Survived the Glaciations, Will They Survive the Fish? Allochthonous Ichthyofauna and Alpine Endemic Newts: A Road Map for a Conservation Strategy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
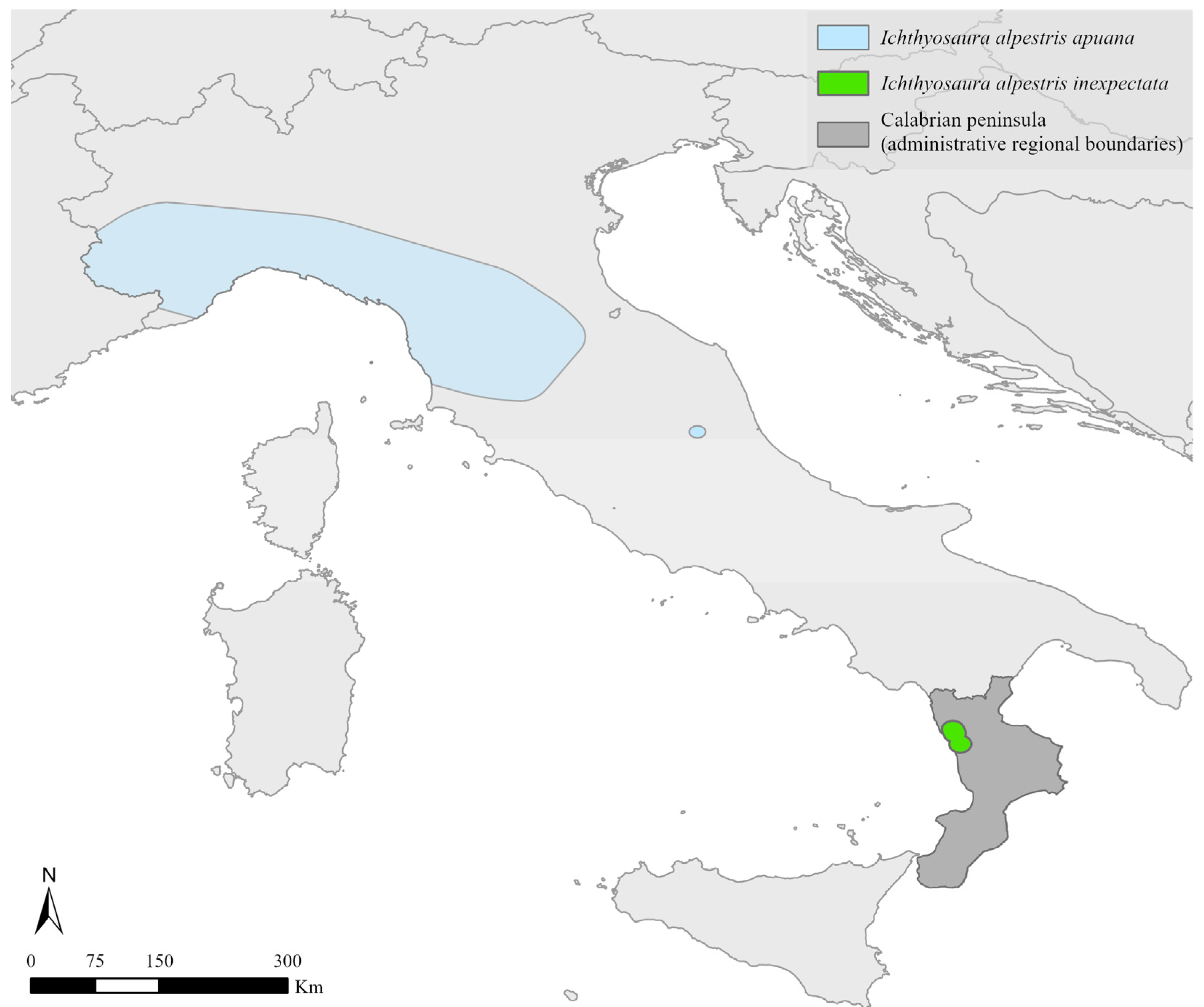
2.1. Study Taxon
2.2. Study Area and Site Description
2.3. Sampling Procedures
2.4. Habitats’ Characteristics and Macroinvertebrates Assemblages
2.5. Data Analysis
3. Results
3.1. Current Distribution Framework and Habitats’ Characteristics
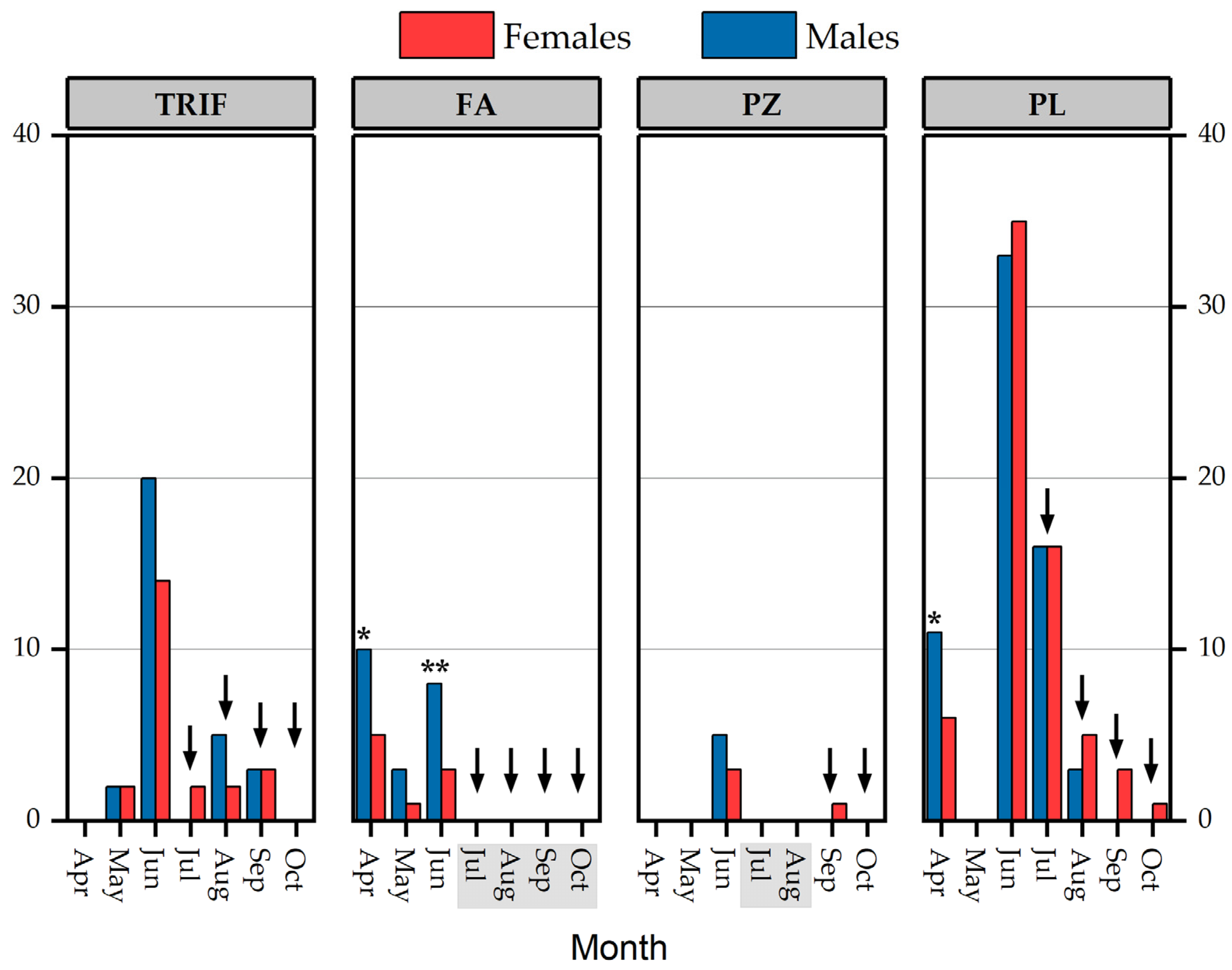
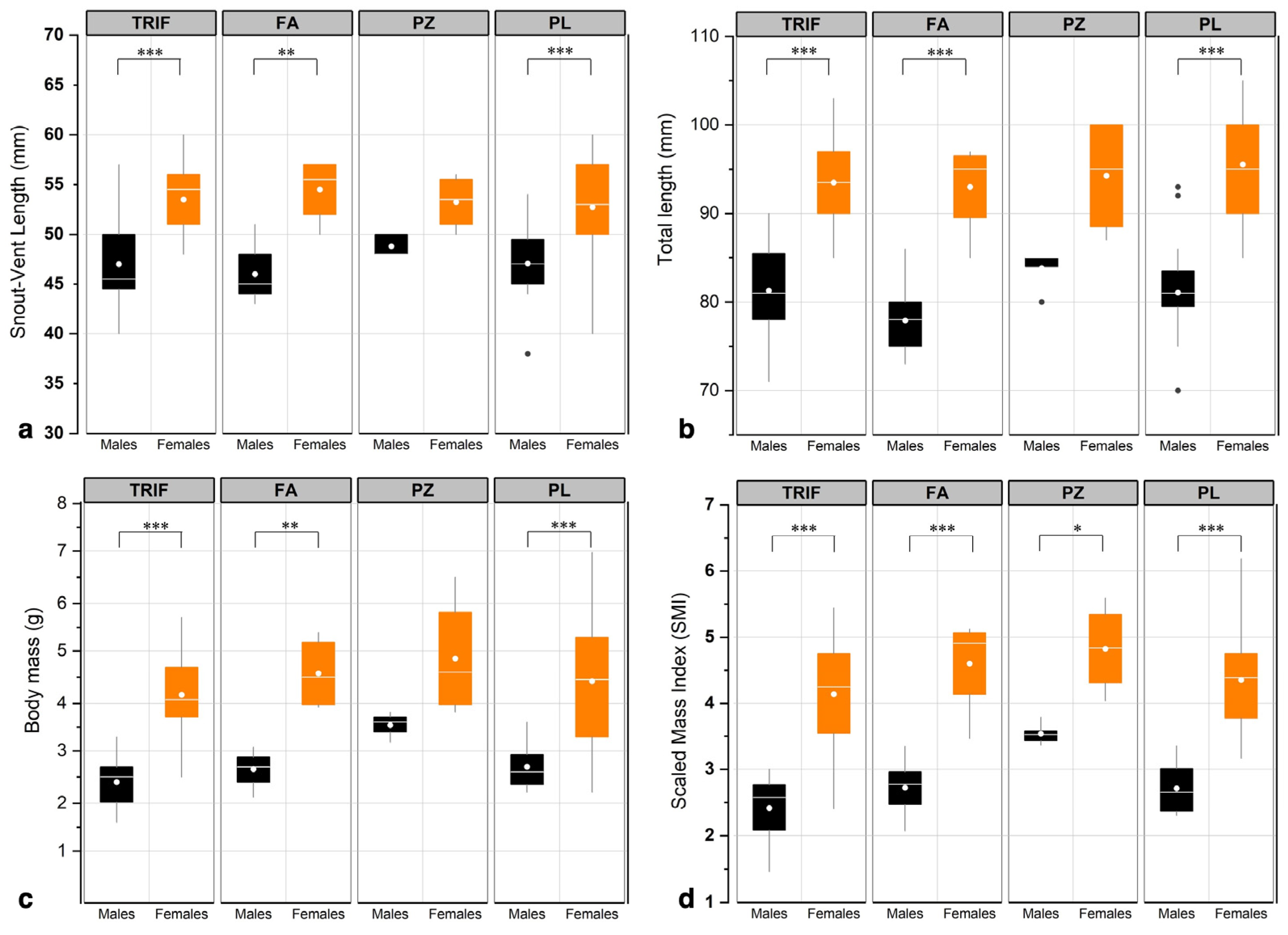
3.2. Population parameters
4. Discussion
4.1. Updated Distribution, Species Occurrence and Habitat Characteristics
4.2. Population Parameters
4.3. Threats, Conservation Measures and Active Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Strayer, D.L.; Dudgeon, D. Freshwater biodiversity conservation: Recent progress and future challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Darwall, W.R.T.; Vie, J.C. Identifying important sites for conservation of freshwater biodiversity: Extending the species-based approach. Fish. Manag. Ecol. 2005, 12, 287–293. [Google Scholar] [CrossRef]
- Groom, M.J.; Meffe, G.K.; Carroll, C.R.; Andelman, S.J. Principles of conservation biology. In Protected Areas: Goals, Limitations and Design; Groom, M.J., Meffe, G.K., Carroll, R., Eds.; Sinauer Associates: Sunderland, MA, USA, 2006. [Google Scholar]
- Bernabò, I.; Biondi, M.; Cittadino, V.; Sperone, E.; Iannella, M. Addressing conservation measures through fine-tuned species distribution models for an Italian endangered endemic anuran. Glob. Ecol. Conserv. 2022, 39, e02302. [Google Scholar] [CrossRef]
- Reid, W.V. Biodiversity hotspots. Trends Ecol. Evol. 1998, 13, 275–280. [Google Scholar] [CrossRef]
- Schmeller, D.S.; Gruber, B.; Budrys, E.; Framsted, E.; Lengyel, S.; Henle, K. National responsibilities in European species conservation: A methodological review. Conserv. Biol. 2008, 22, 593–601. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN: Gland, Switzerland; Cambridge, UK, 2012; pp. iv + 32. [Google Scholar]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Bernabò, I.; Guardia, A.; Macirella, R.; Sesti, S.; Tripepi, T.; Brunelli, E. Tissues injury and pathological changes in Hyla intermedia juveniles after chronic larval exposure to tebuconazole. Ecotoxicol. Environ. Saf. 2020, 205, 111367. [Google Scholar] [CrossRef]
- Bernabò, I.; Guardia, A.; Macirella, R.; Tripepi, S.; Brunelli, E. Chronic exposures to fungicide pyrimethanil: Multi-organ effects on Italian tree frog (Hyla intermedia). Sci. Rep. 2017, 7, 6869. [Google Scholar] [CrossRef]
- Bounas, A.; Keroglidou, M.; Toli, E.A.; Chousidis, I.; Tsaparis, D.; Leonardos, I.; Sotiropoulos, K. Constrained by aliens, shifting landscape, or poor water quality? Factors affecting the persistence of amphibians in an urban pond network. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1037–1049. [Google Scholar] [CrossRef]
- Bosch, J.; Bielby, J.; Martin-Beyer, B.; Rincon, P.; Correa-Araneda, F.; Boyero, L. Eradication of introduced fish allows successful recovery of a stream-dwelling amphibian. PLoS ONE 2019, 14, e0216204. [Google Scholar] [CrossRef]
- Martel, A.; Blooi, M.; Adriaensen, C.; Van Rooij, P.; Beukema, W.; Fisher, M.C.; Farrer, R.A.; Schmidt, B.R.; Tobler, U.; Goka, K.; et al. Recent introduction of a chytrid fungus endangers Western Palearctic salamanders. Science 2014, 346, 630–631. [Google Scholar] [CrossRef]
- Davidson, C.; Knapp, R.A. Multiple Stressors and Amphibian Declines: Dual Impacts of Pesticides and Fish on Yellow-Legged Frogs. Ecol. App. 2007, 17, 587–597. [Google Scholar] [CrossRef]
- Nunes, A.L.; Fill, J.M.; Davies, S.J.; Louw, M.; Rebelo, A.D.; Thorp, C.J.; Vimercati, G.; Measey, J. A global meta-analysis of the ecological impacts of alien species on native amphibians. Proc. R. Soc. B 2019, 286, 20182528. [Google Scholar] [CrossRef]
- Orizaola, G.; Braña, F. Effect of salmonid introduction and other environmental characteristics on amphibian distribution and abundance in mountain lakes of northern Spain. Anim. Conserv. 2006, 9, 171–178. [Google Scholar] [CrossRef]
- Pilliod, S.D.; Peterson, C.R. Local and Landscape Effects of introduced Trout on Amphibians in Historically Fishless Watersheds. Ecosystems 2001, 4, 322–333. [Google Scholar] [CrossRef]
- Tiberti, R. Can satellite ponds buffer the impact of introduced fish on newts in a mountain pond network? Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 28, 457–465. [Google Scholar] [CrossRef]
- Falaschi, M.; Muraro, M.; Gibertini, C.; Delle Monache, D.; Lo Parrino, E.; Faraci, F.; Belluardo, F.; Di Nicola, M.R.; Manenti, R.; Ficetola, G.F. Explaining declines of newt abundance in northern Italy. Freshw. Biol. 2022, 67, 1174–1187. [Google Scholar] [CrossRef]
- Chiocchio, A.; Bisconti, R.; Zampiglia, M.; Nascetti, G.; Canestrelli, D. Quaternary history; population genetic structure and diversity of the cold-adapted Alpine newt Ichthyosaura alpestris in peninsular Italy. Sci. Rep. 2017, 7, 2955. [Google Scholar] [CrossRef]
- Dubois, A.; Breuil, M. Découverte de Triturus alpestris (Laurenti, 1768) en Calabre (Sud de l’Italie). Alytes 1983, 2, 9–18. [Google Scholar]
- Dubois, A. Le Triton alpestre de Calabre: Une forme rare et menacée d’extinction. Alytes 1983, 2, 55–62. [Google Scholar]
- Giacoma, C.; Picariello, O.; Puntillo, D.; Rossi, F.; Tripepi, S. The distribution and habitats of the newt (Triturus, Amphibia) in Calabria (southern Italy). Monit. Zool. Ital.-Ital. J. Zool. 1988, 22, 449–464. [Google Scholar]
- Tripepi, S.; Rossi, F.; Serroni, P.; Brunelli, E. Distribuzione altitudinale degli Anfibi in Calabria. Studi Trent. Sci. Nat. Acta Biol. 1996, 71, 97–101. [Google Scholar]
- Sperone, E.; Bonacci, A.; Brunelli, E.; Corapi, B.; Tripepi, S. Ecologia e conservazione dell’erpetofauna della Catena Costiera calabra. Studi Trent. Sci. Nat. Acta Biol. 2007, 83, 99–104. [Google Scholar]
- Dubois, A.; Ohler, A. A quick method for a rough estimate of the size of a small and threatened animal population: The case of the relict Ichthyosaura alpestris inexpectata in southern Italy. Bull. Soc. Nat. Luxemb. 2009, 110, 115–124. [Google Scholar]
- Rondinini, C.; Battistoni, A.; Teofili, C. (Eds.) Lista Rossa IUCN dei Vertebrati Italiani 2022; Comitato Italiano IUCN e Ministero dell’Ambiente e della Sicurezza Energetica: Roma, Italy, 2022.
- Sillero, N.; Campos, J.; Bonardi, A.; Corti, C.; Creemers, R.; Crochet, P.A.; Crnobrnja Isailović, J.; Denoël, M.; Ficetola, G.F.; Gonçalves, J.; et al. Updated distribution and biogeography of amphibians and reptiles of Europe. Amphib. Reptil. 2014, 35, 1–31. [Google Scholar] [CrossRef]
- Sindaco, R.; Doria, G.; Razzetti, E.; Bernini, F. Atlante Degli Anfibi e dei Rettili d’Italia; Societas Herpetologica Italica, Edizioni Polistampa: Firenze, Italy, 2006. [Google Scholar]
- Lanza, B.; Andreone, F.; Bologna, M.A.; Corti, C.; Razzetti, E. Fauna d’Italia Amphibia; Edizioni Calderini de Il Sole 24 ORE Editoria Specializzata S.r.l.: Bologna, Italy, 2007; Volume XLII, pp. 254–265. [Google Scholar]
- Di Nicola, M.R.; Cavigioli, L.; Luiselli, L.; Andreone, F. Anfibi & Rettili d’Italia; Edizione Belvedere: Latina, Italy, 2021; pp. 202–207. [Google Scholar]
- Andreone, F. Triturus alpestris alpestris, Triturus alpestris apuanus, Tritone alpestre e Tritone appenninico. In Erpetologia del Piemonte e Della Valle d’Aosta—Atlante Degli Anfibi e dei Rettili; Andreone, F., Sindaco, R., Eds.; Monografie—Museo Regionale di Scienze Naturali: Torino, Italy, 1999; Volume 26, pp. 162–163. [Google Scholar]
- Joly, P.; Giacoma, C. Limitation of similarity and feeding habits in three syntopic species of newts (Triturus, Amphibia). Ecography 1992, 15, 401–411. [Google Scholar] [CrossRef]
- Coppari, L.; Ferri, V.; Marini, D.; Di Nicola, M.; Notomista, T. (Eds.) Le Aree di Rilevanza Erpetologica in Italia 1995–2021; Commissione Conservazione della Societas Herpetologica Italica. Available online: http://www-9.unipv.it/webshi/images/files/Volume_ARE_2021.pdf:2021 (accessed on 1 December 2022).
- de Beaulieu, J.-L.; Brugiapaglia, E.; Joannin, S.; Guiter, F.; Zanchetta, G.; Wulf, S.; Peyron, O.; Bernardo, L.; Didier, J.; Stock, A.; et al. Lateglacial-Holocene abrupt vegetation changes at Lago Trifoglietti in Calabria, Southern Italy: The setting of ecosystems in a refugial zone. Quat. Sci. Rev. 2017, 158, 44–57. [Google Scholar] [CrossRef]
- Aramini, G.; Bernabò, I.; Brandmayr, P.; Brusco, A.; Costa, R.M.S.; Fusillo, R.; Gangale, C.; Infusino, M.; Greco, R.; Musarella, C.M.; et al. Rete Natura 2000. Biodiversità in Calabria; Rubbettino Editore: Soveria Mannelli, Italy, 2021; Volume 2, ISBN 978-88-94601-30-5. [Google Scholar]
- Pesaresi, S.; Biondi, E.; Casavecchia, S. Bioclimates of Italy. J. Maps 2017, 13, 955–960. [Google Scholar] [CrossRef]
- Andreone, F.; Dore, B. New data on paedomorphism in Italian populations of alpine newts, Triturus alpestris (Laurenti, 1768) (Caudata: Salamandridae). Herpetozoa 1991, 4, 149–156. [Google Scholar]
- Andreone, F. Variabilità Morfologica e Riproduttiva in Popolazioni di Triturus alpestris (Laurenti, 1768) (Amphibia, Urodela, Salamandridae); Tesi di Dottorato di Ricerca, Università di Bologna: Bologna, Italy, 1990. [Google Scholar]
- Bernabò, I.; Brunelli, E. Comparative morphological analysis during larval development of three syntopic newt species (Urodela: Salamandridae). Eur. Zool. J. 2019, 86, 38–53. [Google Scholar] [CrossRef]
- Campaioli, S.; Ghetti, P.F.; Minelli, A.; Ruffo, S. Manuale per il Riconoscimento dei Macroinvertebrati Delle Acque Dolci Italiane; Provincia Autonoma di Trento: Bari, Italy, 1994; Volume I, p. 357. [Google Scholar]
- Sansoni, G. Atlante per il Riconoscimento dei Macroinvertebrati Bentonici dei Corsi D’acqua Italiani; Provincia Autonoma di Trento: Bari, Italy, 1988; p. 191. [Google Scholar]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés d’Eau Douce; CNRS Editions: Paris, Italy, 2002. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Urbana, IL, USA, 1949; pp. 1–117. [Google Scholar]
- Margalef, R. Temporal succession and spatial heterogeneity in phytoplankton. In Perspectives in Marine Biology; Buzzati-Traverso, A.A., Ed.; University of California Press: Berkeley, CA, USA, 1958; pp. 323–347. [Google Scholar]
- MacCracken, J.G.; Stebbings, J.L. Test of a body condition index with amphibians. J. Herpetol. 2012, 46, 346–350. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. New perspectives for estimating body condition from mass/length data: The scaled mass index as an alternative method. Oikos 2009, 118, 1883–1891. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Funct. Ecol. 2010, 24, 1323–1332. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 15 June 2022).
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. Smatr 3—An R package for estimation and inference about allometric lines. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- Moran, P.A.P. A mathematical theory of animal trapping. Biometrica 1951, 38, 307–311. [Google Scholar] [CrossRef]
- Zippin, C. The removal method of population estimation. J. Wildl. 1958, 22, 82–90. [Google Scholar] [CrossRef]
- Ogle, D.H.; Doll, J.C.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis. R Package Version 0.9.3. 2022. Available online: https://github.com/fishR-Core-Team/FSA (accessed on 1 December 2022).
- Orizaola, G.E.; Brana, F.L. Oviposition behaviour and vulnerability of eggs to predation in four newt species (genus Triturus). Herpetol. J. 2003, 13, 121–124. [Google Scholar]
- Teplitsky, C.; Plénet, S.; Joly, P. Tadpoles’ responses to risk of fish introduction. Oecologia 2003, 134, 270–277. [Google Scholar] [CrossRef]
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Winandy, L.; Darnet, E.; Denoël, M. Amphibians forgo aquatic life in response to alien fish introduction. Anim. Behav. 2015, 109, 209–216. [Google Scholar] [CrossRef]
- Winandy, L.; Legrand, P.; Denoël, M. Habitat selection and reproduction of newts in networks of fish and fishless aquatic patches. Anim. Behav. 2017, 123, 107–115. [Google Scholar] [CrossRef]
- Šunje, E.; Stroil, K.B.; Raffaëlli, J.; Zimić, A.; Marquis, O. A revised phylogeny of Alpine newts unravels the evolutionary distinctiveness of the Bosnian Alpine newt—Ichthyosaura alpestris reiseri (Werner, 1902). Amphib. Reptil. 2021, 42, 481–490. [Google Scholar] [CrossRef]
- Tanadini, L.G.; Schmidt, B.R. Population Size Influences Amphibian Detection Probability: Implications for Biodiversity Monitoring Programs. PLoS ONE 2011, 6, e28244. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; McCarthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Fusillo, R.; Esse, E.; Marcelli, M.; Mastronardi, D.; Bernabò, I. New record of Lissotriton vulgaris meridionalis (Boulenger, 1882) at the southernmost edge of its distribution in Italy. Herpetol. Notes 2021, 14, 923–926. [Google Scholar]
- Bernabò, I.; Cittadino, V.; Tripepi, S.; Marchianò, V.; Piazzini, S.; Biondi, M.; Iannella, M. Updating Distribution, Ecology, and Hotspots for Three Amphibian Species to Set Conservation Priorities in A European Glacial Refugium. Land 2022, 11, 1292. [Google Scholar] [CrossRef]
- Joly, P.; Miaud, C. Fidelity to the breeding site in the alpine newt Triturus alpestris. Behav. Processes 1989, 19, 47–56. [Google Scholar] [CrossRef]
- Vilter, A.; Vilter, V. Migration de reproduction chez le triton alpestre des Alpes vaudoises. C. R. Soc. Biol. 1962, 156, 2005–2006. [Google Scholar]
- Jehle, R.; Sinsch, U. Wanderleistung und rientierung von Amphibien: Eine Übersicht. Z. Feldherpetologie 2007, 14, 137–152. [Google Scholar]
- Ghetti, P.L. I Macroinvertebrati nel Controllo Della Qualità Degli Ambienti di Acque Correnti; Provincia Autonoma di Trento: Trento, Italy, 1997; p. 222. [Google Scholar]
- Thornhill, I.; Batty, L.; Death, R.G.; Friberg, N.R.; Ledger, M.E. Local and landscape scale determinants of macroinvertebrate assemblages and their conservation value in ponds across an urban land-use gradient. Biodivers. Conserv. 2017, 26, 1065–1086. [Google Scholar] [CrossRef]
- Smith, G.R.; Vaala, D.A.; Dingfelder, H.A. Distribution and abundance of macroinvertebrates within two temporary ponds. Hydrobiologia 2003, 497, 161–167. [Google Scholar] [CrossRef]
- Motchié, F.E.; Konan, Y.A.; Koffi, K.B.; Etilé, N.D.R.; Gooré, B.G. Diversity and structure of benthic macroinvertebrates community in relation to environmental variables in Lake Ehuikro, Côte d’Ivoire. Int. J. Res. Environ. Stud. 2020, 7, 1–13. [Google Scholar]
- Hill, M.J.; Mathers, K.L.; Wood, P.J. The aquatic macroinvertebrate biodiversity of urban ponds in a medium-sized European town (Loughborough, UK). Hydrobiologia 2015, 760, 225–238. [Google Scholar] [CrossRef]
- Hill, M.J.; Sayer, C.D.; Wood, P.J. When is the best time to sample aquatic macroinvertebrates in ponds for biodiversity assessment? Environ. Monit. Assess. 2016, 188, 194. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W. Trophic relations of macroinvertebrates. In Methods in Stream Ecology; Hauer, F.R., Lamberti, G.A., Eds.; Academic Press: Burlington, NJ, USA, 2006; pp. 585–609. [Google Scholar]
- Fasola, M.; Canova, L. Feeding habits of Triturus vulgaris, T. cristatus and T. alpestris (Amphibia, Urodela) in the northern Apennines (Italy). Ital. J. Zool. 1992, 59, 273–280. [Google Scholar]
- Vignoli, L.; Bologna, M.A.; Luiselli, L. Seasonal patterns of activity and community structure in an amphibian assemblage at a pond network with variable hydrology. Acta Oecol. 2007, 31, 185–192. [Google Scholar] [CrossRef]
- Denoël, M.; Andreone, F. Trophic habits and aquatic microhabitat use in gilled immature, paedomorphic and metamorphic Alpine newts (Triturus alpestris apuanus) in a pond in central Italy. Belg. J. Zool. 2003, 133, 95–102. [Google Scholar]
- Salvidio, S.; Costa, A.; Crovetto, F. Individual trophic specialisation in the Alpine newt increases with increasing resource diversity. Ann. Zool. Fenn. 2019, 56, 17–24. [Google Scholar] [CrossRef]
- Careddu, G.; Carlini, N.; Romano, A.; Rossi, L.; Calizza, E.; Sporta Caputi, S.; Costantini, M.L. Diet composition of the Italian crested newt (Triturus carnifex) in structurally different artificial ponds based on stomach contents and stable isotope analyses. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1505–1520. [Google Scholar] [CrossRef]
- Schabetsberger, R.; Jersabek, C.D. Alpine newts (Triturus alpestris) as top predators in a high-altitude karst lake: Daily food consumption and impact on the copepod Arctodiaptomus alpinus. Freshw. Biol. 1995, 33, 47–61. [Google Scholar] [CrossRef]
- Aronsson, S.; Stenson, J.A. Newt–fish interactions in a small forest lake. Amphib. Reptil. 1995, 16, 177–184. [Google Scholar]
- Iannella, M.; Console, G.; D’Alessandro, P.; Cerasoli, F.; Mantoni, C.; Ruggieri, F.; Di Donato, F.; Biondi, M. Preliminary Analysis of the Diet of Triturus carnifex and Pollution in Mountain Karst Ponds in Central Apennines. Water 2020, 12, 44. [Google Scholar] [CrossRef]
- Solé, M.; Rödder, D. Dietary assessments of adult amphibians. In Amphibian Ecology and Conservation: A Handbook of Techniques; Dodd, J., Ed.; Oxford University Press: Oxford, UK, 2010; pp. 167–184. [Google Scholar]
- Romano, A.; Costa, A.; Basile, M. Skewed sex ratio in a forest salamander: Artefact of the different capture probabilities between sexes or actual ecological trait? Amphib. Reptil. 2018, 39, 79–86. [Google Scholar] [CrossRef]
- Kalezić, M.L.; Džukić, G.; Popadić, A. Paedomorphosis in Yugoslav Alpine newt (Triturus alpestris) populations: Morphometric variability and sex ratio. Arhiv. Bioloških. Nauka. 1989, 41, 67–79. [Google Scholar]
- Schabetsberger, R.; Goldschmid, A. Age structure and survival rate in alpine newts (Triturus alpestris). Alytes 1994, 12, 41–47. [Google Scholar]
- Naumov, B.Y.; Popgeorgiev, G.S.; Kornilev, Y.V.; Plachiyski, D.G.; Stojanov, A.J.; Tzankov, N.D. Distribution and Ecology of the Alpine Newt Ichthyosaura alpestris (Laurenti, 1768) (Amphibia: Salamandridae) in Bulgaria. Acta Zool. Bulg. 2020, 72, 83–102. [Google Scholar]
- Arntzen, J.W. Seasonal variation in sex ratio and asynchronous presence at ponds of male and female Triturus newts. J. Herpetol. 2002, 36, 30–35. [Google Scholar] [CrossRef]
- Sztatecsny, M.; Schabetsberger, R. Into thin air: Vertical migration, body condition, and quality of terrestrial habitats of alpine common toads, Bufo bufo. Can. J. Zool. 2005, 83, 788–796. [Google Scholar] [CrossRef]
- Bancila, R.I.; Hartel, T.; Plaiasu, R.; Smets, J.; Cogalniceanu, D. Comparing three body condition indices in amphibians: A case study of yellow-bellied toad Bombina variegata. Amphib. Reptil. 2010, 31, 558–562. [Google Scholar]
- Vilizzi, L.; Tarkan, A.S.; Copp, G.H. Experimental Evidence from Causal Criteria Analysis for the Effects of Common Carp Cyprinus carpio on Freshwater Ecosystems: A Global Perspective. Rev. Fish. Sci. Aquacult. 2015, 23, 253–290. [Google Scholar] [CrossRef]
- Kloskowski, J.; Nieoczym, M. Strong behavioral effects of omnivorous fish on Amphibian oviposition habitat selection: Potential consequences for ecosystem shifts. Front. Ecol. Evol. 2022, 10, 323. [Google Scholar] [CrossRef]
- Segev, O.; Mangel, M.; Blaustein, L. Deleterious effects by mosquitofish (Gambusia affinis) on the endangered fire salamander (Salamandra infraimmaculata). Anim. Conserv. 2009, 12, 29–37. [Google Scholar] [CrossRef]
- Vannini, A.; Bruni, G.; Ricciardi, G.; Platania, L.; Mori, E.; Tricarico, E. Gambusia holbrooki, the “tadpole fish”: The impact of its predatory behaviour on four protected species of European amphibians. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2018, 28, 476–484. [Google Scholar] [CrossRef]
- Gamradt, S.C.; Kats, L.B. Effect of introduced crayfish and mosquitofish on California newts. Conserv. Biol. 1996, 10, 1155–1162. [Google Scholar] [CrossRef]
- Monello, R.J.; Wright, R.G. Predation by goldfish (Carassius auratus) on eggs and larvae of the eastern long-toed salamander (Ambystoma macrodactylum columbianum). J. Herpetol. 2001, 35, 350–353. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Blaustein, A.R.; Miller, C.L. Transfer of a pathogen from fish to amphibians. Conserv. Biol. 2001, 15, 1064–1070. [Google Scholar] [CrossRef]
- Fasola, M. Resource partitioning by three species of newts during their aquatic phase. Ecography 1993, 16, 73–81. [Google Scholar] [CrossRef]
- Denoël, M.; Duguet, R.; Džukić, G.; Kalezić, M.L.; Mazzotti, S. Biogeography and ecology of paedomorphosis in Triturus alpestris (Amphibia, Caudata). J. Biogeogr. 2001, 28, 1271–1280. [Google Scholar] [CrossRef]
- Denoël, M.; Džukić, G.; Kalezić, M.L. Effect of widespread fish introductions on paedomorphic newts in Europe. Conserv. Biol. 2005, 19, 162–170. [Google Scholar] [CrossRef]
- Toli, E.A.; Chavas, C.; Denoël, M.; Bounas, A.; Sotiropoulos, K. A subtle threat: Behavioral and phenotypic consequences of invasive mosquitofish on a native paedomorphic newt. Biol. Invasions 2020, 22, 1299–1308. [Google Scholar] [CrossRef]
- Fried, L.M.; Boyer, M.C.; Brooks, M.J. Amphibian response to rotenone treatment of ten alpine lakes in Northwest Montana. N. Am. J. Fish. Manag. 2018, 38, 237–246. [Google Scholar] [CrossRef]
- Schnee, M.E.; Clancy, N.G.; Boyer, M.C.; Bourret, S.L. Recovery of freshwater invertebrates in alpine lakes and streams following eradication of non-native trout with rotenone. J. Fish Wildl. Manag. 2021, 12, 475–484. [Google Scholar] [CrossRef]
- Tiberti, R.; Bogliani, G.; Brighenti, S.; Iacobuzio, R.; Liautaud, K.; Rolla, M.; von Hardenberg, A.; Bassano, B. Recovery of high mountain Alpine lakes after the eradication of introduced brook trout Salvelinus fontinalis using non-chemical methods. Biol. Invasions 2019, 21, 875–894. [Google Scholar] [CrossRef]
- Denoël, M.; Winandy, L. The importance of phenotypic diversity in conservation: Resilience of palmate newt morphotypes after fish removal in Larzac ponds (France). Biol. Conserv. 2015, 192, 402–408. [Google Scholar] [CrossRef]
- Miró, A.; O’Brien, D.; Tomàs, J.; Buchaca, T.; Sabás, I.; Osorio, V.; Lucati, F.; Pou-Rovira, Q.; Ventura, M. Rapid amphibian community recovery following removal of non-native fish from high mountain lakes. Biol. Conserv. 2020, 251, 108783. [Google Scholar] [CrossRef]
- Denoël, M.; Scimè, P.; Zambelli, N. Newt life after fish introduction: Extirpation of paedomorphosis in a mountain fish lake and newt use of satellite pools. Curr. Zool. 2016, 62, 61–69. [Google Scholar] [CrossRef]
- Recuero, E.; Buckley, D.; García-París, M.; Arntzen, J.W.; Cogălniceanu, D.; Martínez-Solano, I. Evolutionary history of Ichthyosaura alpestris (Caudata, Salamandridae) inferred from the combined analysis of nuclear and mitochondrial markers. Mol. Phylogenet. Evol. 2014, 81, 207–220. [Google Scholar] [CrossRef]
- Casacci, L.P.; Barbero, F.; Balletto, E. The “Evolutionarily Significant Unit” concept and its applicability in biological conservation. Ital. J. Zool. 2014, 81, 182–193. [Google Scholar] [CrossRef]
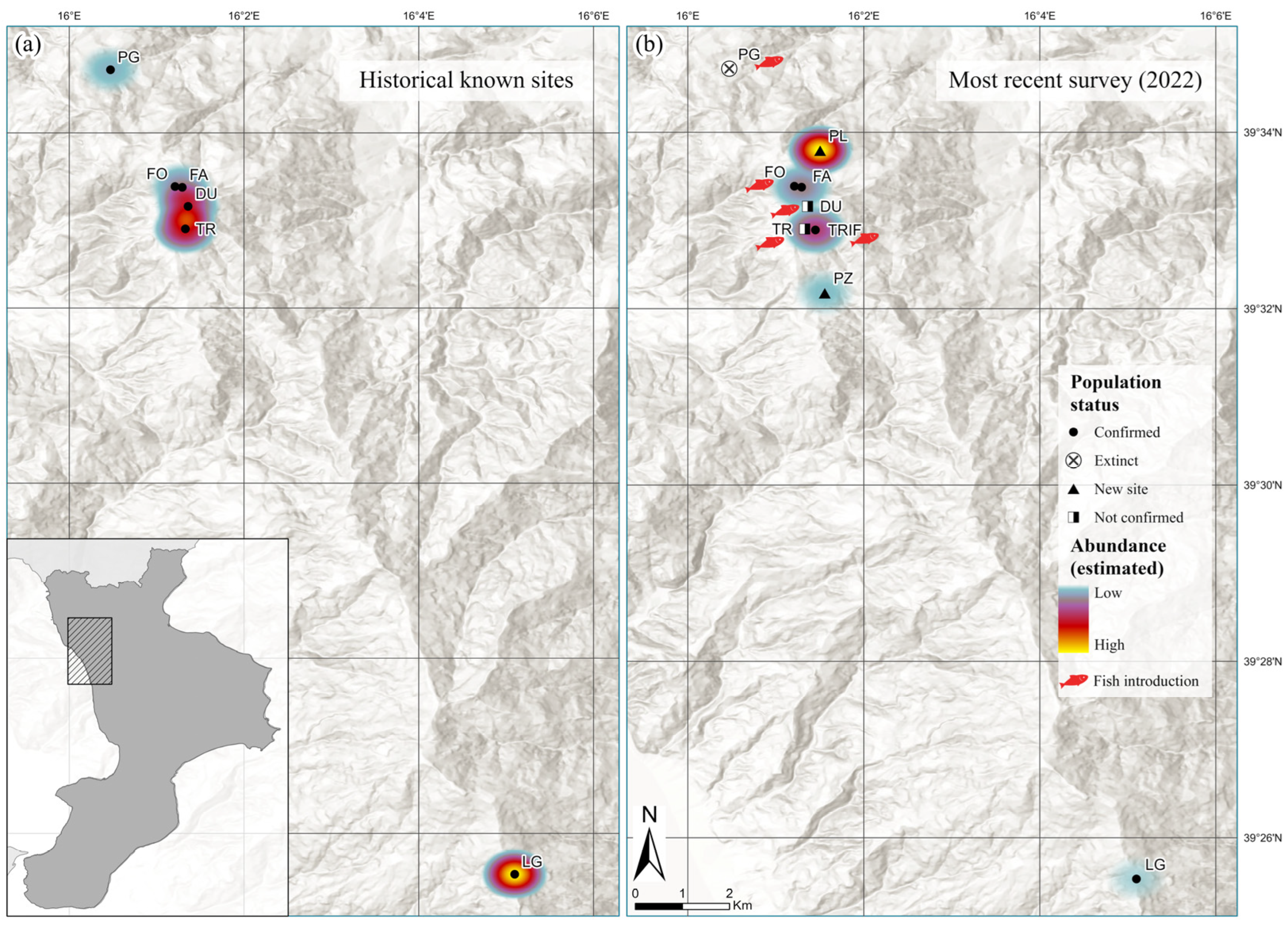

| Site Code N2000 | Municipality | Locality | Year of Last Observation | Maximum Number Captured/Observed (1983–2018) | References |
|---|---|---|---|---|---|
| IT9310058 Pantano della Giumenta | Malvito | Stagno C/Pantano dorato | 1983 | 4 | [24,25] |
| IT9310060 Laghi di Fagnano | Fagnano Castello | Trifoglietti (pond TR and pond TRIF) | 2018 (TR) and 2022 (TRIF) | 23 | [22,23,24,25,26] |
| Due Uomini | 2018 | 21 | |||
| Fonnente (lake FO and pond FA) | 2022 | 25 | [25,26] | ||
| IT9310061 Laghicello | San Benedetto Ullano | Laghicello | 2022 | 69 | [22,23,24,25,27] |
| Site Name | |||||||
|---|---|---|---|---|---|---|---|
| FO | DU | TR | TRIF | FA | PZ | PL | |
| Lat. N Long. E | 39°33′24″ 16°1′13″ | 39°33′9″ 16°1′22″ | 39°32′54″ 16°1′2″ | 39°32′53″ 16°1′27″ | 39°33′23″ 16°1′18″ | 39°32′11″ 16°1′34″ | 39°33′48″ 16°1′30″ |
| Altitude (m a.s.l.) | 1050 | 1077 | 1048 | 1045 | 1055 | 880 | 1010 |
| Area (m2) | 3561 | 20,679 | 10,800 | 520 | 827 | 742 1 | 235 1 |
| Depth (m) | 1 | - | 1.5 2 | 0.9 | 1.2 | 0.7 | 1.1 |
| Permanence | P/T 3 | P | P | P | T | T | P |
| Shading (%) | 20 | 20 | 30 | 80 | 20 | 90 | 90 |
| Fish presence | Carassius sp. | Cyprinus carpio | G. holbrooki | G. holbrooki | - | - | - |
| Aquatic vegetation | Tla, Pna | Pna, Pau, Cve | Sau, Cro, Cpa, Jef, Lvu, Eca, Epa, Pna | Cve, Jef | Jef | absent | absent |
| I. a. inexpectata | L | - | - | A, L, P | A, L | A, L | A, L, OL |
| Other breeding species | Tca, Lit, Pes, Rda, Hin | Tca, Bbu, Pes, Rda | Hin, Pes, Rda | Lit, Tca, Ssa, Pes, Rda, Rit, Bbu | Lit, Tca, Ssa, Hin, Pes, Rda, Rit | Lit, Tca, Bbu, Rda | Lit, Ssa, Rda, Rit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernabò, I.; Iannella, M.; Cittadino, V.; Corapi, A.; Romano, A.; Andreone, F.; Biondi, M.; Gallo Splendore, M.; Tripepi, S. Survived the Glaciations, Will They Survive the Fish? Allochthonous Ichthyofauna and Alpine Endemic Newts: A Road Map for a Conservation Strategy. Animals 2023, 13, 871. https://doi.org/10.3390/ani13050871
Bernabò I, Iannella M, Cittadino V, Corapi A, Romano A, Andreone F, Biondi M, Gallo Splendore M, Tripepi S. Survived the Glaciations, Will They Survive the Fish? Allochthonous Ichthyofauna and Alpine Endemic Newts: A Road Map for a Conservation Strategy. Animals. 2023; 13(5):871. https://doi.org/10.3390/ani13050871
Chicago/Turabian StyleBernabò, Ilaria, Mattia Iannella, Viviana Cittadino, Anna Corapi, Antonio Romano, Franco Andreone, Maurizio Biondi, Marcellino Gallo Splendore, and Sandro Tripepi. 2023. "Survived the Glaciations, Will They Survive the Fish? Allochthonous Ichthyofauna and Alpine Endemic Newts: A Road Map for a Conservation Strategy" Animals 13, no. 5: 871. https://doi.org/10.3390/ani13050871
APA StyleBernabò, I., Iannella, M., Cittadino, V., Corapi, A., Romano, A., Andreone, F., Biondi, M., Gallo Splendore, M., & Tripepi, S. (2023). Survived the Glaciations, Will They Survive the Fish? Allochthonous Ichthyofauna and Alpine Endemic Newts: A Road Map for a Conservation Strategy. Animals, 13(5), 871. https://doi.org/10.3390/ani13050871









