Luteal Function, Biometrics, and Echotextural Attributes in Santa Inês Ewes Superovulated with Different Total Doses of Porcine Follicle-Stimulating Hormone
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Animals
2.2. Experimental Design
2.3. Ultrasound and Videolaparoscopic Evaluations
2.4. Computerized Analysis of Ultrasound CL Images
2.5. Blood Collection and Measurements of Serum P4 Concentrations
2.6. Embryo Recovery
2.7. Statistical Analysis
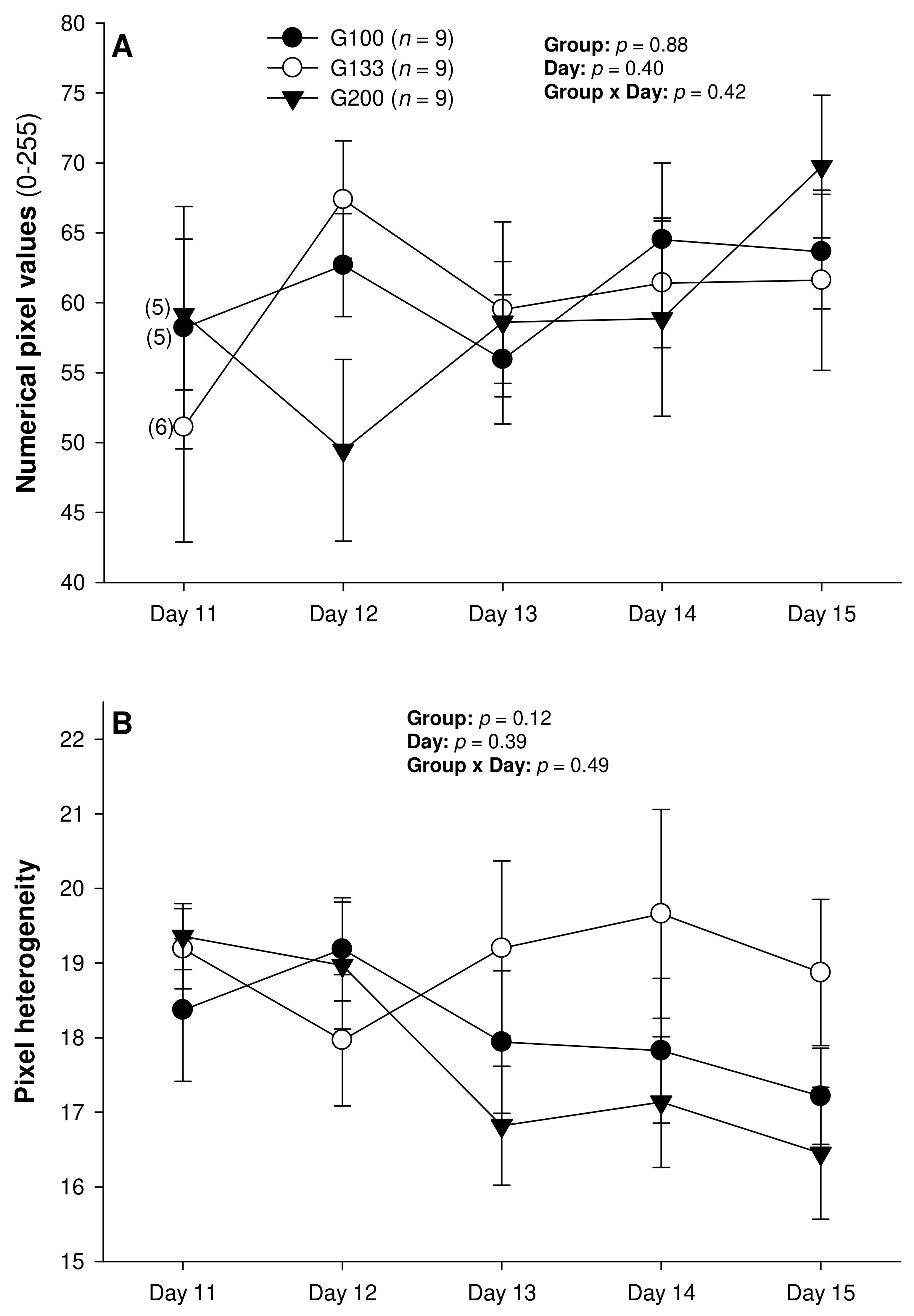
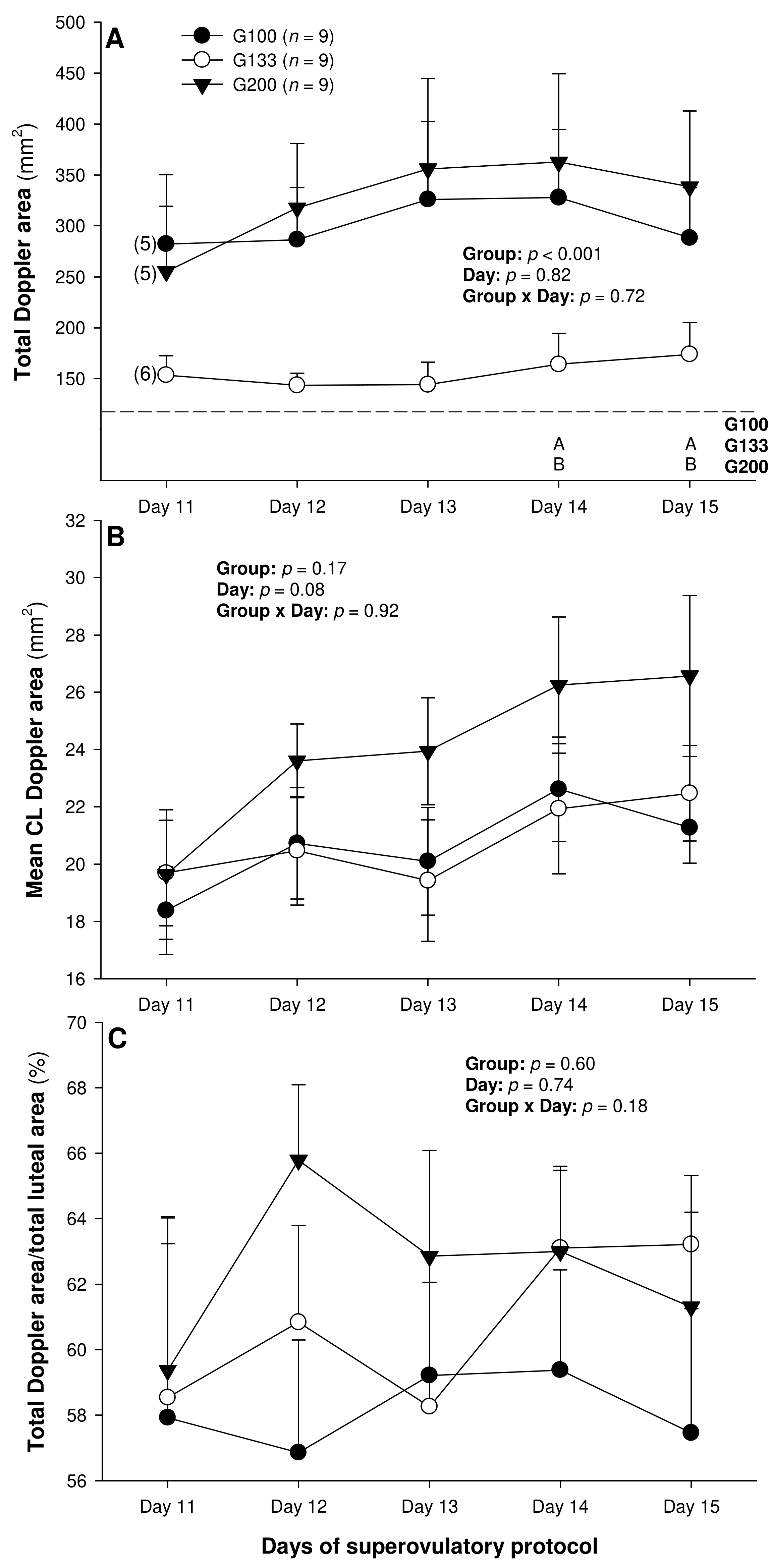
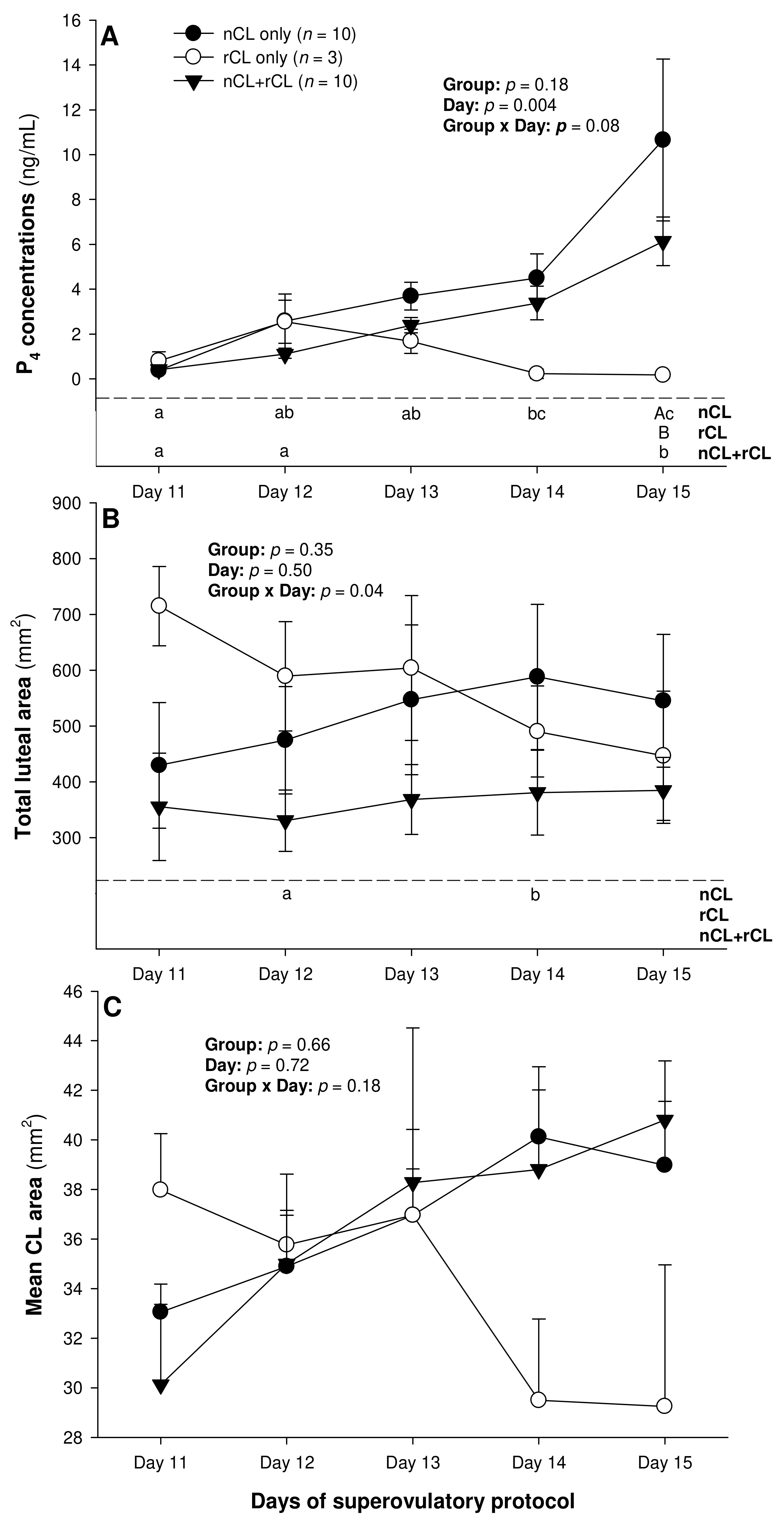
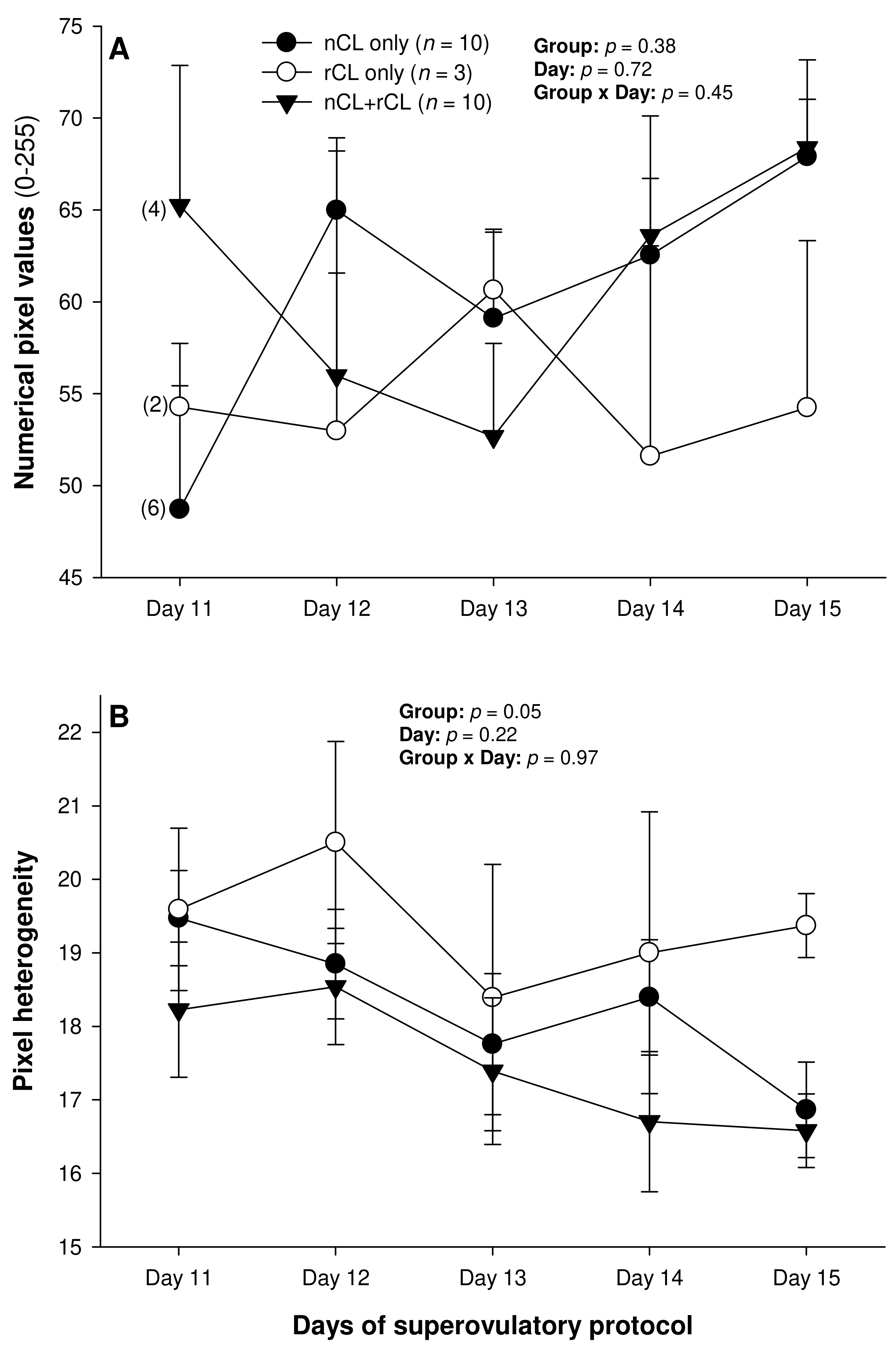
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartlewski, P.M.; Seaton, P.; Oliveira, M.E.F.; Kridli, R.T.; Murawski, M.; Schwarz, T. Intrinsic determinants and predictors of superovulatory yields in sheep: Circulating concentrations of reproductive hormones, ovarian status and antral follicular blood flow. Theriogenology 2016, 86, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Maciel, G.S.; Rodriguez, M.G.K.; Santos, V.J.C.; Uscategui, R.A.R.; Nociti, R.P.; Maronezi, M.C.; Oliveira, C.S.; Feliciano, M.A.R.; Vicente, W.R.R.; Fonseca, J.F.; et al. Follicular dynamics and in vivo embryo production in Santa Inês ewes treated with smaller doses of pFSH. Anim. Reprod. Sci. 2019, 209, 106137. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.E.F.; Figueira, L.M.; Souza-Fabjan, J.M.G.; Fonseca, J.F.; Bartlewski, P.M. New approaches to superovulation in goats and sheep. Rev. Bras. Reprod. Anim. 2021, 45, 229–235. [Google Scholar] [CrossRef]
- Oliveira, M.E.F.; Feliciano, M.A.R.; D’Amatoa, C.C.; Oliveira, L.G.; Sony, D.; Bicudo, S.D.; Fonseca, J.F.; Vicente, W.R.R.; Visco, E.; Bartlewski, P.M. Correlations between ovarian follicular blood flow and superovulatory responses in ewes. Anim. Reprod. Sci. 2014, 144, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.P.; Matteri, R.L.; Kastelic, J.P. Association between surges of follicle stimulating hormone and the emergence of follicular waves in heifers. J. Reprod. Infertil. 1992, 94, 177–188. [Google Scholar] [CrossRef]
- Fortune, J.E. Ovarian follicular growth and development in mammals. Biol. Reprod. 1994, 50, 225–232. [Google Scholar] [CrossRef]
- Ginther, O.J.; Beg, M.A.; Bergfelt, D.R.; Donadeu, F.X.; Knot, K. Follicle selection in monovular species. Biol. Reprod. 2001, 65, 638–647. [Google Scholar] [CrossRef]
- Christenson, L.K.; Stouffer, R.L. Follicle-stimulating hormone and luteinizing hormone/chorionic gonadotropin stimulation of vascular endothelial growth factor production by macaque granulosa cells from pre- and periovulatory follicles. J. Clin. Endocrinol. Metab. 1997, 82, 2135–2142. [Google Scholar] [CrossRef]
- Laitinen, M.; Ristimäki, A.; Honkasalo, M.; Narko, K.; Paavonen, K.; Ritvos, O. Differential hormonal regulation of vascular endothelial growth factors VEGF, BEGF-B, and VEGF-C messenger ribonucleic acid levels in cultured human granulosa-luteal cells. Endocrinology 1997, 138, 4748–4756. [Google Scholar] [CrossRef]
- Sanchez-Dávila, F.; Ledezma-Torres, R.A.; Padilla-Rivas, G.; del Bosque-González, A.S.; Gonzaléz Gomez, A.; Bernal-Barragán, H. Effect of three pFSH doses on superovulation and embryo quality in goats during two breeding seasons in north-eastern Mexico. Reprod. Domest. Anim. 2014, 49, 40–43. [Google Scholar] [CrossRef]
- Loiola Filho, J.B.; Monte, A.P.O.D.; Souza, T.T.D.S.; Miranda, M.D.S.; Magalhães, L.C.; Barros, C.H.S.C.; Silva, A.A.A.; Santos, A.O.; Guimarães, A.S.L.; Costa, J.M.S.; et al. Effect of pFSH dose reduction on in vivo embryo production in Dorper ewes. Semin. Ciências Agrárias 2015, 36, 4215–4224. [Google Scholar] [CrossRef]
- Oliveira, M.E.F.; Cordeiro, M.F.; Ferreira, R.M.; Souza, S.F.; Pieroni, J.S.P.; Rodrigues, L.F.S.; Fonseca, J.F.; Vicente, W.R.R. Does supplemental LH changes rate and time to ovulation and embryo yield in Santa Inês ewes treated for superovulation with FSH plus eCG. Ciência Rural. 2012, 42, 1077–1082. [Google Scholar] [CrossRef]
- Rodriguez, M.G.K.; Maciel, G.S.; Uscategui, R.A.R.; Santos, V.J.C.; Nociti, R.P.; Da Silva, P.A.; Feliciano, M.A.R.; Brandão, F.Z.; Fonseca, J.F.; Oliveira, M.E.F. Early luteal development in Santa Inês ewes superovulated with reduced doses of porcine follicle-stimulating hormone. Reprod. Domest. Anim. 2019, 54, 456–463. [Google Scholar] [CrossRef]
- Okada, A.; Kamada, S.; Jeon, C.W.; Miyamoto, A.; Fukui, Y. Incidence of abnormal corpus luteum in superovulated ewes. J. Reprod. Dev. 2000, 46, 397–402. [Google Scholar] [CrossRef]
- Bevers, M.M.; Dieleman, S.J.; Blankestein, D.M.; Van Tol, H.T.M.; Broek, J. Changes in pulsatile secretion patterns of LH, FSH, progesterone, androstenedione and oestradiol in cows after superovulation with PMSG. J. Reprod. Infertil. 1989, 87, 745–754. [Google Scholar] [CrossRef]
- Oliveira, M.E.F.; Fonseca, J.F.; Pieroni, J.S.P.; Ferreira, R.M.; Cordeiro, M.F.; Souza, S.F.; Teixeira, P.P.M.; Vicente, W.R.R. Occurrence of subnormal corpus luteum in superovulated Santa Inês sheep using protocols with or without LH administrated at the end of the FSH treatment. Anim. Reprod. 2009, 6, 231. [Google Scholar]
- Oliveira, M.E.F.; Bartlewski, P.M.; Feliciano, M.A.R. Controle do ciclo estral. In Biotécnicas Reprodutivas em Ovinos e Caprinos, 1st ed.; MedVet: São Paulo, Brazil, 2013; pp. 71–89. [Google Scholar]
- Rodriguez, M.G.K.; Campanholi, S.P.; Maciel, G.S.; Oliveira, M.E.F. Regressão luteal prematura em pequenos ruminantes. Rev. Bras. Reprod. Anim. 2015, 39, 270–276. [Google Scholar]
- Cervantes, M.J.; Juaréz, M.L.; Mejía, V.O.; Berruecos, V.J.M.; Vera, A.H.; Valencia, J. Use of fluorogestone acetate after breeding to reduce the effect of premature luteal regression in dairy goats when superovulation is induced with FSH. Anim. Reprod. Sci. 2007, 97, 47–54. [Google Scholar] [CrossRef]
- Connell, A.R.O.; Hurst, P.R.; Davis, G.H.; McNatty, K.P.; Taylor, S.L.; Juengel, J.L. An earlier rise in systemic progesterone and increased progesterone in the uterine vein during early pregnancy are associated with enhanced embryonic survival in the ewe. Theriogenology 2013, 80, 269–274. [Google Scholar] [CrossRef]
- Kraisoon, A.; Redmer, D.A.; Bass, C.S.; Navanukraw, C.; Dorsam, S.T.; Valkov, R.A.; Grazul-Bilska, A.T. Corpora lutea in superovulated ewes fed different planes of nutrition. Domest. Anim. Endoc. 2018, 62, 16–23. [Google Scholar] [CrossRef]
- Bartlewski, P.M.; Beard, A.P.; Rawlings, N.C. An ultrasonographic study of luteal function in breeds of sheep with different ovulation rates. Theriogenology 1999, 52, 115–130. [Google Scholar] [CrossRef]
- Gonzalez de Bulnes, A.; Santiago Moreno, J.; Gomez Brunet, A.; Lopez Sebastian, A. Relationship between ultrasonographic assessment of the corpus luteum and plasma progesterone concentration during the oestrous cycle in monovular ewes. Reprod. Domest. Anim. 2000, 35, 65–68. [Google Scholar] [CrossRef]
- Figueira, L.M.; Fonseca, J.F.; Arashiro, E.K.N.; Souza-Fabjan, J.M.G.; Ribeiro, A.C.S.; Oba, E.; Viana, J.H.M.; Brandão, F.Z. Colour Doppler Ultrasonography as a Tool to Assess Luteal Function in Santa Inês Ewes. Reprod. Domest. Anim. 2015, 50, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, L.G.B.; Viana, J.H.M.; Souza, E.D.; Camargo, L.S.A.; Fonseca, J.F.; Fernandes, C.A.C.; Torres, C.A.A. Use of computer-assisted ultrasound image analysis in embryo recipient selection. Reprod. Fertil. Dev. 2007, 19, 323–324. [Google Scholar] [CrossRef]
- Siqueira, L.G.B.; Torres, C.A.A.; Amorim, L.S.; Souza, E.D.; Camargo, L.S.A.; Fernandes, C.A.C.; Viana, J.H.M. Interrelationships among morphology, echotexture, and function of the bovine corpus luteum during the estrous cycle. Anim. Reprod. Sci. 2009, 115, 18–28. [Google Scholar] [CrossRef]
- Bartlewski, P.M.; Sohal, J.; Paravinja, V.; Baby, T.; Oliveira, M.E.F.; Murawski, M.; Schwarz, T.; Zieba, D.A.; Keisler, D.H. Is progesterone the key regulatory factor behind ovulation rate in sheep? Domest. Anim. Endocrinol. 2017, 58, 30–38. [Google Scholar] [CrossRef]
- Rubianes, E.; Ungerfeld, R.; Ibarra, D. Serum anti-eCG improves luteal function and increases ova/embryos recovery in eCG-superovulated ewes. Small Ruminant. Res. 1996, 21, 105–111. [Google Scholar] [CrossRef]
- Gusmão, A.L.; Biscarde, C.E.A.; Kiya, C.K. Superovulação e transferência de embriões em ovelhas. Rev. Bras. Reprod. Anim. 2013, 37, 226–231. [Google Scholar]
- Viana, J.H.M.; Siqueira, L.G.B.; Diniz, E.S.; Camargo, L.S.; Oliveira, E.R.; Fonseca, J.F.; Fernandes, C.A.C. Definição de área mínima representativa para análise de imagens ultrassonográficas de corpos lúteos bovinos. Acta Sci. Vet. 2006, 34, 578. [Google Scholar]
- Brasil, O.O.; Moreira, N.H.; Santos Júnior, G.; Silva, B.D.M.; Mariante, A.S.; Ramos, A.F. Superovulatory and embryo yielding in sheep using increased exposure time to progesterone associated with a GnRH agonist. Small Rumin. Res. 2016, 136, 54–58. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Q.; Ma, J.; Yi, X.; Zhu, Y.; Xi, X.; Feng, Y.; Jin, Z. Reactive oxygen species regulate FSH-induced expression of vascular endothelial growth factor via Nrf2 and HIF1α signaling in human epithelial ovarian cancer. Oncol. Rep. 2013, 50, 1429–1434. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Grazul-Bilska, A.T.; Redmer, D.A. Angiogenesis in the corpus luteum. Endocrine 2000, 12, 1–9. [Google Scholar] [CrossRef]
- Balaro, M.F.A.; Santos, A.S.; Moura, L.F.G.M.; Fonseca, J.F.; Brandão, F.Z. Luteal dynamic and functionality assessment in dairy goats by luteal blood flow, luteal biometry, and hormonal assay. Theriogenology 2017, 95, 118–126. [Google Scholar] [CrossRef]
- Smith, M.F.; McIntush, E.W.; Smith, G.W. Mechanism associated with corpus luteum development. J. Anim. Sci. 1994, 72, 857–872. [Google Scholar] [CrossRef]
- Sangha, G.K.; Sharma, R.K.; Guraya, S.S. Biology of corpus luteum in small ruminants. Small Rumin. Res. 2002, 43, 53–64. [Google Scholar] [CrossRef]
- Hazzard, T.M.; Stouffer, R.L. Angiogenesis in ovarian follicular and luteal development. Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 883–900. [Google Scholar] [CrossRef]
- Vrisman, D.P.; Bastos, N.M.; Rossi, G.F.; Rodrigues, N.N.; Borges, L.P.B.; Taira, A.R.; De Paz, C.C.; Nogueira, G.P.; Teixeira, P.P.; Monteiro, F.M.; et al. Corpus luteum dynamics after ovulation induction with or without previous exposure to progesterone in prepubertal Nellore heifers. Theriogenology 2018, 106, 60–68. [Google Scholar] [CrossRef]
- Davies, K.L.; Bartlewski, P.M.; Pierson, R.A.; Rawlings, N.C. Computer-assisted image analyses of corpora lutea in relation to peripheral concentrations of progesterone: A comparison between breeds of sheep with different ovulation rates. Anim. Reprod. Sci. 2006, 96, 165–175. [Google Scholar] [CrossRef]
- Simões, J.; Almeida, J.C.; Baril, G.; Azevedo, J.; Fontes, P.; Mascarenhas, R. Assessment of luteal function by ultrasonographic appearance and measurement of corpora lutea in goats. Anim. Reprod. Sci. 2007, 97, 36–46. [Google Scholar] [CrossRef]
- Scully, S.; Evans, A.C.O.; Carter, F.; Duffy, P.; Lonergan, P.; Crowe, M.A. Ultrasound monitoring of blood flow and echotexture of the corpus luteum and uterus during early pregnancy of beef heifers. Theriogenology 2015, 83, 449–458. [Google Scholar] [CrossRef]
- Duggavathi, R.; Bartlewski, P.M.; Pierson, R.A.; Rawlings, N.C. Luteogenesis in Cyclic Ewes: Echotextural, Histological, and Functional Correlates. Biol. Reprod. 2003, 69, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Stubbings, R.B.; Bosu, W.T.R.; Baker, C.A.V.; King, G.J. Serum progesterone concentration associated with superovulation and premature corpus luteum failure in dairy goats. Can. J. Vet. Res. 1986, 50, 369–373. [Google Scholar] [PubMed]
- Sá Filho, O.G.; Vasconcelos, J.L.M. Regressão prematura do corpo lúteo em bovinos. Rev. Vet. Zootec. 2008, 15, 220–233. [Google Scholar]
- Bartlewski, P.M.; Beard, A.P.; Chapman, C.L.; Nelson, M.L.; Palmer, B.; Aravindakshan, J.; Cook, S.J.; Rawlings, N.C. Ovarian responses in gonadotrophin-releasing hormone-treated anoestrous ewes: Follicular and endocrine correlates with luteal outcome. Reprod. Fertil. Dev. 2011, 13, 133–142. [Google Scholar] [CrossRef]
- Oliveira, M.E.F.; Ribeiro, I.F.; Rodriguez, M.G.K.; Maciel, G.S.; Fonseca, J.F.; Brandão, F.Z.; Bartlewski, P.M. Assessing the usefulness of B-mode and colour Doppler sonography, and measurements of circulating progesterone concentrations for determining ovarian responses in superovulated ewes. Reprod. Domest. Anim. 2018, 53, 742–750. [Google Scholar] [CrossRef]


| Variable/Group | G100 | G133 | G200 |
|---|---|---|---|
| % of ewes with nCL only | 67% (6/9) a | 57% (4/7) | 14% (1/7) b |
| % of ewes with rCL only | 11% (1/9) | 0% (0/7) | 29% (2/7) |
| % of ewes with nCL and rCL | 22% (2/9) | 43% (3/7) | 57% (4/7) |
| Total no. of CL | 13.8 ± 1.8 | 8.7 ± 1.1 | 14.2 ± 2.4 |
| nCL | 11.1 ± 2.9 | 8.6 ± 1.3 | 8.4 ± 3.2 |
| rCL | 2.7 ± 1.7 | 1.7 ± 1.0 | 6.8 ± 3.2 |
| Input Variable (x) | Coefficient of Correlation | p Value |
|---|---|---|
| Total no. of CL | 0.18 | 0.04 |
| nCL | 0.38 | 0.00005 |
| rCL | −0.19 | 0.04 |
| Total luteal area | 0.30 | 0.001 |
| Mean luteal area | 0.41 | 0.000003 |
| Numerical pixel values | 0.15 | 0.12 |
| Pixel heterogeneity | −0.29 | 0.002 |
| Total Doppler area | 0.31 | 0.0006 |
| Mean Doppler area | 0.33 | 0.0003 |
| CL vascularization % | 0.02 | 0.86 |
| Variable/Group | nCL only (n = 10) | rCL only (n = 3) | nCL + rCL (n = 10) | p Value |
|---|---|---|---|---|
| Total no. of CL | 14.3 ± 2.2 | 16.7 ± 2.9 | 10.6 ± 1.4 | 0.21 |
| nCL | 14.3 ± 2.2 a | - | 8.1 ± 1.4 b | 0.002 |
| rCL * | - | 16.7 ± 2.9 a | 2.5 ± 0.8 b | <0.001 |
| Variable/Groups | G100 (n = 8) | G133 (n = 8) | G200 (n = 7) | p Values |
|---|---|---|---|---|
| No. of recovered structures | 6.0 ± 1.3 | 4.1 ± 1.4 | 7.1 ± 1.4 | 0.32 |
| No. of unfertilized eggs * | 2.1 ± 1.0 | 1.0 ± 0.9 | 1.0 ± 0.7 | 0.58 |
| No. of embryos | 3.9 ± 1.5 | 3.1 ± 1.3 | 6.1 ± 1.3 | 0.31 |
| No. of viable embryos (grades I–III) | 2.6 ± 1.0 | 1.5 ± 0.9 | 4.4 ± 1.3 | 0.18 |
| No. of degenerated embryos (grade IV) * | 1.2 ± 0.6 | 1.6 ± 0.7 | 1.7 ± 0.5 | 0.86 |
| Recovery rate (%) * | 48.3 ± 9.6 | 48.1 ± 14.4 | 54.9 ± 12.6 | 0.91 |
| Unfertilized eggs (%) * | 39.3 ± 16.3 | 20.0 ± 17.0 | 12.7 ± 8.8 | 0.41 |
| Viability rate (%) | 42.3 ± 13.0 | 35.8 ± 14.9 | 61.9 ± 11.8 | 0.39 |
| Degenerated embryos (%) | 18.4 ± 7.6 | 44.2 ± 15.2 | 25.3 ± 5.6 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bevilaqua, J.R.; Rodriguez, M.G.K.; Maciel, G.S.; Vergani, G.B.; Fonseca, J.F.d.; Bartlewski, P.M.; Oliveira, M.E.F. Luteal Function, Biometrics, and Echotextural Attributes in Santa Inês Ewes Superovulated with Different Total Doses of Porcine Follicle-Stimulating Hormone. Animals 2023, 13, 873. https://doi.org/10.3390/ani13050873
Bevilaqua JR, Rodriguez MGK, Maciel GS, Vergani GB, Fonseca JFd, Bartlewski PM, Oliveira MEF. Luteal Function, Biometrics, and Echotextural Attributes in Santa Inês Ewes Superovulated with Different Total Doses of Porcine Follicle-Stimulating Hormone. Animals. 2023; 13(5):873. https://doi.org/10.3390/ani13050873
Chicago/Turabian StyleBevilaqua, Júlia Ribeiro, Mariana Garcia Kako Rodriguez, Giovanna Serpa Maciel, Gabriel Brun Vergani, Jeferson Ferreira da Fonseca, Pawel Mieczyslaw Bartlewski, and Maria Emilia Franco Oliveira. 2023. "Luteal Function, Biometrics, and Echotextural Attributes in Santa Inês Ewes Superovulated with Different Total Doses of Porcine Follicle-Stimulating Hormone" Animals 13, no. 5: 873. https://doi.org/10.3390/ani13050873
APA StyleBevilaqua, J. R., Rodriguez, M. G. K., Maciel, G. S., Vergani, G. B., Fonseca, J. F. d., Bartlewski, P. M., & Oliveira, M. E. F. (2023). Luteal Function, Biometrics, and Echotextural Attributes in Santa Inês Ewes Superovulated with Different Total Doses of Porcine Follicle-Stimulating Hormone. Animals, 13(5), 873. https://doi.org/10.3390/ani13050873







