animal predation on sea turtle nests in Northwest Costa Rica. PeerJ 2022, 10, e12925. [Google Scholar] [CrossRef]
- Stancyk, S.E. Non-Human Predators of Sea Turtles and Their Control. In Biology and Conservation of Sea Turtles; Bjorndal, K.A., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1995; pp. 139–152. [Google Scholar]
- Engeman, R.M.; Martin, R.E.; Smith, H.T.; Woolard, J. Dramatic reduction in predation on marine turtle nests through improved predator monitoring and management. Oryx 2005, 39, 318–326. [Google Scholar] [CrossRef]
- Donlan, E.M.; Townsend, J.H.; Golden, E.A. Predation of Caretta caretta (Testudines: Cheloniidae) eggs by larvae of Lanelater sallei on Key Biscayne, Florida. Caribbean. J. Sci. 2004, 40, 415–420. [Google Scholar]
- Wetterer, J.; Wood, L.; Johnson, C.; Krahe, H.; Fitchett, S. Predacious ants, beach replenishment, and nest placement by sea turtles. Environ. Entomol. 2007, 36, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, S. Status of sea turtles along the Pacific coast of Middle America. In Biology and Conservation of Sea Turtles; Bjorndal, K.A., Ed.; Smithsonian Institution Press: Washington, DC, USA, 1982; pp. 211–219. [Google Scholar]
- Viejobueno, S.; Adams, C.; Arauz, R. Conservación e Investigación de Tortugas Marinas en el Pacífico de Costa Rica. Reporte Técnico Julio 2011–Febrero 2012; Publicaciones no Periódicas del Programa de Restauración de Tortugas Marinas: San Jose, Costa Rica, 2012; pp. 1–56. [Google Scholar]
- Tripathy, B.; Rajasekhar, P.S. Natural and anthropogenic threats to olive ridley sea turtles (Lepidochelys olivacea) at the Rushikulya rookery of Orissa coast, India. Indian J. Mar. Sci. 2009, 38, 439–443. [Google Scholar]
- Rojas-Cañizales, D.; Mejías-Balsalobre, C.; Naranjo, I.; Arauz, D. Reporte Final: Proyectos de Conservación de Playas de Anidación de Tortugas Marinas en el Sureste de la Península de Nicoya; Publicaciones no periódicas del Centro de Rescate de Especies Marinas Amenazadas: San Jose, Costa Rica, 2019; pp. 1–19. [Google Scholar]
- Rojas-Cañizales, D. Mejías-Balsalobre, C. Protocol Manual for Southern Nicoya Peninsula (SNP) Sea Turtle Nesting Beach Conservation Projects; Publicaciones no Periódicas del Centro de Rescate de Especies Marinas Amenazadas: San Jose, Costa Rica, 2019; pp. 1–21. [Google Scholar]
- Reichart, H.A. Synopsis of Biological Data on the Ridley Sea Turtles Lepidochelys olivacea (Eschscholtz 1829) in the Western Atlantic; NMFS-SEFSC-336; NOAA Technical Memorandum: Miami, FL, USA, 1993; pp. 1–86.
- Nelson, K.; Mo, C. Predation of Olive Ridley (Lepidochelys olivacea) Nests by Vertebrates and Crabs at Playa Nancite, Costa Rica; Report ACM Tropical Field Research; Área de Conservación de Guanacaste, Programa de Investigación: Liberia, Costa Rica, 1996; pp. 1–16. [Google Scholar]
- Chaves-Cordero, G.A. Tendencia Poblacional y Éxito de Eclosión de las Anidaciones Masivas de Tortugas Lora (Lepidochelys olivacea ESCHSCHOLTZ 1829) en el Refugio Nacional de Vida Silvestre de Ostional, Guanacaste. Master’s Thesis, Universidad de Costa Rica, San Jose, Costa Rica, 2007. [Google Scholar]
- Viejobueno, S.; Adams, C.; Arauz, R. Oportunidades para el desarrollo sostenible de las comunidades costeras de Nandayure (Nicoya sur, Guanacaste). Ambientales 2011, 41, 37–46. [Google Scholar] [CrossRef]
- StatSoft, Inc. STATISTICA (Data Analysis Software System). Version 7. 2004. Available online: www.statsoft.com (accessed on 23 June 2022).
- Blamires, S.J.; Guinea, M.L. Implications of Nest Site Selection on egg predation at the Sea Turtle Rookery at Fog Bay. In Proceedings of the Workshop Marine Turtle Conservation and Management, Northern Territory University Darwin, Darwin, Australia, 3–4 June 1997. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: https://www.R-project.org/ (accessed on 28 January 2023).
- Cortes-Briceno, J.V. Efecto de la Depredación por la Aplicación de la NOM-162-SEMARNAT en el Manejo de Nidos de los Campamentos de Tortugueros de la Isla Aguada y Chenkan, Campeche, México. Bachelor’s Thesis, Universidad de Guadalajara, Guadalajara, Mexico, August 2015. [Google Scholar]
- Bowen, B.; Avise, J.C.; Richardson, J.I.; Meylan, A.B.; Margaritoulis, D.; Hopkins-Murphy, S.R. Population Structure of Loggerhead Turtles (Caretta caretta) in the Northwestern Atlantic Ocean and Mediterranean Sea. Conserv. Biol. 1993, 7, 834–844. [Google Scholar] [CrossRef]
- Behera, S.; Tripathy, B.; Sivakumar, K.; Choudhury, B.C. A case study on Olive Ridley (Lepidochelys olivacea) solitary nest in Gahirmatha Rookery, Odisha, India. Testudo 2013, 7, 49–60. [Google Scholar]
- Burger, J.; Gochfeld, M. Avian Predation on Olive Ridley (Lepidochelys olivacea) Sea Turtle Eggs and Hatchlings: Avian Opportunities, Turtle Avoidance, and Human Protection. Copeia 2014, 1, 109–122. [Google Scholar] [CrossRef]
- Honarvar, S.; O’Connor, M.P.; Spotila, J.R. Density-dependent effects on hatching success of the olive ridley turtle, Lepidochelys olivacea. Oecologia 2008, 157, 221–230. [Google Scholar] [CrossRef]
- Stewart, K.R.; Wyneken, J. Predation risk to loggerhead hatchlings at a high-density nesting beach in southeast Florida. Bull. Mar. Sci. 2004, 74, 325–335. [Google Scholar]
- Eckrich, C.E.; Owens, D.W. Solitary versus Arribada Nesting in the Olive Ridley Sea Turtles (Lepidochelys olivacea): A Test of the Predator-Satiation Hypothesis. Herpetologica 1995, 51, 349–354. [Google Scholar]
- Kolbe, J.J.; Janzen, F.J. Spatial and temporal dynamics of turtle nest predation: Edge effects. Oikos 2002, 99, 538–544. [Google Scholar] [CrossRef]
- Lal Mohan, R.S. Observations on the ecology of nest and on some aspects of reproductive behavior of the ridley turtle Lepidochelys olivacea from Calicut coast. Indian J. Fish. 1986, 33, 39–44. [Google Scholar]
- Dornfeld, T.C.; Robinson, N.J.; Santidrian Tomillo, P.; Paladino, F.V. Ecology of solitary nesting olive ridley sea turtles at Playa Grande, Costa Rica. Mar. Biol. 2014, 162, 123–139. [Google Scholar] [CrossRef]
- Spencer, R.J.; Thompson, M.B. The significance of predation in nest site selection of turtles: An experimental consideration of macro- and microhabitat preferences. Oikos 2003, 102, 592–600. [Google Scholar] [CrossRef]
- Lopez-Castro, M.C.; Carmona, R.; Nichols, W.J. Nesting characteristics of the olive ridley turtle (Lepidochelys olivacea) in Cabo Pulmo, southern Baja California. Mar. Biol. 2004, 145, 811–820. [Google Scholar] [CrossRef]
- Avila-Aguilar, A. Selección de sitios de anidación de Lepidochelys olivacea (Testudines: Cheloniidae) en el Pacifico Sur de Costa Rica. Rev. Biol. Trop. 2015, 63 (Suppl. 1), 375–381. [Google Scholar] [CrossRef]
- Fonseca, L.G.; Villachica, W.N.; Rangel, E.; Palola, E.; Gilbert, M.; Valverde, R.A. Reassessment of the olive ridley sea turtle Lepidochelys olivacea (Testudines: Cheloniidae) nesting population at Nancite beach, Costa Rica. Mar. Biol. 2023. submitted. [Google Scholar]
- James, R.; Melero, D. Anidación y conservación de la tortuga lora (Lepidochelys olivacea) en playa Drake, península de Osa, Costa Rica (2006 a 2012). Rev. Biol. Trop. 2015, 63, 117–129. [Google Scholar] [CrossRef]
- Tripathy, B. An assessment of solitary and arribada nesting of olive ridley sea turtles (Lepidochelys olivacea) at the Rushikulya Rookery of Orissa, India. Asiatic Herpetol. Res. 2008, 11, 136–142. [Google Scholar]
- Davis, G.E.; Whiting, M.C. Loggerhead sea turtle nesting in Everglades National Park. Florida, USA. Herpetological 1977, 33, 18–28. [Google Scholar]
- Worth, D.F.; Smith, J.B. Marine Turtle Nesting on Hutchison Island, Florida in 1973; Florida Department of Natural Resources. Marine Research Laboratory: St. Teresa, FL, USA, 1976; pp. 1–16.
- Picón Cruz, J.C.; Rodríguez Quirós, R.; Bravo Chacón, J. Diagnóstico socio ambiental en los sectores Refugio Nacional de Vida Silvestre Camaronal, Refugio Nacional de Vida Silvestre Caletas-Arío y humedales de la Cuenca Nandamojo. In Experiencias en Investigación y Manejo de Humedales Interiores y Marino-Costeros en Costa Rica; Picón Cruz, J.C., Rodríguez Quirós, R., Bravo Chacón, J., Eds.; Universidad Nacional Sede Regional Chorotega (SRCH) Centro Mesoamericano de Desarrollo Sostenible del Trópico Seco (CEMEDE): Liberia, Costa Rica, 2014; pp. 36–69. [Google Scholar]
- Drake, D.L.; Behm, J.E.; Hagerty, M.A.; Mayor, P.A.; Goldenberg, S.J.; Spotila, J. Marine Turtle Nesting Activity at Playa Naranjo, Santa Rosa National Park, Costa Rica, for the 1998–1999 Season. Chelonian Conserv. Biol. 2003, 4, 675–678. [Google Scholar]
- Mejías-Balsalobre, C.; Rojas-Cañizales, D.; Fusté-Mach, R.; Webster, E.G.; Arauz, D.; Naranjo, I.; Barrios-Garrido, H.; Arauz, R. Corozalito: Socio-economic and conservation impacts of a young growing arribada beach on a small Costa Rican community. In Proceedings of the Thirty-Ninth Annual Sea Turtle Symposium on Sea Turtle Biology and Conservation, Charleston, SC, USA, 2–8 February 2019. [Google Scholar]
- Boulon, R.H. Reducing Threats to Eggs and Hatchlings: In Situ Protection. In Research and Management Techniques for the Conservation of Sea Turtles; Eckert, K.L., Bjorndal, K.A., Abreu-Grobois, F.A., Donnelly, M., Eds.; IUCN/SSC Marine Turtle Specialist Group: Ross, CA, USA, 1999; pp. 169–174. [Google Scholar]
- Mortimer, J.A. Reducing Threats to Eggs and Hatchlings: Hatcheries. In Research and Management Techniques for the Conservation of Sea Turtles; Eckert, K.L., Bjorndal, K.A., Abreu-Grobois, F.A., Donnelly, M., Eds.; IUCN/SSC Marine Turtle Specialist Group: Ross, CA, USA, 1999; pp. 175–178. [Google Scholar]
- Binhammer, M.R.; Beange, M.; Arauz, R. Sand Temperature, Sex Ratios, and Nest Success in Olive Ridley Sea Turtles. Mar. Turt. Newsl. 2019, 159, 5–9. [Google Scholar]
- Mejías Balsalobre, C.; Bride, I.G. Assessing the impacts of hatcheries on green turtle hatchlings. Mar. Turtle Newsl. 2016, 151, 16–21. [Google Scholar]
- Castro, J.C. Contribución de las Tortugas Loras Solitarias (Lepidochelys olivacea Eschscholtz) en el Mantenimiento de las Poblaciones de esta Especie. Bachelor’s Thesis, Universidad de Costa Rica, San Jose, Costa Rica, 1986. [Google Scholar]
- Marquez, R.; Villanueva, A.; Peñaflores, C.; Rios, D. Situación actual y recomendaciones para el manejo de las tortugas marinas de la costa occidental Mexicana, en especial la tortuga golfina Lepidochelys olivacea. Ciencia Pesquera Ins. Nal. Pesca. Sria. Pesca México 1982, 3, 83–91. [Google Scholar]
- Pheasey, H.; Roberts, D.L.; Rojas-Cañizales, D.; Mejías-Balsalobre, C.; Griffiths, R.A.; Williams-Guillen, K. Using GPS-enabled decoy turtle eggs to track illegal trade. Curr. Biol. 2020, 30, R1066–R1068. [Google Scholar] [CrossRef] [PubMed]
- Spencer, R.J.; Van Dyke, J.U.; Thompson, M.B. The ethological trap: Functional and numerical responses of highly efficient invasive predators driving prey extinctions. Ecol. Appl. 2016, 26, 1969–1983. [Google Scholar] [CrossRef]
- Garcia, A.; Ceballos, G.; Adaya, R. Intensive beach management as an improved sea turtle conservation strategy in Mexico. Biol. Conserv. 2003, 111, 253–261. [Google Scholar] [CrossRef]


| Season | Total Nesting Events | Total Predation Events | Annual Predation Rate (%) |
|---|---|---|---|
| 2008 | 1119 | 89 | 7.95 |
| 2009 | 1782 | 162 | 9.09 |
| 2010 | 1763 | 98 | 5.56 |
| 2011 | 1512 | 150 | 9.92 |
| 2012 | 1588 | 275 | 17.32 |
| 2013 | 1772 | 453 | 25.56 |
| 2014 | 1918 | 107 | 5.58 |
| 2015 | 2923 | 445 | 15.22 |
| 2016 | 2403 | 157 | 6.53 |
| 2017 | 3396 | 160 | 4.71 |
| 2018 | 2313 | 489 | 21.14 |
| 2019 | 2656 | 500 | 18.83 |
| 2020 | 2303 | 653 | 28.35 |
| 2021 | 2700 | 712 | 26.37 |
| Total | 30,148 | 4450 | 14.80 |
| Class | Scientific Name | Common Name (% of Occurrence) |
|---|---|---|
| Mammals | Procyon spp. Nasua narica Conepatus semitriatus Eira barbara Didelphis marsupialis Canis lupus familiaris | Raccoon (55.69%) Coati (6.14%) Stripped Skunk (0.45%) Tayra (0.12%) Common Opossum (0.05%) Dog (0.89%) |
| Birds | Caragyps atratus Caracara cheriway | Black Vulture (22.77%) Caracara (0.36%) |
| Crustaceans | Coenobitidae compressus | Ecuadorian Hermit Crab (13.28%) |
| Insects | Unidentified | Maggots (0.12%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinoza-Rodríguez, N.; Rojas-Cañizales, D.; Mejías-Balsalobre, C.; Naranjo, I.; Arauz, R. Predation Rate on Olive Riley Sea Turtle (Lepidochelys olivacea) Nests with Solitary Nesting Activity from 2008 to 2021 at Corozalito, Costa Rica. Animals 2023, 13, 875. https://doi.org/10.3390/ani13050875
Espinoza-Rodríguez N, Rojas-Cañizales D, Mejías-Balsalobre C, Naranjo I, Arauz R. Predation Rate on Olive Riley Sea Turtle (Lepidochelys olivacea) Nests with Solitary Nesting Activity from 2008 to 2021 at Corozalito, Costa Rica. Animals. 2023; 13(5):875. https://doi.org/10.3390/ani13050875
Chicago/Turabian StyleEspinoza-Rodríguez, Nínive, Daniela Rojas-Cañizales, Carmen Mejías-Balsalobre, Isabel Naranjo, and Randall Arauz. 2023. "Predation Rate on Olive Riley Sea Turtle (Lepidochelys olivacea) Nests with Solitary Nesting Activity from 2008 to 2021 at Corozalito, Costa Rica" Animals 13, no. 5: 875. https://doi.org/10.3390/ani13050875
APA StyleEspinoza-Rodríguez, N., Rojas-Cañizales, D., Mejías-Balsalobre, C., Naranjo, I., & Arauz, R. (2023). Predation Rate on Olive Riley Sea Turtle (Lepidochelys olivacea) Nests with Solitary Nesting Activity from 2008 to 2021 at Corozalito, Costa Rica. Animals, 13(5), 875. https://doi.org/10.3390/ani13050875






