Social Reward Behaviour in Two Groups of European Grey Wolves (Canis lupus lupus)—A Case Study
Abstract
Simple Summary
Abstract
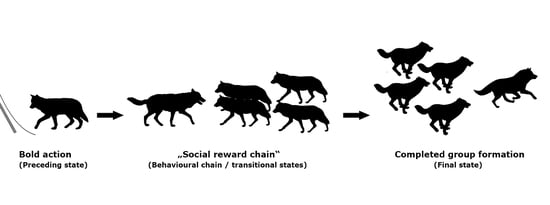
1. Introduction
2. Materials and Methods
2.1. Study Animals
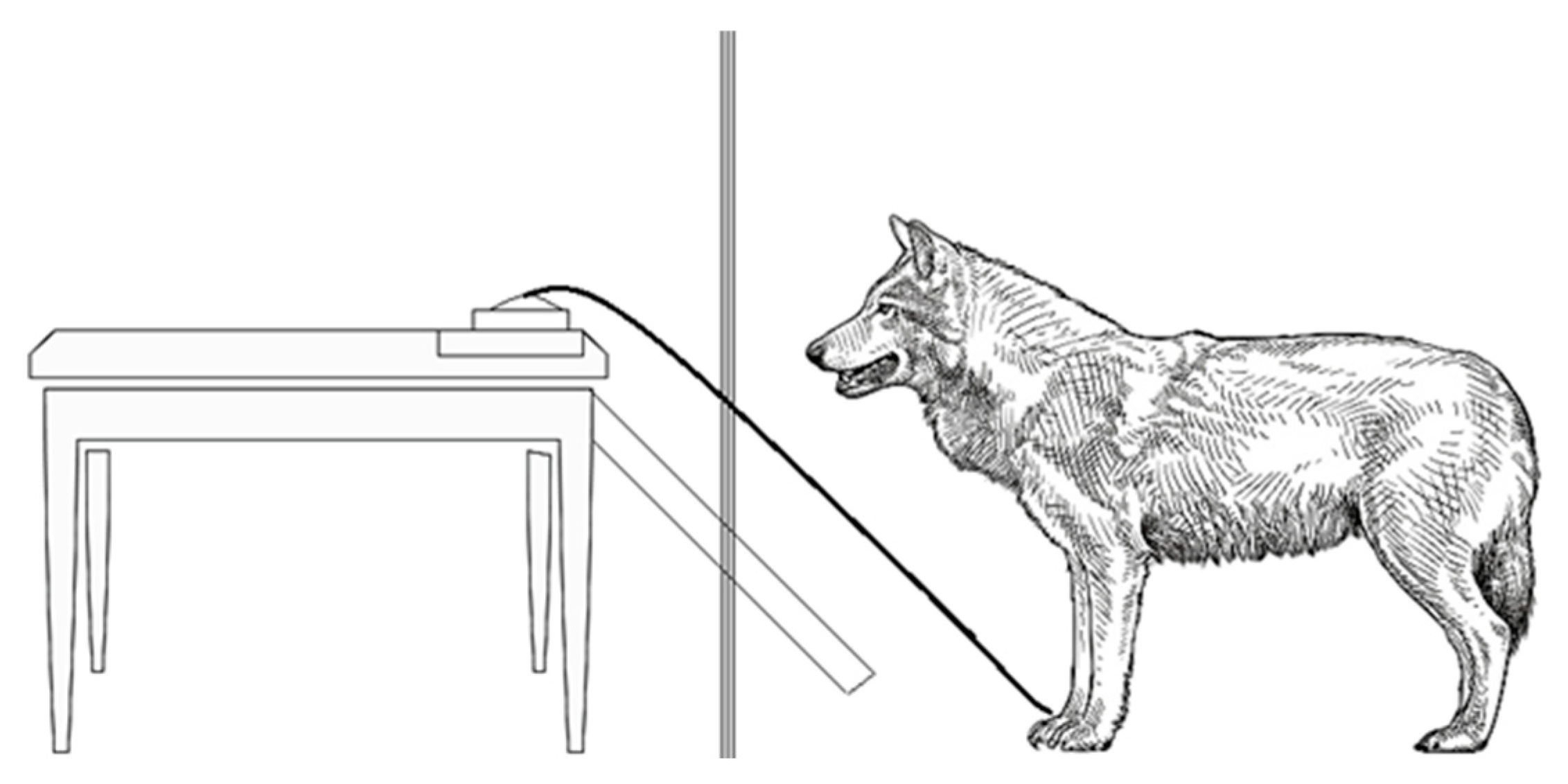
2.2. Novel Object
2.3. Observational Methodology
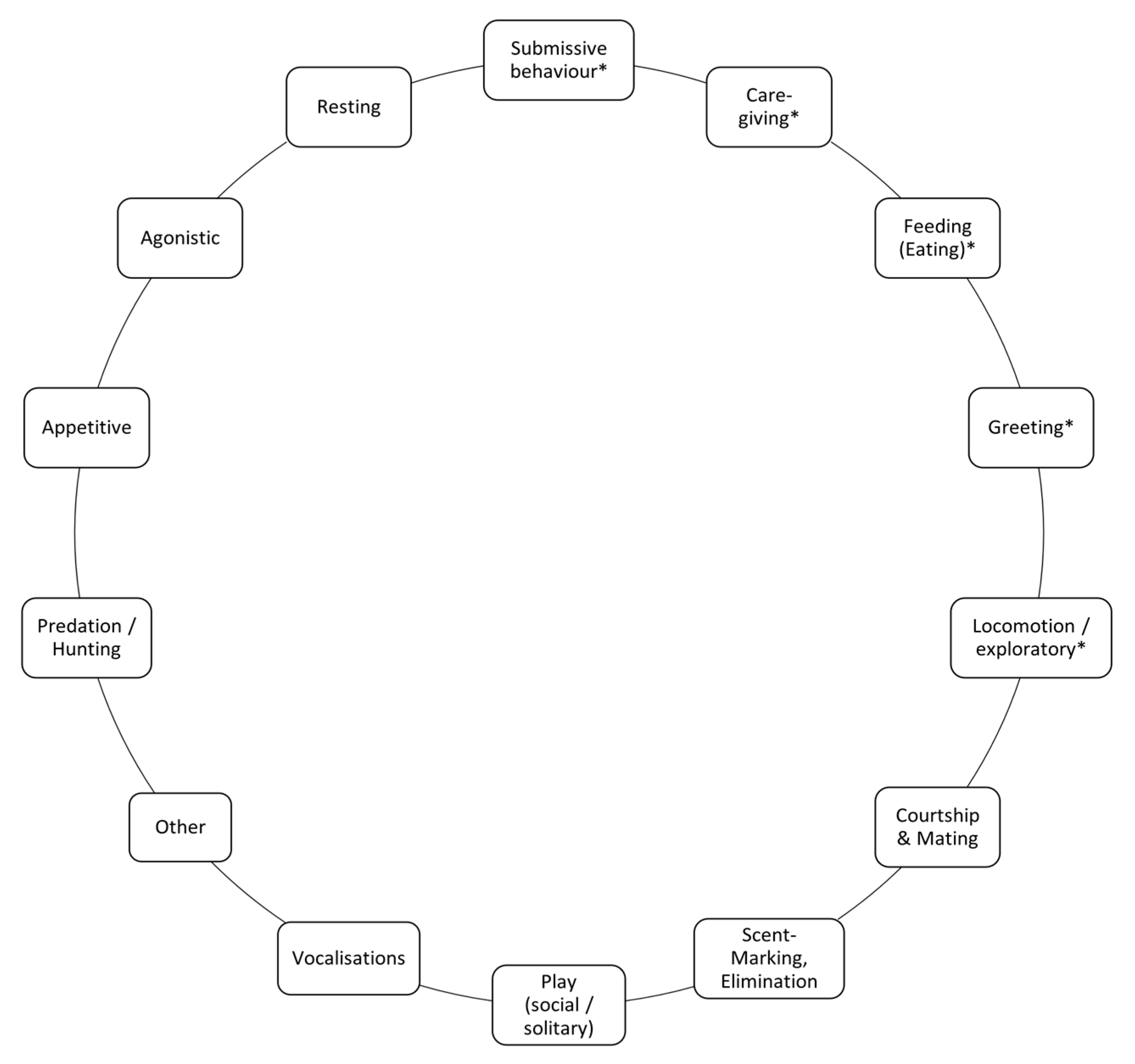
Definitions of Behaviours of Interest
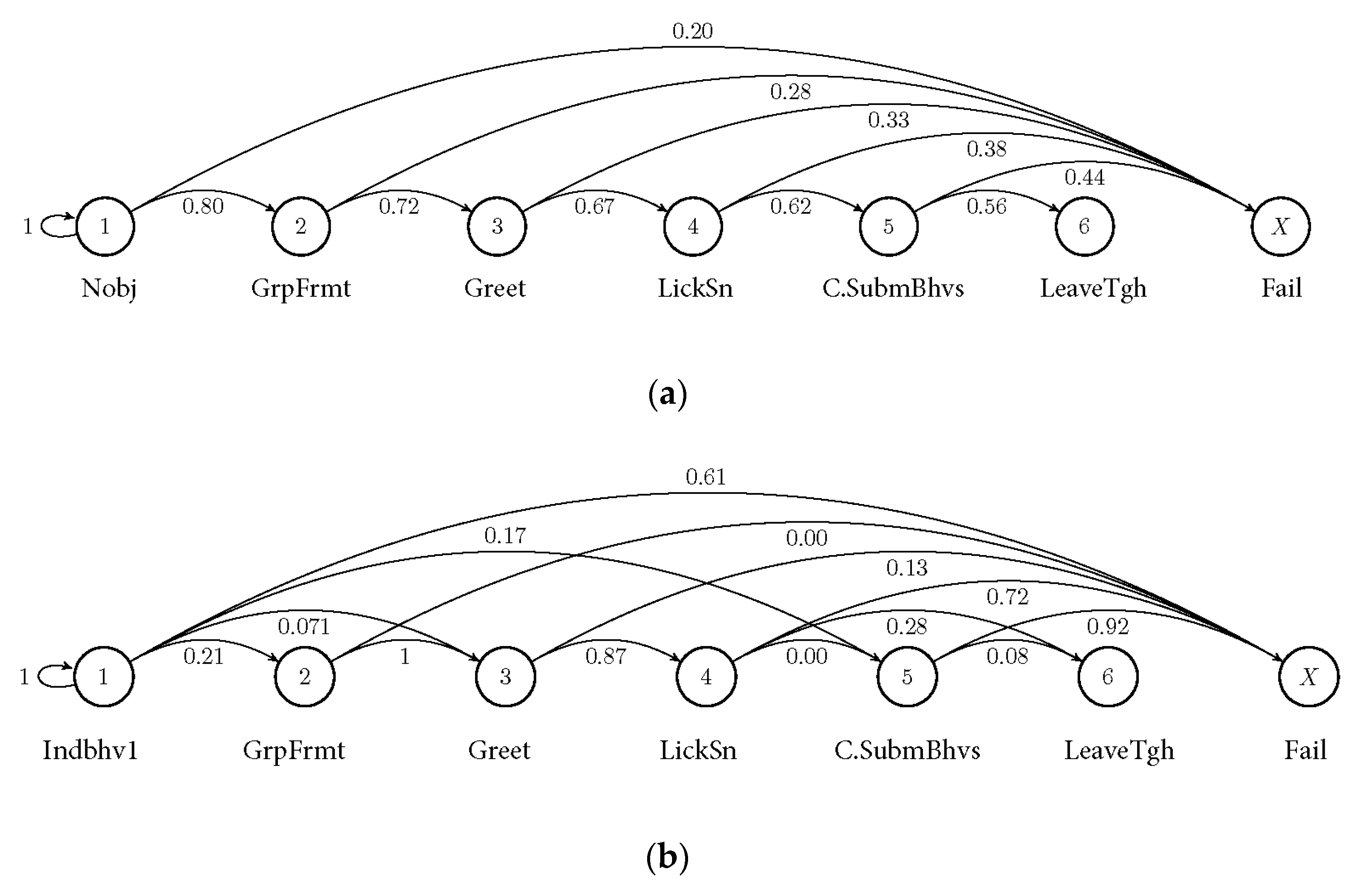
2.4. Markov Chain Modelling
2.5. Statistical Evaluation of Markov Chain Probabilities
2.6. Influence Factors on the Frequency of Novel Object Interaction
3. Results
3.1. Observations
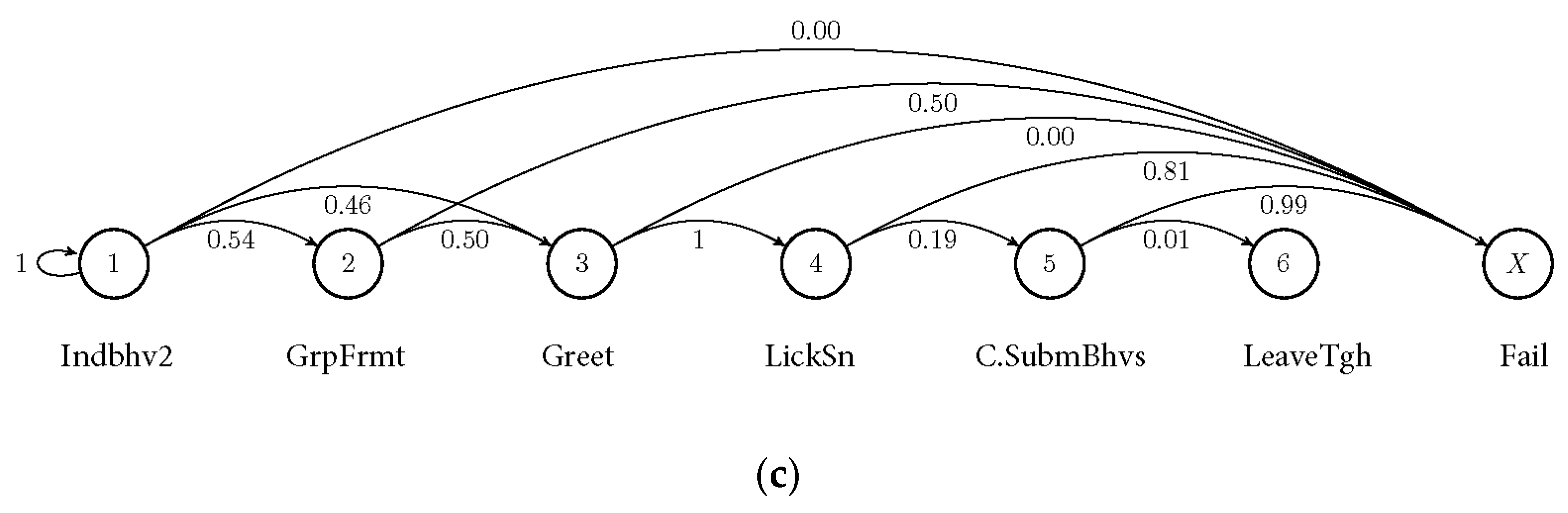
3.2. Markov Probabilities of Initial Behaviours on the Succeeding Behaviours
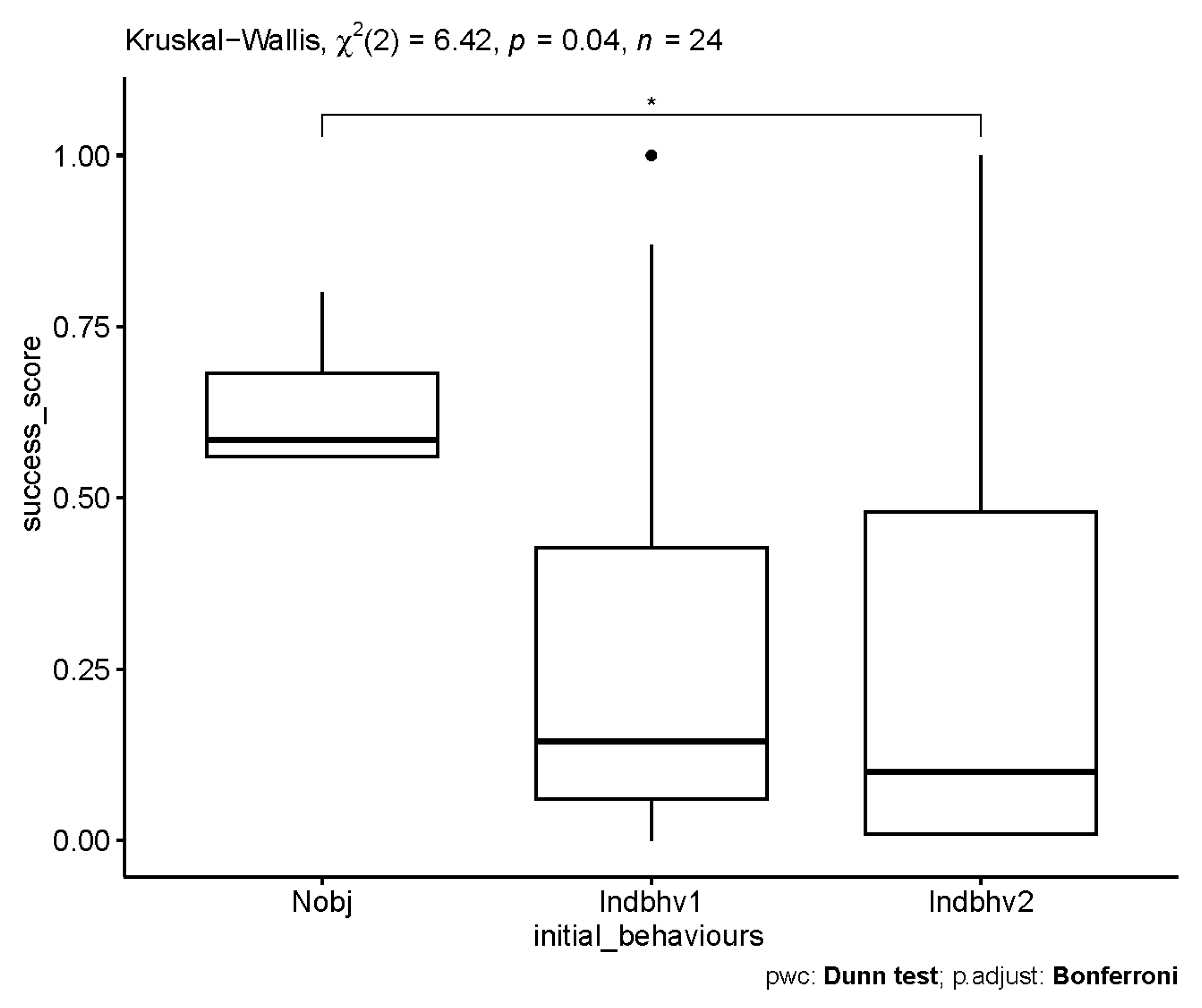
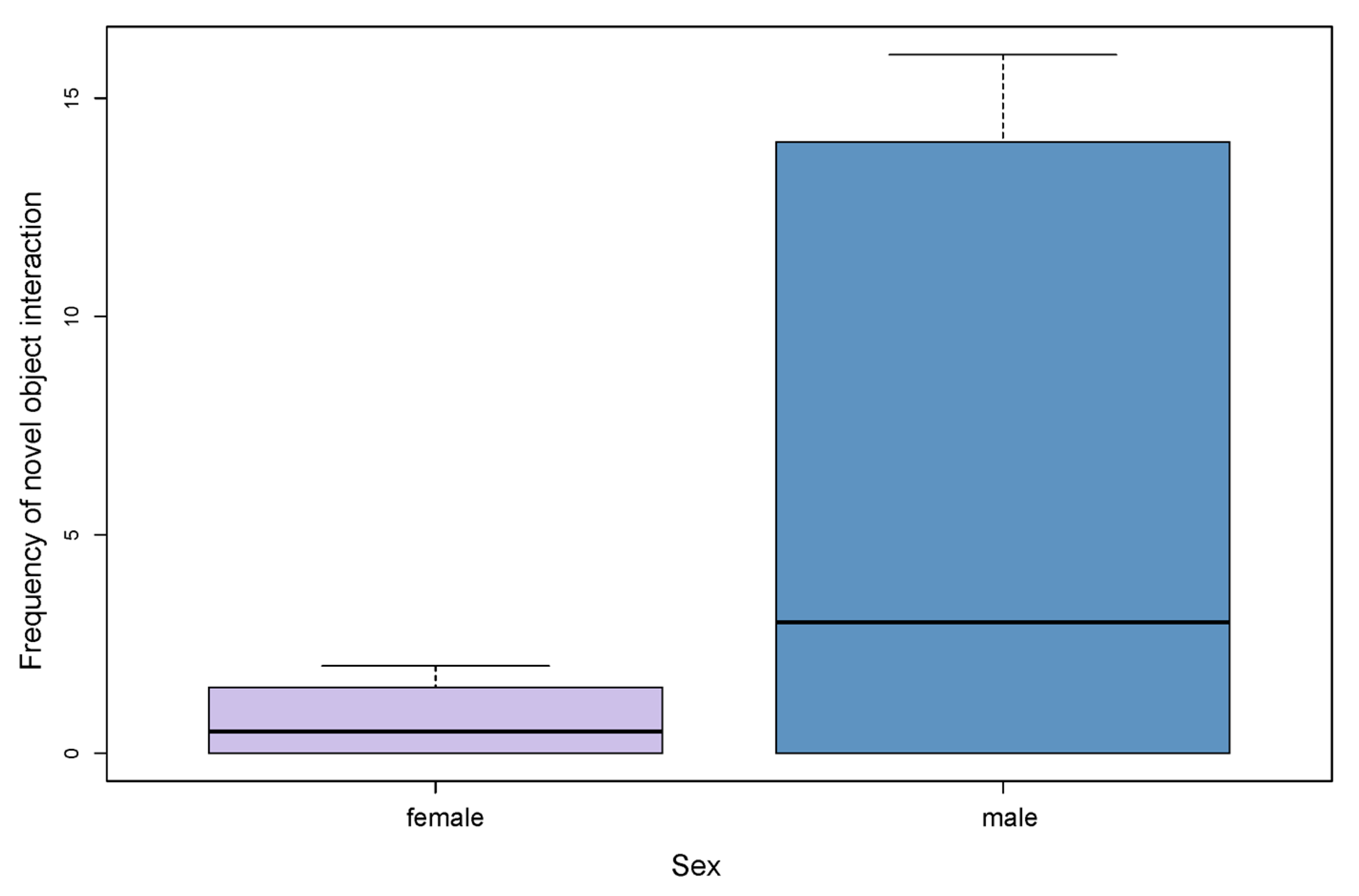
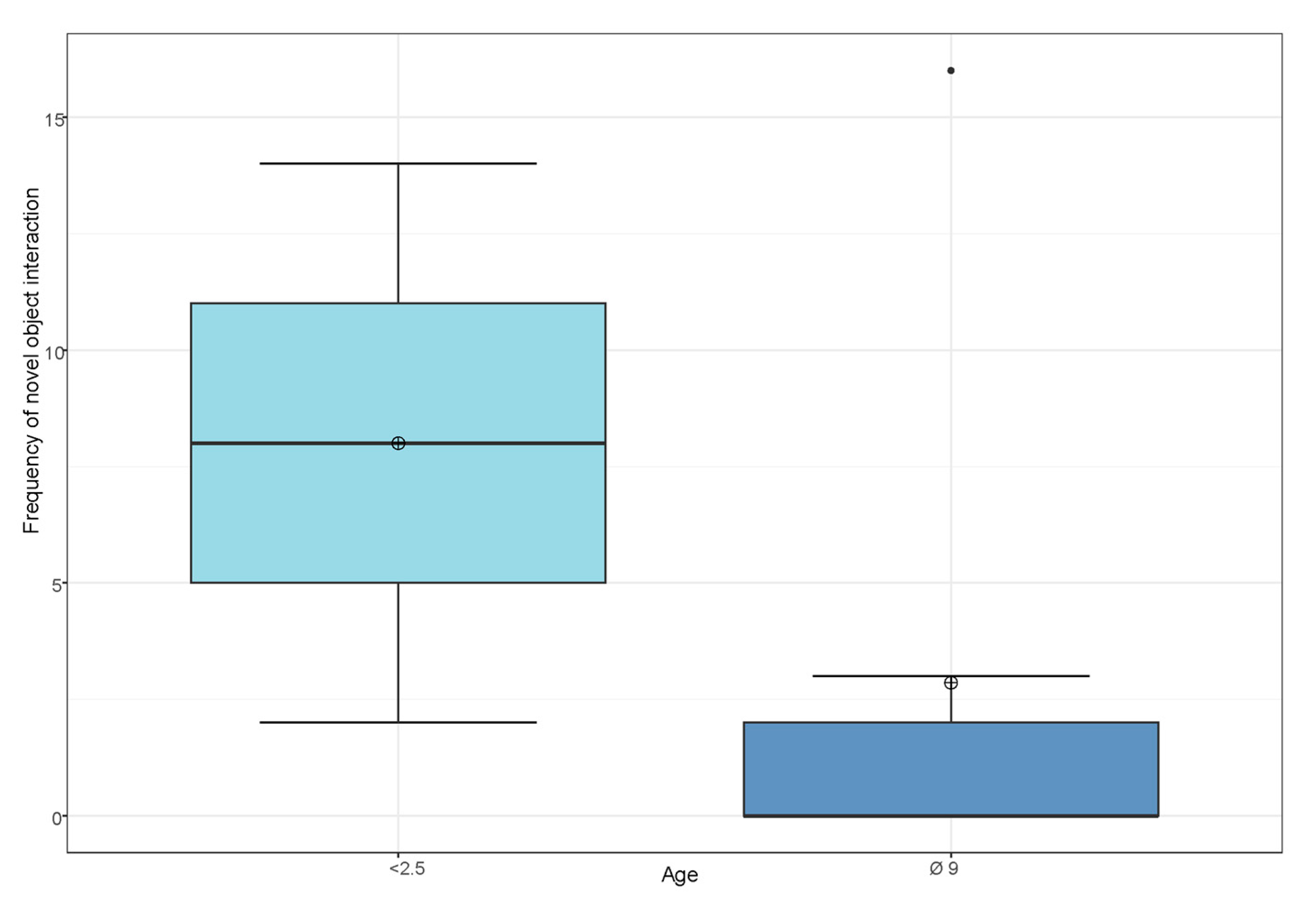
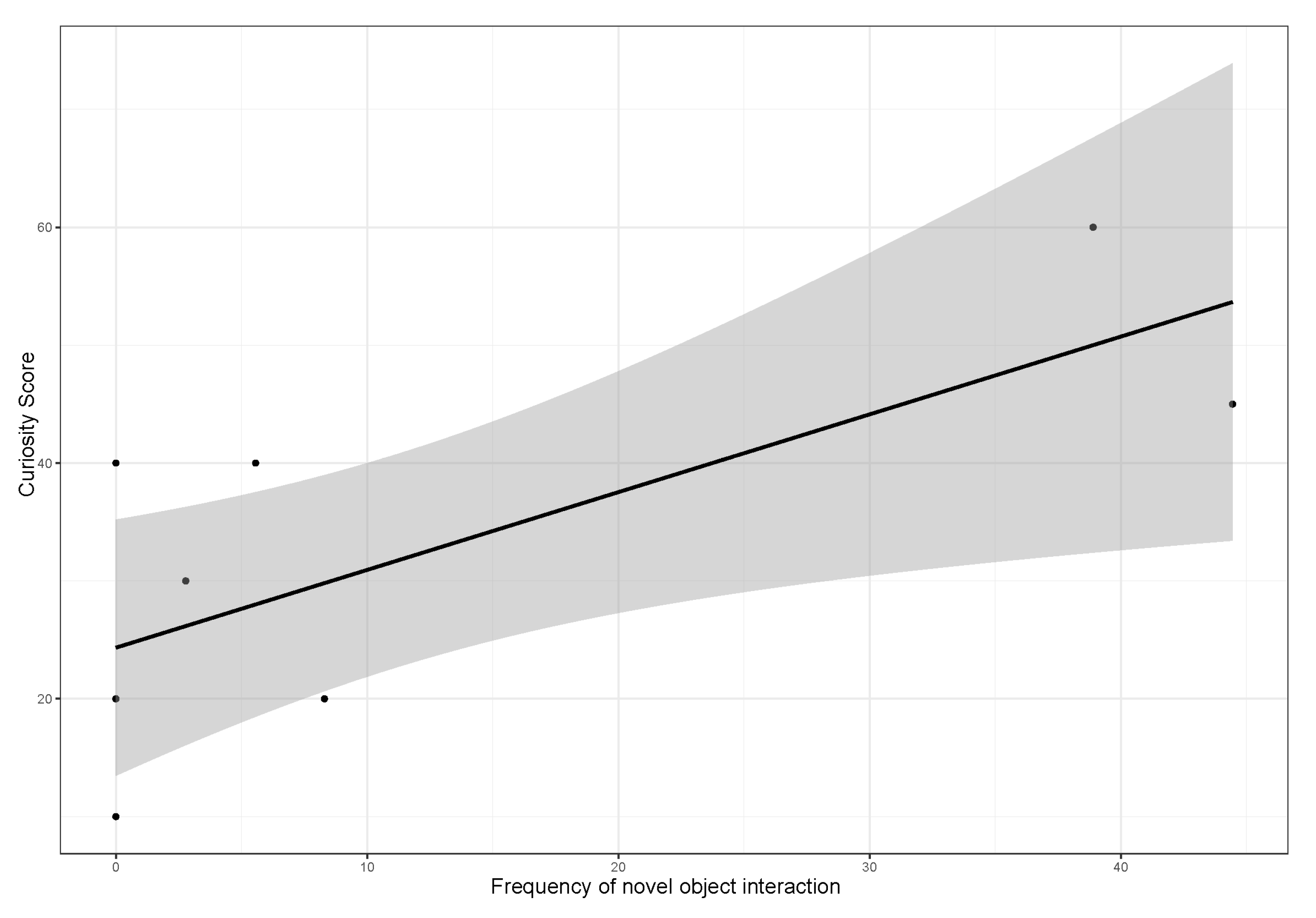
3.3. Influence of Sex, Age and Personality Traits on the Frequency of Novel Object Interaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Réale, D.; Reader, S.M.; Sol, D.; McDougall, P.T.; Dingemanse, N.J. Integrating Animal Temperament within Ecology and Evolution. Biol. Rev. 2007, 82, 291–318. [Google Scholar] [CrossRef]
- Bell, A.M.; Hankison, S.J.; Laskowski, K.L. The Repeatability of Behaviour: A Meta-Analysis. Anim. Behav. 2009, 77, 771–783. [Google Scholar] [CrossRef]
- Réale, D.; Martin, J.; Coltman, D.W.; Poissant, J.; Festa-Bianchet, M. Male Personality, Life-history Strategies and Reproductive Success in a Promiscuous Mammal. J. Evol. Biol. 2009, 22, 1599–1607. [Google Scholar] [CrossRef]
- Groothuis, T.G.G.; Claudio, C. Avian personalities: Characterization and epigenesis. Neuroscience and biobehavioral reviews. Neurosci. Biobehav. Rev. 2005, 29, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.M.; Stevens, E.D. Consistency in Context-Specific Measures of Shyness and Boldness in Rainbow Trout, Oncorhynchus Mykiss. Ethology 2005, 111, 849–862. [Google Scholar] [CrossRef]
- Ariyomo, T.O.; Watt, P.J. The Effect of Variation in Boldness and Aggressiveness on the Reproductive Success of Zebrafish. Anim. Behav. 2012, 83, 41–46. [Google Scholar] [CrossRef]
- Reaney, L.T.; Backwell, P.R.Y. Risk-Taking Behavior Predicts Aggression and Mating Success in a Fiddler Crab. Behav. Ecol. 2007, 18, 521–525. [Google Scholar] [CrossRef]
- Mowles, S.L.; Cotton, P.A.; Briffa, M. Consistent Crustaceans: The Identification of Stable Behavioural Syndromes in Hermit Crabs. Behav. Ecol. Sociobiol. 2012, 66, 1087–1094. [Google Scholar] [CrossRef]
- Toscano, B.J.; Gownaris, N.J.; Heerhartz, S.M.; Monaco, C.J. Personality, Foraging Behavior and Specialization: Integrating Behavioral and Food Web Ecology at the Individual Level. Oecologia 2016, 182, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Bridger, D.; Bonner, S.J.; Briffa, M. Individual Quality and Personality: Bolder Males Are Less Fecund in the Hermit Crab Pagurus bernhardus. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142492. [Google Scholar] [CrossRef]
- Belgrad, B.A.; Karan, J.; Griffen, B.D. Individual Personality Associated with Interactions between Physiological Condition and the Environment. Anim. Behav. 2017, 123, 277–284. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral Syndromes: An Ecological and Evolutionary Overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.L.; Gosling, S.D.; Moltschaniwskyj, N.A. Development of Shy/Bold Behaviour in Squid: Context-Specific Phenotypes Associated with Developmental Plasticity. Anim. Behav. 2008, 75, 433–442. [Google Scholar] [CrossRef]
- Réale, D.; Gallant, B.Y.; Leblanc, M.; Festa-Bianchet, M. Consistency of Temperament in Bighorn Ewes and Correlates with Behaviour and Life History. Anim. Behav. 2000, 60, 589–597. [Google Scholar] [CrossRef]
- Gosling, S.D.; John, O.P. Personality Dimensions in Nonhuman Animals. Curr. Dir. Psychol. Sci. 1999, 8, 69–75. [Google Scholar] [CrossRef]
- Finkemeier, M.-A.; Langbein, J.; Puppe, B. Personality Research in Mammalian Farm Animals: Concepts, Measures, and Relationship to Welfare. Front. Vet. Sci. 2018, 5, 131. [Google Scholar] [CrossRef]
- Chapman, B.B.; Hulthén, K.; Blomqvist, D.R.; Hansson, L.-A.; Nilsson, J.-Å.; Brodersen, J.; Anders Nilsson, P.; Skov, C.; Brönmark, C. To Boldly Go: Individual Differences in Boldness Influence Migratory Tendency. Ecol. Lett. 2011, 14, 871–876. [Google Scholar] [CrossRef]
- Dammhahn, M.; Almeling, L. Is Risk Taking during Foraging a Personality Trait? A Field Test for Cross-Context Consistency in Boldness. Anim. Behav. 2012, 84, 1131–1139. [Google Scholar] [CrossRef]
- Beckmann, C.; Biro, P.A. On the Validity of a Single (Boldness) Assay in Personality Research. Ethology 2013, 119, 937–947. [Google Scholar] [CrossRef]
- Herde, A.; Eccard, J.A. Consistency in Boldness, Activity and Exploration at Different Stages of Life. BMC Ecol. 2013, 13, 49. [Google Scholar] [CrossRef]
- Bevan, P.A.; Gosetto, I.; Jenkins, E.R.; Barnes, I.; Ioannou, C.C. Regulation between Personality Traits: Individual Social Tendencies Modulate Whether Boldness and Leadership Are Correlated. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180829. [Google Scholar] [CrossRef] [PubMed]
- McDonald, N.D.; Rands, S.A.; Hill, F.; Elder, C.; Ioannou, C.C. Consensus and Experience Trump Leadership, Suppressing Individual Personality during Social Foraging. Sci. Adv. 2016, 2, e1600892. [Google Scholar] [CrossRef] [PubMed]
- Biro, P.A.; Stamps, J.A. Are Animal Personality Traits Linked to Life-History Productivity? Trends Ecol. Evol. 2008, 23, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Jolles, J.W.; Ostojić, L.; Clayton, N.S. Dominance, Pair Bonds and Boldness Determine Social-Foraging Tactics in Rooks, Corvus Frugilegus. Anim. Behav. 2013, 85, 1261–1269. [Google Scholar] [CrossRef]
- Kurvers, R.H.J.M.; Krause, J.; Croft, D.P.; Wilson, A.D.M.; Wolf, M. The Evolutionary and Ecological Consequences of Animal Social Networks: Emerging Issues. Trends Ecol. Evol. 2014, 29, 326–335. [Google Scholar] [CrossRef]
- Bell, A.M.; Sih, A. Exposure to Predation Generates Personality in Threespined Sticklebacks (Gasterosteus Aculeatus). Ecol. Lett. 2007, 10, 828–834. [Google Scholar] [CrossRef]
- Krause, J.; James, R.; Croft, D.P. Personality in the Context of Social Networks. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 4099–4106. [Google Scholar] [CrossRef]
- Harcourt, J.L.; Biau, S.; Johnstone, R.; Manica, A. Boldness and Information Use in Three-Spined Sticklebacks. Ethology 2010, 116, 440–447. [Google Scholar] [CrossRef]
- Pike, T.W.; Samanta, M.; Lindström, J.; Royle, N.J. Behavioural Phenotype Affects Social Interactions in an Animal Network. Proc. R. Soc. B Biol. Sci. 2008, 275, 2515–2520. [Google Scholar] [CrossRef]
- Bergman, T.J.; Sheenan, M.J. Social Knowledge and Signals in Primates. Am. J. Primatol. 2013, 75, 683–694. [Google Scholar] [CrossRef]
- Wittig, R.M.; Crockford, C.; Langergraber, K.E.; Zuberbühler, K. Triadic Social Interactions Operate across Time: A Field Experiment with Wild Chimpanzees. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133155. [Google Scholar] [CrossRef] [PubMed]
- Massen, J.J.M.; Pašukonis, A.; Schmidt, J.; Bugnyar, T. Ravens Notice Dominance Reversals among Conspecifics within and Outside Their Social Group. Nat. Commun. 2014, 5, 3679. [Google Scholar] [CrossRef] [PubMed]
- Engh, A.L.; Siebert, E.R.; Greenberg, D.A.; Holekamp, K.E. Patterns of Alliance Formation and Postconflict Aggression Indicate Spotted Hyaenas Recognize Third-Party Relationships. Anim. Behav. 2005, 69, 209–217. [Google Scholar] [CrossRef]
- Chase, I.D.; Tovey, C.; Spangler-Martin, D.; Manfredonia, M. Individual Differences versus Social Dynamics in the Formation of Animal Dominance Hierarchies. Proc. Natl. Acad. Sci. USA 2002, 99, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
- Earley, R.L.; Dugatkin, L.A. Eavesdropping on Visual Cues in Green Swordtail (Xiphophorus Helleri) Fights: A Case for Networking. Proc. R. Soc. London. Ser. B Biol. Sci. 2002, 269, 943–952. [Google Scholar] [CrossRef]
- Reimert, I.; Bolhuis, J.E.; Kemp, B.; Rodenburg, T.B. Emotions on the Loose: Emotional Contagion and the Role of Oxytocin in Pigs. Anim. Cogn. 2015, 18, 517–532. [Google Scholar] [CrossRef] [PubMed]
- Reimert, I.; Fong, S.; Rodenburg, T.B.; Bolhuis, J.E. Emotional States and Emotional Contagion in Pigs after Exposure to a Positive and Negative Treatment. Appl. Anim. Behav. Sci. 2017, 193, 37–42. [Google Scholar] [CrossRef]
- Taborsky, B.; English, S.; Fawcett, T.W.; Kuijper, B.; Leimar, O.; McNamara, J.M.; Ruuskanen, S.; Sandi, C. Towards an Evolutionary Theory of Stress Responses. Trends Ecol. Evol. 2021, 36, 39–48. [Google Scholar] [CrossRef]
- Mann, J. Behavioral Sampling Methods for Cetaceans.: A Rewie and Critique. Mar. Mammal Sci. 1999, 15, 102–122. [Google Scholar] [CrossRef]
- Goodmann, P.A.K.E.; Wolf Ethogram, W.J. Ethology Series No. 3; Eckhard, H., Ed.; Hess Institute of Ethology: Battle Ground, IN, USA, 2002. [Google Scholar]
- Guttorp, P. Stochastic Modeling of Scientific Data; CRC: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Caswell. Matrix Population Models; Construction, Analysis, and Interpretation Inc.: Sunderland, MA, USA, 2001. [Google Scholar]
- Turcsán, B.; Kubinyi, E.; Miklósi, Á. Trainability and boldness traits differ between dog breed clusters based on conventional breed categories and genetic relatedness. Appl. Anim. Behav. Sci. 2011, 132, 61–70. [Google Scholar] [CrossRef]
- Ley, J.; Bennett, P.; Coleman, G. A refinement and validation of the Monash Canine Personality Questionnaire (MCPQ). Appl. Anim. Behav. Sci. 2009, 116, 220–227. [Google Scholar] [CrossRef]
- Draper, T.W. Canine analogs of human personality factors. J. Gen. Psychol. 1995, 122, 241–252. [Google Scholar] [CrossRef] [PubMed]
- de Vries, H. Finding a dominance order most consistent with a linear hierarchy: A new procedure and review. Anim. Behav. 1998, 55, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Gammell, M.P.; de Vries, H.; Jennings, D.J.; Carlin, C.M.; Hayden, T.J. David’s score: A more appropriate dominance ranking method than Clutton-Brock et al.’s index. Anim. Behav. 2003, 66, 601–605. [Google Scholar] [CrossRef]
- Cordoni, G.; Palagi, E. Back to the Future: A Glance Over Wolf Social Behavior to Understand Dog–Human Relationship. Animals 2019, 9, 991. [Google Scholar] [CrossRef]
- Nunn, C.L.; Deaner, R.O. Patterns of Participation and Free Riding in Territorial Conflicts among Ringtailed Lemurs (Lemur Catta). Behav. Ecol. Sociobiol. 2004, 57, 50–61. [Google Scholar] [CrossRef]
- Bonanni, R.; Valsecchi, P.; Natoli, E. Pattern of Individual Participation and Cheating in Conflicts between Groups of Free-Ranging Dogs. Anim. Behav. 2010, 79, 957–968. [Google Scholar] [CrossRef]
- Rault, J.-L. Friends with Benefits: Social Support and Its Relevance for Farm Animal Welfare. Appl. Anim. Behav. Sci. 2012, 136, 1–14. [Google Scholar] [CrossRef]
- Beery, A.K.; Kaufer, D. Stress, Social Behavior, and Resilience: Insights from Rodents. Neurobiol. Stress. 2015, 1, 116–127. [Google Scholar] [CrossRef]
- Panksepp, J.B.; Jochman, K.A.; Kim, J.U.; Koy, J.J.; Wilson, E.D.; Chen, Q.; Wilson, C.R.; Lahvis, G.P. Affiliative behavior, ultrasonic communication and social reward are influenced by genetic variation in adolescent mice. PLoS ONE 2007, 2, e351. [Google Scholar] [CrossRef]
- Wilson, A.D.; Godin, J.-G.J. Boldness and Behavioral Syndromes in the Bluegill Sunfish, Lepomis Macrochirus. Behav. Ecol. 2009, 20, 231–237. [Google Scholar] [CrossRef]
- Dahlbom, S.J.; Lagman, D.; Lundstedt-Enkel, K.; Sundström, L.F.; Winberg, S. Boldness Predicts Social Status in Zebrafish (Danio Rerio). PLoS ONE 2011, 6, e23565. [Google Scholar] [CrossRef] [PubMed]
- Gosling, S.D. From mice to men: What can we learn about personality from animal research? APA PsycNet 2001, 127, 45–86. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.L. Extraversion—Introversion. In The Wiley Encyclopedia of Personality and Individual Differences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 159–163. [Google Scholar] [CrossRef]
- Kubinyi, E.; Turcsán, B.; Miklósi, Á. Dog and owner demographic characteristics and dog personality trait associations. Behav. Process. 2009, 81, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Øverli, Ø.; Sorensen, C.; Nilsson, G.E. Behavioral indicators of stress-coping style in rainbow trout: Do males and females react differently to novelty? Psysiol. Behav. 2006, 87, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Schuett, W.; Dall, S. Sex differences, social context and personality in zebra finches, Taeniopygia guttata. Anim. Behav. 2009, 77, 1041–1050. [Google Scholar] [CrossRef]
- Ward-Fear, G.; Brown, G.P.; Pearson, D.J.; West, A.; Rollins, L.A.; Shine, R. The ecological and life history correlates of boldness in free-ranging lizards. Ecosphere 2018, 9, e02125. [Google Scholar] [CrossRef]
- Smith, B.P. Living with Wild Dogs: Personality Dimensions in Captive Dingoes (Canis Dingo) and Implications for Ownership. Anthrozoos 2014, 27, 423–433. [Google Scholar] [CrossRef]

| Name | Abbreviation | Sex | Birth Date | Facility |
|---|---|---|---|---|
| Runa | EU1 | Female | 2018 | Schwarze Berge |
| Yuuki | EU2 | Male | 2018 | Schwarze Berge |
| Dunja | EU3 | Female | 2013 | Schwarze Berge |
| Django | EU4 | Male | 2014 | Schwarze Berge |
| Skadi | EU5 | Female | 2017 | Schwarze Berge |
| Wolfgang | EUW1 | Male | 2011 | Zoo Wingst |
| Rudolf | EUW2 | Male | 2011 | Zoo Wingst |
| Anfa | EUW3 | Female | 2011 | Zoo Wingst |
| Wolle | EUW4 | Male | 2013 | Zoo Wingst |
| Andra | EUW5 | Female | 2011 | Zoo Wingst |
| Behavioural Category | Original Category [40] | Subcategories | Behaviours in the Social Reward Chain |
|---|---|---|---|
| Agonistic behaviours | Agonistic behaviours | Elicited Aggression Food-related Aggression Sex-related Aggression All-Out Attack Defence and Submission Offensive Threat Ritualised Attack, Counterattack, Fight | None |
| Play behaviours | Play behaviours | Agonistic Play Social Play Solitary Play | None |
| Caregiving | Caregiving, Care Solicitation | None | Approach Sniffle Face Wipe Follow Hold Out Face, Airplane Ears |
| Submissive behaviours | Not existent | None | Expose Belly Down and Lick Lick Snout Low Posture Follow |
| Feeding (Eating) | Feeding (EAT) | None | Approach Cache Carry Object Drag Eat Forage Grab Lick Mouth Paw Tug |
| Greeting | Greeting | None | Airplane Ears Approach Body Rub Ears Back Ears Pricked Greet Group Formation/Group Together Grin Hug Hum Lick Leave Together Parallel Gait Parallel Walk Sniff Noses Tail Wag |
| Locomotion/exploratory | Locomotion | None | Amble Approach Avoid Follow Observation Object Interaction Observation Jump Run |
| Scent-Marking, Elimination | Scent-Marking, Elimination | None | None |
| Other | Other | None | Explore Indirect Approach Orient Wander |
| Predation, Hunting | Predation, Hunting | None | None |
| Resting | Resting | None | None |
| Initial Behaviours | Description of Observed Behaviours |
|---|---|
| Novel object interaction (Nobj) | Approaching the novel object (apparatus) up to maximum 1 m distance, and sniffing on the rope or interacting with it by trying to pull the rope/pulling the rope |
| Forage | Walking around the enclosure, searching for leftovers of food or dead animals, eating, or hiding food |
| Run | Running around individually in the enclosure from one area to another |
| State | Description of Observed Behaviours |
|---|---|
| Group formation (GrpFrmt) | Individuals grouping together, observing the individual performing an “initial behaviour” |
| Greeting (Greet) | Tail wagging, body rubbing and face/body sniffing of group formation towards “active performer” |
| Submissive behaviour (LickSn) | Snout licking, lowered body posture, whimpering, lying on back/exposing belly towards the individual performing the “initial behaviour” |
| Combined submissive behaviours (C.SubmBhv) | Body rubbing, ears back, opening group formation and including the individual performing the “initial behaviour” to the group |
| Leaving together (LeaveTgh) | Leaving proximity of “initial behaviour performance location” as a united group |
| Factor | Questions and Items to Determine Factor Values |
|---|---|
| Curiosity/Openness | “is inventive and resourceful when it comes to finding or reaching hidden food or toys” “does not have many interests apart from eating and sleeping, e.g., is unexplorative, uncurious, show limited interest in new objects, humans or animals” “has a good grasp of things and learns quickly” “attentive” “intelligent” “clever” |
| Playfulness | “is enthusiastic and encourages his peers to play” |
| “is easy to get excited about new play ideas” | |
| “often does not understand what is being asked of him in play situations” |
| Initial State | Success Score (Mean ± SD) |
|---|---|
| Nobj (novel object interaction) | 0.63 * ± 0.091 |
| Indhbhv1 (“run”) | 0.318 ± 0.394 |
| Indbhv2 (“forage”) | 0.279 ± 0.362 |
| Personality Trait/Factor | Result (Mean ± SD) |
|---|---|
| Playfulness | 31.66 ± 15.81 |
| Extraversion | 42.22 ± 24.25 |
| Curiosity | 42.77 ± 18.72 |
| Sociability | 40.75 ± 14.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tebelmann, H.; Gansloßer, U. Social Reward Behaviour in Two Groups of European Grey Wolves (Canis lupus lupus)—A Case Study. Animals 2023, 13, 872. https://doi.org/10.3390/ani13050872
Tebelmann H, Gansloßer U. Social Reward Behaviour in Two Groups of European Grey Wolves (Canis lupus lupus)—A Case Study. Animals. 2023; 13(5):872. https://doi.org/10.3390/ani13050872
Chicago/Turabian StyleTebelmann, Hana, and Udo Gansloßer. 2023. "Social Reward Behaviour in Two Groups of European Grey Wolves (Canis lupus lupus)—A Case Study" Animals 13, no. 5: 872. https://doi.org/10.3390/ani13050872
APA StyleTebelmann, H., & Gansloßer, U. (2023). Social Reward Behaviour in Two Groups of European Grey Wolves (Canis lupus lupus)—A Case Study. Animals, 13(5), 872. https://doi.org/10.3390/ani13050872