Effects of Water Temperature on Gonads Growth in Ambystoma mexicanum Axolotl Salamanders
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Gonads Collection
2.3. Statistical Analysis
3. Results
3.1. Females
3.2. Males
4. Discussion
4.1. Females
4.2. Males
4.3. Effects of High Rearing Temperature and Climate Warming
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Alvarez, D.; Nicieza, A.G. Effects of temperature and food quality on anuran larval growth and metamorphosis. Funct. Ecol. 2002, 16, 640–648. [Google Scholar] [CrossRef]
- Merila, J.; Laurila, A.; Laugen, A.T.; Rasanen, K.; Pahkala, M. Plasticity in age and size at metamorphosis in Rana temporaria—Comparison of high and low latitude populations. Ecography 2000, 23, 457–465. [Google Scholar] [CrossRef]
- Flament, S. Sex reversal in amphibians. Sex Dev. 2016, 10, 267–278. [Google Scholar] [CrossRef]
- Ruthsatz, K.; Peck, M.A.; Dausmann, K.H.; Sabatino, N.M.; Glos, J. Patterns of temperature induced developmental plasticity in anuran larvae. J. Therm. Biol. 2018, 74, 123–132. [Google Scholar] [CrossRef]
- Sinai, N.; Glos, J.; Mohan, A.V.; Lyra, M.L.; Riepe, M.; Thöle, E.; Zummach, C.; Ruthsatz, K. Developmental plasticity in amphibian larvae across the world: Investigating the roles of temperature and latitude. J. Therm. Biol. 2022, 106, 103233. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; Scott, D.E.; Pechmann, H.K. Time and size at metamorphosis related to adult fitness in Ambystoma talpoideum. Ecology 1988, 69, 184–192. [Google Scholar] [CrossRef]
- Earl, J.E.; Whiteman, H.H. Are Commonly Used Fitness Predictors Accurate? A Meta-analysis of Amphibian Size and Age at Metamorphosis. Copeia 2015, 103, 297–309. [Google Scholar] [CrossRef]
- Dupre, R.K.; Petranka, J.W. Ontogeny of temperature selection in larval amphibians. Copeia 1985, 1985, 462–467. [Google Scholar] [CrossRef]
- Ruthsatz, K.; Dausmann, K.H.; Peck, M.A.; Glos, J. Thermal tolerance and acclimation capacity in the European common frog (Rana temporaria) change throughout ontogeny. J. Exper. Zool. Part A Ecol. Int. Phys. 2022, 337, 477–490. [Google Scholar] [CrossRef]
- Fontenot, C.L., Jr.; Lutterschmidt, W.I. Thermal Selection and Temperature Preference of the Aquatic Salamander, Amphiuma tridactylum. Herp. Cons. Biol. 2011, 6, 395–399. [Google Scholar]
- Hutchison, V.H.; Hill, L.G. Thermal Selection in the Hellbender, Cryptobranchus alleganiensis, and the Mudpuppy, Necturus maculosus. Herpetologica 1976, 32, 327–331. [Google Scholar]
- Moalli, R.; Meyers, R.S.; Ultsch, G.R.; Jackson, D.C. Acid-base balance and temperature in a predominantly skin-breathing salamander, Cryptobranchus alleganiensis. Resp. Phys. 1981, 43, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Terrell, K.A.; Quintero, R.P.; Galicia, V.A.; Bronikowski, E.; Evans, M.; Kleopfer, J.D.; Murray, S.; Murphy, J.B.; Nissen, B.D.; Gratwicke, B. Physiological impacts of temperature variability and climate warming in hellbenders (Cryptobranchus alleganiensis). Cons. Phys. 2021, 9, coab079. [Google Scholar] [CrossRef] [PubMed]
- Finkler, M.S. Effects of temperature, sex, and gravidity on the metabolism of small-mouthed salamanders, Ambystoma texanum, during the reproductive season. J. Herp. 2006, 40, 103–106. [Google Scholar] [CrossRef]
- Zhang, L.; Kouba, A.; Wang, Q.; Zhao, H.; Jiang, W.; Willard, S.; Zhang, H. The Effect of Water Temperature on the Growth of Captive Chinese Giant Salamanders (Andrias davidianus) Reared for Reintroduction: A Comparison with Wild Salamander Body Condition. Herpetologica 2014, 70, 369–377. [Google Scholar] [CrossRef]
- Hu, Q.; Tian, H.; Xiao, H. Effects of temperature and sex steroids on sex ratio, growth, and growth-related gene expression in the Chinese giant salamander Andrias davidianus. Aquat. Biol. 2019, 28, 79–90. [Google Scholar] [CrossRef]
- Moore, F.L. Regulation of reproductive behaviors. In Hormones and Reproduction in Fishes, Amphibians, and Reptiles; Springer: Boston, MA, USA, 1987; pp. 505–522. [Google Scholar]
- Yartsev, V.V.; Kuranova, V.N. Seasonal Dynamics of Male and Female Reproductive Systems in the Siberian Salamander, Salamandrella keyserlingii (Caudata, Hynobiidae). Asian Herp. Res. 2015, 6, 169–183. [Google Scholar] [CrossRef]
- Rastogi, R.K.; Iela, L.; di Meglio, M.; Di Fiore, M.M.; D’Aniello, B.; Pinelli, C.; Fiorentino, M. Hormonal regulation of reproductive cycles in amhibians. In Amphibian Biology; Heatwole, H., Ed.; Surrey Beatty and Sons: Chipping Norton, NSW, Australia, 2005; Volume 6, Endocrinology; pp. 2045–2177. [Google Scholar]
- Rastogi, R.K.; Pinelli, C.; Polese, G.; D’Aniello, B.; Chieffi-Baccari, G. Hormones and reproductive cycles in anuran amphibians. In Hormones and Reproduction of Vertebrates; Academic Press: Cambridge, MA, USA, 2011; pp. 171–186. [Google Scholar]
- Recuero, E.; Cruzado-Cortes, J.; Parra-Olea, G.; Zamudio, K.R. Urban aquatic habitats and conservation of highly endangered species: The case of Ambystoma mexicanum (Caudata, Ambystomatidae). Annales Zoologici. Fennici. 2010, 47, 223–239. [Google Scholar] [CrossRef]
- IUCN SSC Amphibian Specialist Group. 2020. Ambystoma mexicanum. The IUCN Red List of Threatened Species 2020: E.T1095A53947343. Available online: https://dx.doi.org/10.2305/IUCN.UK.2020-3.RLTS.T1095A53947343.en (accessed on 25 January 2023).
- Alcaraz, G.; López-Portela, X.; Robles-Mendoza, C. Response of a native endangered axolotl, Ambystoma mexicanum (Amphibia), to exotic fish predator. Hydrobiologia 2015, 753, 73–80. [Google Scholar] [CrossRef]
- Contreras, V.; Martínez-Meyer, E.; Valiente, E.; Zambrano, L. Recent decline and potential distribution in the last remnant area of the microendemic Mexican axolotl (Ambystoma mexicanum). Biol. Cons. 2009, 142, 2881–2885. [Google Scholar] [CrossRef]
- Figiel, C.R., Jr. Cryopreservation of Sperm from the Axolotl Ambystoma mexicanum: Implications for Conservation. Herp. Cons. Biol. 2013, 8, 748–755. [Google Scholar]
- Figiel, C.R., Jr. Cold Storage of Sperm from the Axolotl, Ambystoma mexicanum. Herp. Cons. Biol. 2020, 15, 367–371. [Google Scholar]
- Veith, I.A.; Figiel, C.R., Jr. Sex hormones in the Axolotl, Ambystoma mexicanum: Potential method for sex determination. Amph. Rept. Cons. 2022, 16, 71–75.e302. [Google Scholar]
- Bordzilovskaya, N.P.; Dettlaff, T.A. Table of stages of the normal development of axolotl embryos. Axolotl Newsl. 1979, 7, 2–22. [Google Scholar]
- Andreone, F.; Dore, B. Adaptation of the reproductive cycle in Triturus alpestris apuanus to an unpredictable habitat. Amph. Rept. 1992, 13, 251–261. [Google Scholar]
- Gosch, B.; Fischer, K. Seasonal changes of testis volume and sperm quality in adult fallow deer (Dama dama) and their relationship to the antler cycle. J. Reprod. Infertil. 1989, 85, 7–17. [Google Scholar] [CrossRef]
- Guarino, F.M.; Caputo, V.; Angelini, F. The reproductive cycle of the newt Triturus italicus. Amph. Rept. 1992, 13, 121–133. [Google Scholar] [CrossRef]
- Stéquert, B.; Rodriguezb, J.N.; Cuisset, B.; Le Menn, F. Gonadosomatic index and seasonal variations of plasma sex steroids in skipjack tuna (Katsuwonus pelamis) and yellowfin tuna (Thunnus albacares) from the western Indian Ocean. Aquat. Living Resour. 2001, 14, 313–318. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statist. Methods; Iowa State University Press: Ames, IA, USA, 1989; p. 503. [Google Scholar]
- Lahnsteiner, F.; Kletzl, M. The effect of water temperature on gamete maturation and gamete quality in the European grayling (Thymalus thymallus) based on experimental data and on data from wild populations. Fish Physiol. Biochem. 2012, 38, 455–467. [Google Scholar] [CrossRef]
- Dadras, H.; Dzyuba, B.; Cosson, J.; Golpour, A.; Siddique, M.A.M.; Linhart, O. Effect of water temperature on the physiology of fish spermatozoon function: A brief review Aquacul. Res. 2017, 48, 729–740. [Google Scholar] [CrossRef]
- Tsutsui, Y. Notes on the Behavior of the Japanese newt, Diemyctylus pyrrhogaster Boie; (1) Breeding habits. Mem. Coll. Sci. Kyoto. Imp. Univ. 1931, 7, 159–167. [Google Scholar]
- Werner, J.K. Temperature-photoperiod effects on spermatogenesis in the salamander Plethodon cinereus. Copeia 1969, 1969, 592–602. [Google Scholar] [CrossRef]
- Armstrong, J.B.; Duhon, S.T.; Malacinski, G.M. Raising the axolotl in captivity. In Developmental Biology of the Axolotl; Armstrong, J.B., Malacinski, G.M., Eds.; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
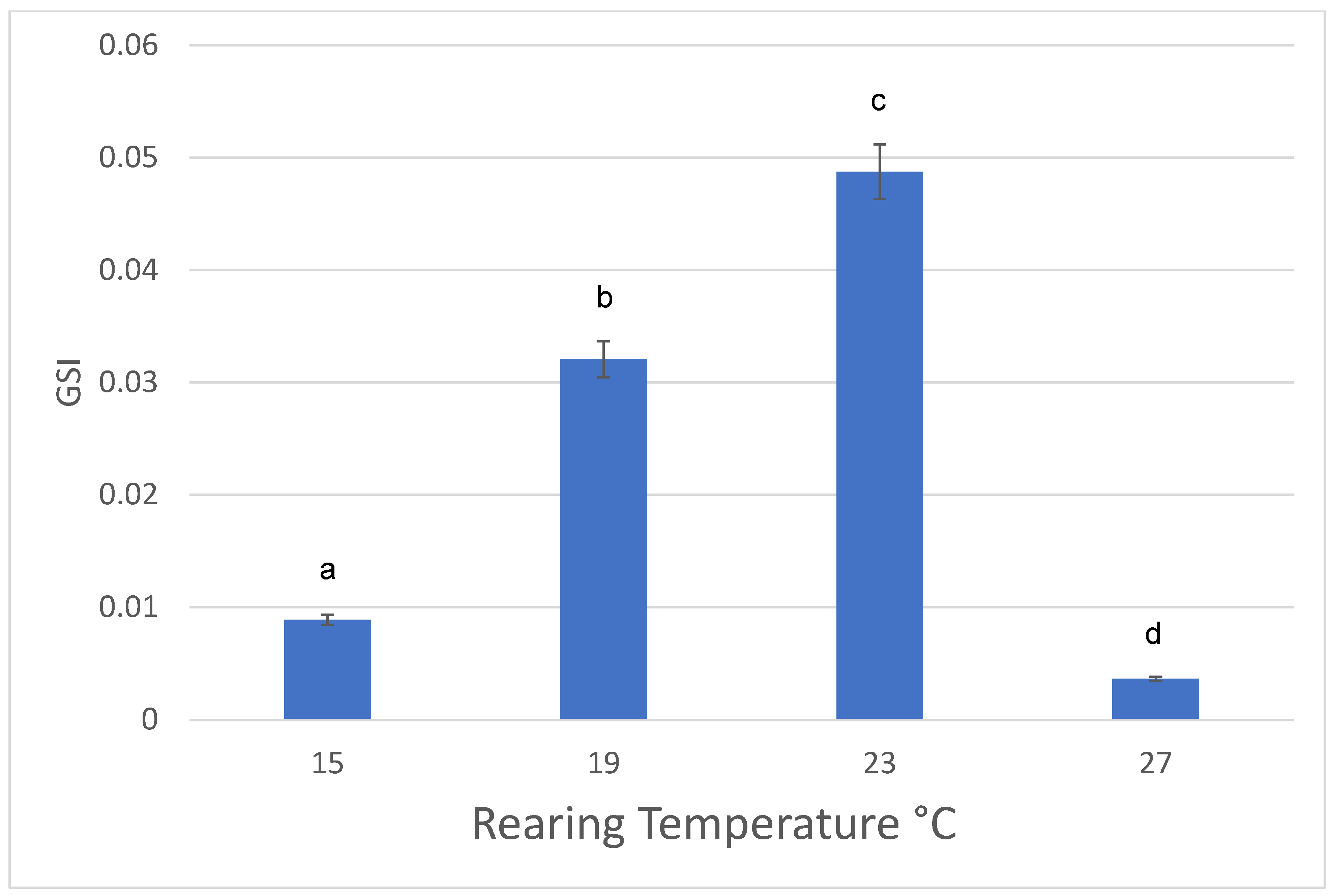
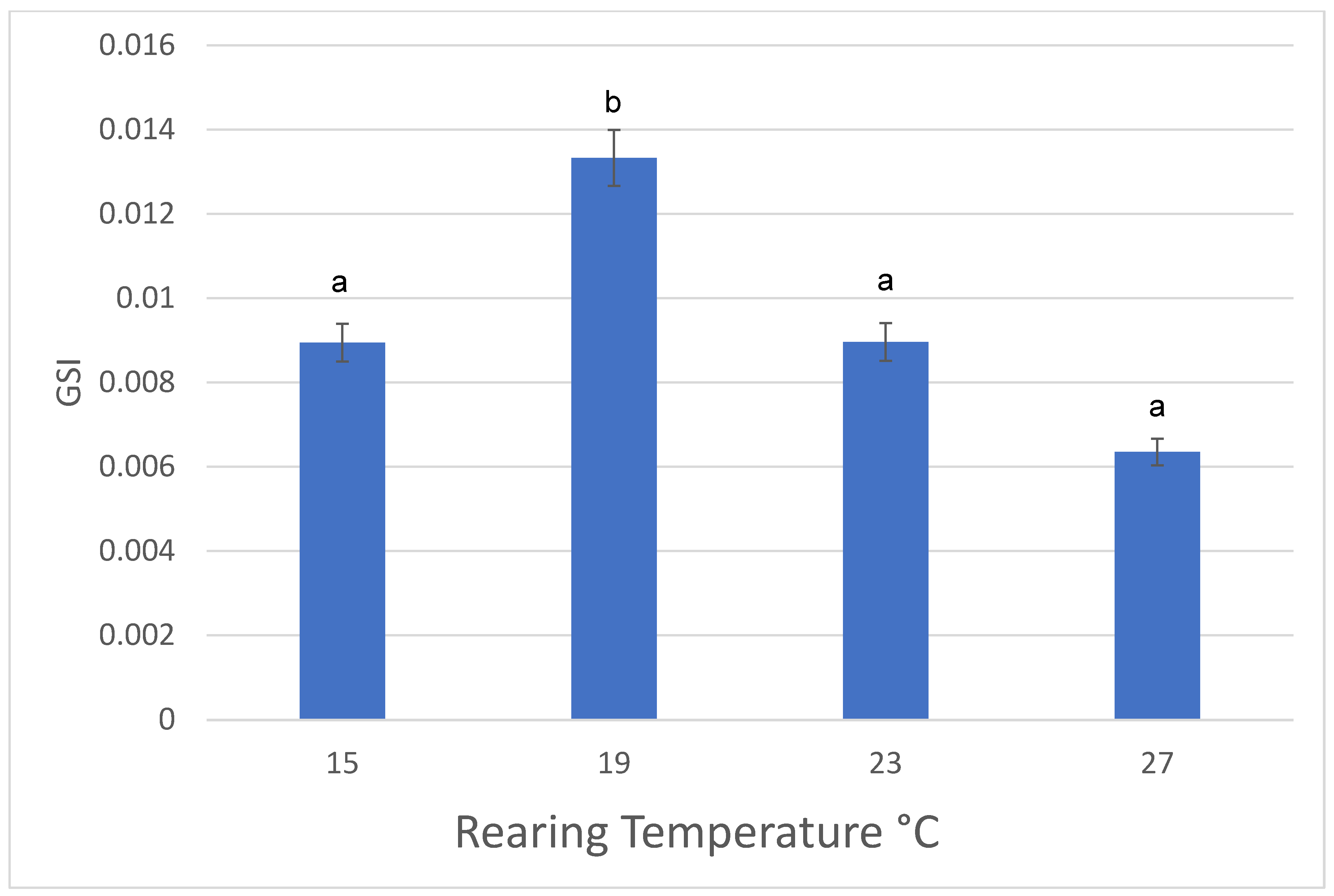
| Females | 15 °C | 19 °C | 23 °C | 27 °C |
| n | 25 | 21 | 16 | 21 |
| SVL | 110.6 ± 12.6 a | 103.8 ± 5.4 b | 114.4 ± 5.4 a | 101.6 ± 8.9 b |
| Body Mass | 95.9 ± 12.6 a | 70.6 ± 7.9 b | 93.1 ± 12.7 a | 57.0 ± 18.3 b |
| Males | 15 °C | 19 °C | 23 °C | 27 °C |
| n | 27 | 30 | 20 | 12 |
| SVL | 111.1 ± 4.1 a | 109.5 ± 5.6 a | 115.0 ± 6.1 b | 109.2 ± 8.6 a |
| Body Mass | 86.5 ± 7.8 a | 72.3 ± 12.3 a | 92.1 ± 17.8 b | 72.1 ± 13.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figiel, C.R., Jr. Effects of Water Temperature on Gonads Growth in Ambystoma mexicanum Axolotl Salamanders. Animals 2023, 13, 874. https://doi.org/10.3390/ani13050874
Figiel CR Jr. Effects of Water Temperature on Gonads Growth in Ambystoma mexicanum Axolotl Salamanders. Animals. 2023; 13(5):874. https://doi.org/10.3390/ani13050874
Chicago/Turabian StyleFigiel, Chester R., Jr. 2023. "Effects of Water Temperature on Gonads Growth in Ambystoma mexicanum Axolotl Salamanders" Animals 13, no. 5: 874. https://doi.org/10.3390/ani13050874
APA StyleFigiel, C. R., Jr. (2023). Effects of Water Temperature on Gonads Growth in Ambystoma mexicanum Axolotl Salamanders. Animals, 13(5), 874. https://doi.org/10.3390/ani13050874





