Immunity and Growth Plasticity of Asian Short-Toed Lark Nestlings in Response to Changes in Food Conditions: Can It Buffer the Challenge of Climate Change-Induced Trophic Mismatch?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
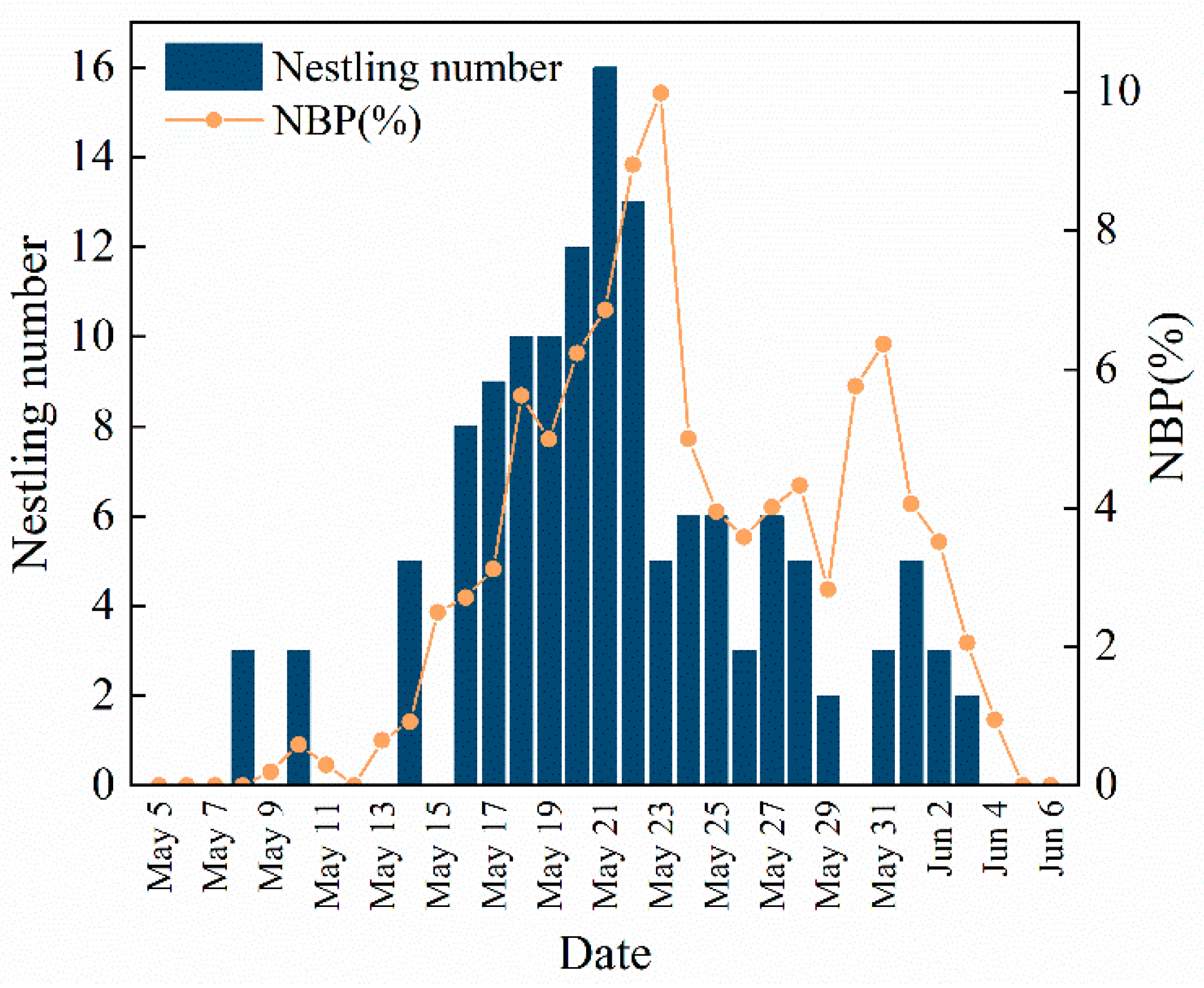
2.2. Data Collection
2.3. Nestling Blood Samples
2.4. Cytokine Gene Expression Analysis
2.5. Plasma IGF-1 Analysis
2.6. Statistical Analysis
3. Results
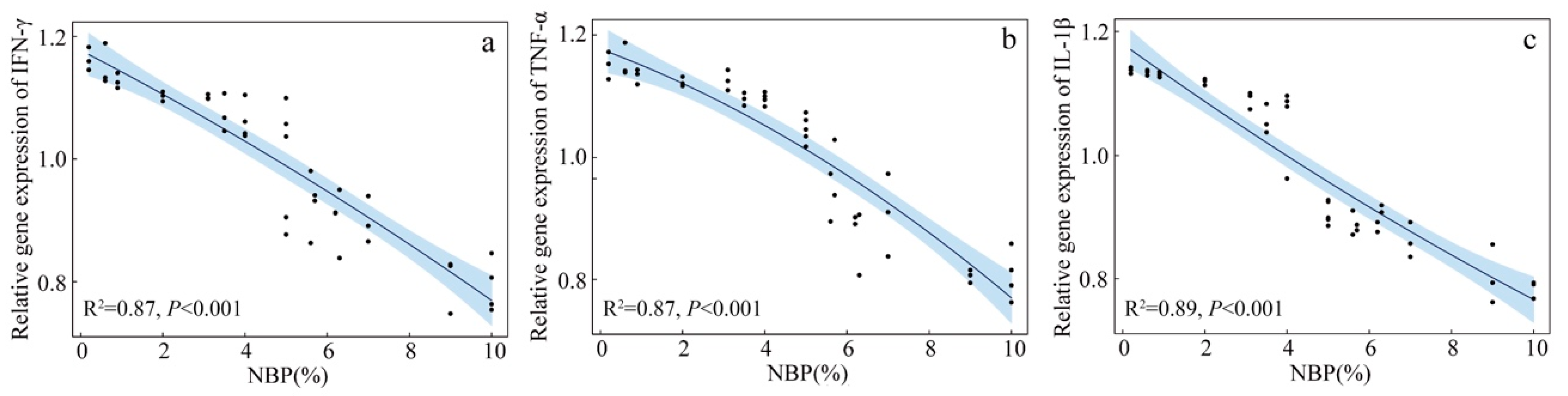
3.1. Blood Cell Cytokine Gene Expression of Nestlings
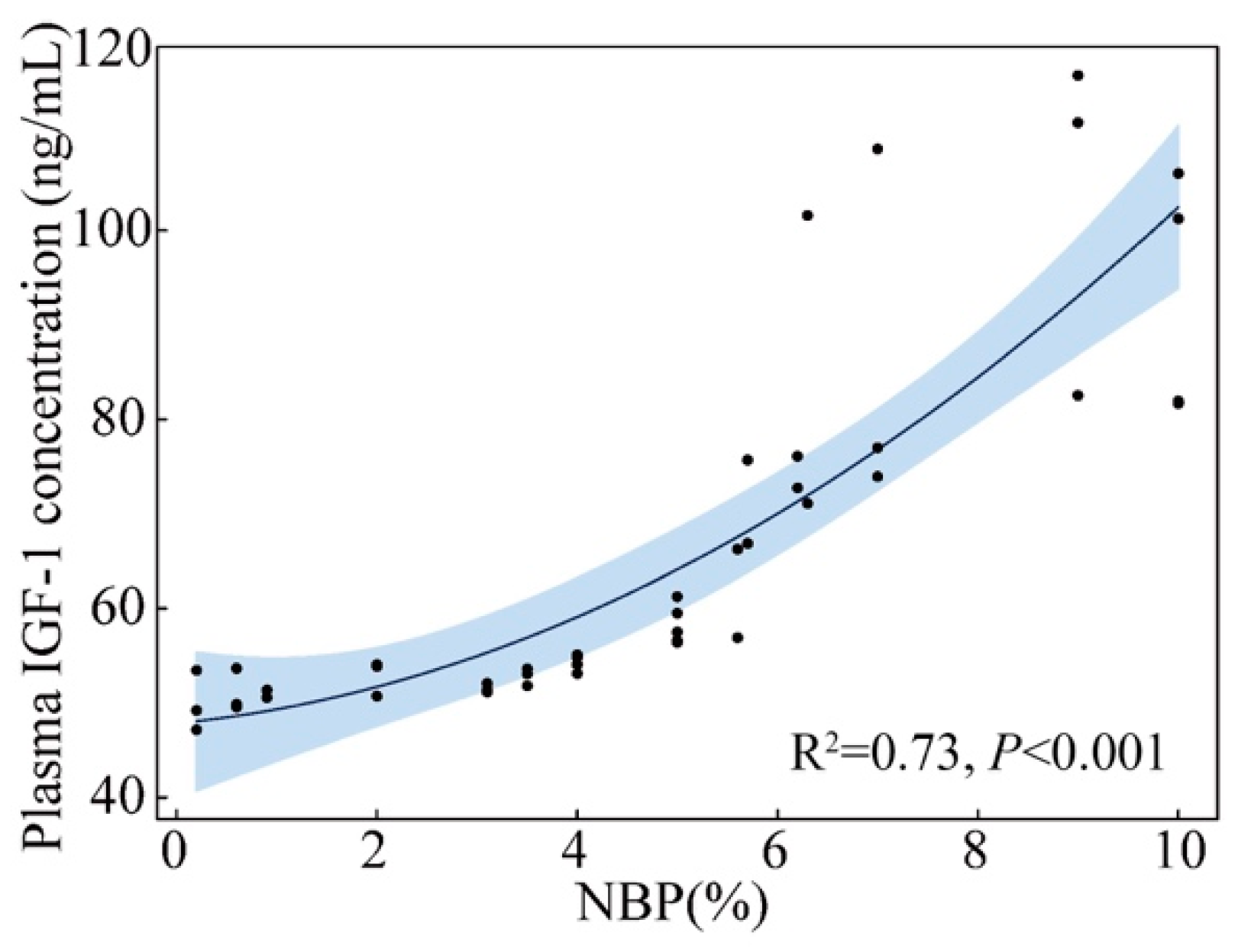
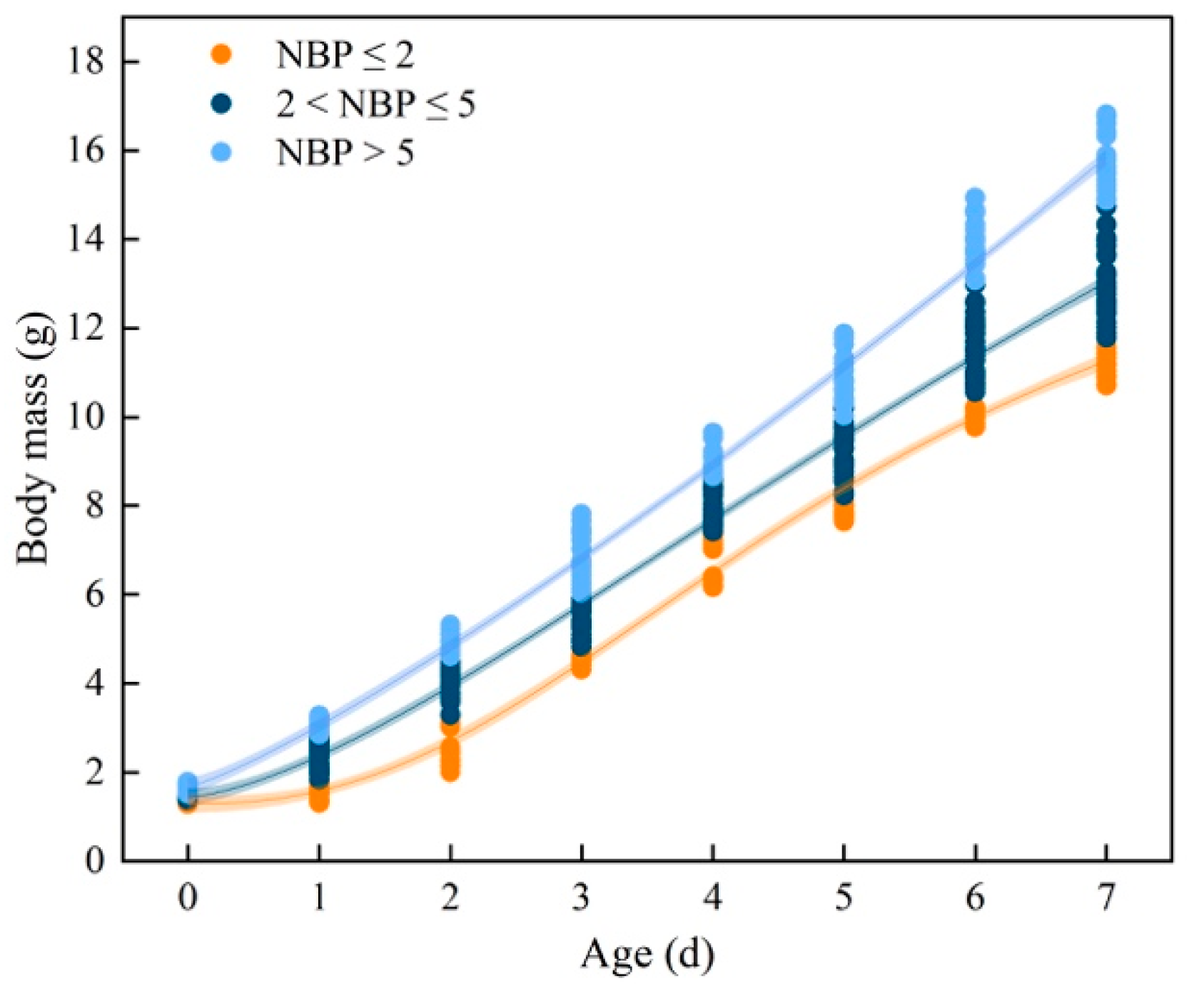
3.2. Plasma IGF-1 Levels and Body Mass of Nestlings
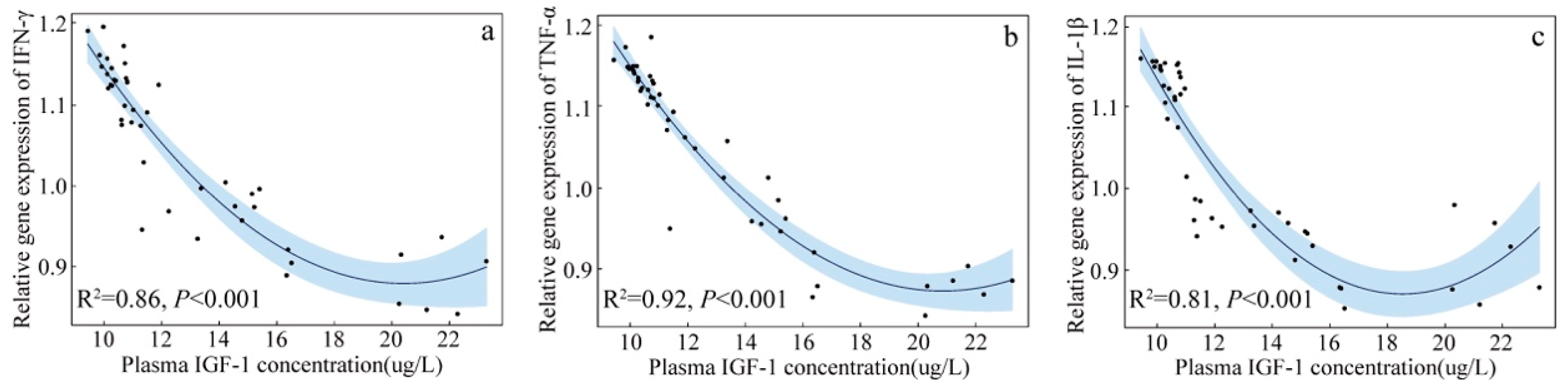
3.3. Correlation between Blood Cell Cytokine Gene Expression and Plasma IGF-1 of Nestlings
3.4. Fledge Rate of Nestlings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, L.F.; Van Noordwijk, A.J. A method to isolate environmental effects on nestling growth, illustrated with examples from the Great Tit (Parus major). Funct. Ecol. 1993, 7, 493–502. [Google Scholar] [CrossRef]
- Dias, P.C.; Blondel, J. Breeding time, food supply and fitness components of Blue Tits (Parus caeruleus) in Mediterranean habitats. Ibis 1996, 38, 644–649. [Google Scholar] [CrossRef]
- Naef-Daenzer, B.; Keller, L.F. The foraging performance of great and blue tits (Parus major and P. caeruleus) in relation to cater-pillar development, and its consequences for nestling growth and fledging weight. J. Anim. Ecol. 1999, 68, 708–718. [Google Scholar] [CrossRef]
- Rossmanith, E.; Höntsch, K.; Blaum, N.; Jeltsch, F. Reproductive success and nestling diet in the Lesser spotted woodpecker (Picoides minor): The early bird gets the caterpillar. J. Ornithol. 2007, 148, 323–332. [Google Scholar] [CrossRef]
- Murray, M.H.; Kidd, A.D.; Curry, S.E.; Hepinstall, J.A.; Yabsley, M.; Adams, H.C.; Ellison, T.; Welch, C.; Hernandez, S.M. From wetland specialist to hand-fed generalist: Shifts in diet and condition with provisioning for a recently urbanized wading bird. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170100. [Google Scholar] [CrossRef]
- Buse, A.; Dury, S.; Woodburn, R.J.W.; Perrins, C.M.; Good, J.E.G. Effects of elevated temperature on multi-species interactions: The case of Pedunculate Oak, Winter Moth and Tits. Funct. Ecol. 1999, 13, 74–82. [Google Scholar] [CrossRef]
- Visser, M.E.; van Noordwijk, A.J.; Tinbergen, J.M.; Lessells, C.M. Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. B-Biol. Sci. 1998, 265, 1867–1870. [Google Scholar] [CrossRef]
- van Gils, J.A.; Lisovski, S.; Lok, T.; Meissner, W.; Ożarowska, A.; de Fouw, J.; Rakhimberdiev, E.; Soloviev, M.Y.; Piersma, T.; Klaassen, M. Body shrinkage due to Arctic warming reduces red knot fitness in tropical wintering range. Science 2016, 352, 819–821. [Google Scholar] [CrossRef]
- Both, C.; Bouwhuis, S.; Lessells, C.M.; Visser, M.E. Climate change and population declines in a long-distance migratory bird. Nature 2006, 441, 81–83. [Google Scholar] [CrossRef]
- Miller-Rushing, A.J.; Høye, T.T.; Inouye, D.W.; Post, E. The effects of phenological mismatches on demography. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3177–3186. [Google Scholar] [CrossRef]
- Saino, N.; Ambrosini, R.; Rubolini, D.; von Hardenberg, J.; Provenzale, A.; Hüppop, K.; Hüppop, O. Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proc. R. Soc. B-Biol. Sci. 2011, 278, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.E.; Both, C. Shifts in phenology due to global climate change: The need for a yardstick. Proc. R. Soc. B-Biol. Sci. 2005, 272, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- de Camargo, N.F.; de Camargo, W.R.F.; Corrêa, D.C.V.; de Camargo, A.J.A.; Vieira, E.M. Adult feeding moths (Sphingidae) differ from non-adult feeding ones (Saturniidae) in activity-timing overlap and temporal niche width. Oecologia 2016, 180, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Garcia, A.; Williams, J.B. Developmental plasticity of cutaneous water loss and lipid composition in stratum corneum of desert and mesic nestling house sparrows. Proc. Natl. Acad. Sci. USA 2008, 105, 15611–15616. [Google Scholar] [CrossRef]
- Honarmand, M.; Goymann, W.; Naguib, M. Stressful Dieting: Nutritional conditions but not compensatory growth elevate corticosterone levels in Zebra finch nestlings and fledglings. PLoS ONE 2010, 5, e12930. [Google Scholar] [CrossRef]
- Killpack, T.L.; Karasov, W.H. Growth and development of house sparrows (Passer domesticus) in response to chronic food restriction throughout the nestling period. J. Exp. Biol. 2012, 215, 1806–1815. [Google Scholar] [CrossRef]
- Gatica-Sosa, C.; Brzezk, P.; Chediack, J.G.; Cid, F.D.; Karasov, W.H.; Caviedes-Vidal, E. Differential transcriptional responses underlie dietary induction of intestinal carbohydrase activities in house sparrow nestlings. J. Anim. Physiol. Anim. Nutr. 2016, 100, 236–242. [Google Scholar] [CrossRef]
- Nelson, R.J.; Demas, G.E. Seasonal changes in immune function. Q. Rev. Biol. 1996, 71, 512–548. [Google Scholar] [CrossRef]
- Martin, L.B.; Weil, Z.M.; Nelson, R.J. Seasonal changes in vertebrate immune activity: Mediation by physiological trade-offs. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 321–339. [Google Scholar] [CrossRef]
- Harshman, L.G.; Zera, A.J. The cost of reproduction: The devil in the details. Trends Ecol. Evol. 2006, 22, 80–86. [Google Scholar] [CrossRef]
- Knowles, S.C.L.; Nakagawa, S.; Sheldon, B.C. Elevated reproductive effort increases blood parasitaemia and decreases immune function in birds: A meta-regression approach. Funct. Ecol. 2009, 23, 405–415. [Google Scholar] [CrossRef]
- Sheldon, B.C.; Verhulst, S. Ecological immunology: Costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996, 11, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Owens, I.; Wilson, K. Immunocompetence: A neglected life history trait or conspicuous red herring? Trends Ecol. Evol. 1999, 14, 170–172. [Google Scholar] [CrossRef]
- Saino, N.; Calza, S.; Møller, A.P. Effects of a dipteran ectoparasite on immune response and growth trade-offs in barn swallow, Hirundo rustica, nestling. Oikos 1998, 81, 217–228. [Google Scholar] [CrossRef]
- Fair, J.M.; Hansen, E.S.; Ricklefs, R.E. Growth, developmental stability and immune response in juvenile Japanese quails (Coturnix coturnixjaponica). Proc. R. Soc. B-Biol. Sci. 1999, 266, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Lochmiller, R.L.; Deerenberg, C. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos 2000, 88, 87–98. [Google Scholar] [CrossRef]
- Hoi-Leitner, M.; Romero-Pujante, M.; Hoi, H.; Pavlova, A. Food availability and immune capacity in serin (Serinus serinus) nestlings. Behav. Ecol. Sociobiol. 2001, 49, 333–339. [Google Scholar] [CrossRef]
- Soler, J.J.; Neve, L.D.; Pérez-Contreras, T.; Soler, M.; Sorci, G. Trade off between immunocompetence and growth in magpies: An experimental study. Proc. R. Soc. B-Biol. Sci. 2003, 270, 241–248. [Google Scholar] [CrossRef]
- Eraud, C.; Jacquet, A.; Faivre, B. Survival cost of an early immune soliciting in nature. Evolution 2009, 63, 1036–1043. [Google Scholar] [CrossRef]
- Brommer, J.E. Immunocompetence and its costs during development: An experimental study in blue tit nestlings. Proc. R. Soc. B-Biol. Sci. 2004, 271, S110–S113. [Google Scholar] [CrossRef]
- Brzek, P.; Konarzewski, M. Relationship between avian growth rate and immune response depends on food availability. J. Exp. Biol. 2007, 210, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Killpack, T.L.; Carrel, E.; Karasov, W.H. Impacts of short-term food restriction on immune development in altricial House sparrow nestlings. Physiol. Biochem. Zool. 2015, 88, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef]
- Azevedo, Z.M.A.; Luz, R.A.; Victal, S.H.; Kurdian, B.; Fonseca, V.M.; Fitting, C.; Câmara, E.P. Increased production of tumor necrosis factor-α in whole blood cultures from children with primary malnutrition. Braz. J. Med. Biol. Res. 2005, 38, 171–183. [Google Scholar] [CrossRef]
- Venkatraman, J.T.; Pendergast, D.R. Effect of dietary intake on immune function in athletes. Sports Med. 2002, 32, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Monteleone, M.; Stow, J.L.; Schroder, K. Mechanisms of unconventional secretion of IL-1 family cytokines. Cytokine 2015, 74, 213–218. [Google Scholar] [CrossRef]
- Peng, C.; Sun, J.; Dai, L.; Shan, A. Effects of recombinant chicken Interferon-γ on growth performance immune function of broilers. China Poult. 2011, 33, 11–14. [Google Scholar]
- Lupu, F.; Terwilliger, J.D.; Lee, K.; Segre, G.V.; Efstratiadis, A. Roles of growth hormone and Insulin-like Growth Factor 1 in mouse postnatal growth. Dev. Biol. 2001, 229, 141–162. [Google Scholar] [CrossRef]
- Schlueter, P.J.; Sang, X.; Duan, C.; Wood, A.W. Insulin-like growth factor receptor 1b is required for zebrafish primordial germ cell migration and survival. Dev. Biol. 2007, 305, 377–387. [Google Scholar] [CrossRef]
- Stratikopoulos, E.; Szabolcs, M.; Dragatsis, I.; Klinakis, A.; Efstratiadis, A. The hormonal action of IGF-1 in postnatal mouse growth. Proc. Natl. Acad. Sci. USA 2008, 105, 19378–19383. [Google Scholar] [CrossRef] [PubMed]
- Lodjak, J.; Mägi, M.; Tilgar, V. Insulin-like growth factor 1 and growth rate in nestlings of a wild passerine bird. Funct. Ecol. 2014, 28, 159–166. [Google Scholar] [CrossRef]
- Lodjak, J.; Mägi, M.; Sild, E.; Mänd, R. Causal link between insulin-like growth factor 1 and growth in nestlings of a wild passerine bird. Funct. Ecol. 2017, 31, 184–191. [Google Scholar] [CrossRef]
- Fingar, D.C.; Blenis, J. Target of rapamycin (TOR): An integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 2004, 23, 3151–3171. [Google Scholar] [CrossRef] [PubMed]
- Hack, N.L.; Cordova, K.L.; Glaser, F.L.; Journey, M.L.; Resner, E.J.; Hardy, K.M.; Beckman, B.R.; Lema, S.C. Interactions of long-term food ration variation and short-term fasting on insulin-like growth factor-1 (IGF-1) pathways in copper rockfish (Sebastes caurinus). Gen. Comp. Endocrinol. 2019, 280, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.D.; Janszen, A.W.; Wouterlood, F.G.; Tilders, F.J. Interleukin-1-induced long-lasting changes in hypothalamic corticotropin-releasing hormone (CRH)-neurons and hyperre-sponsiveness of the hypothalamus-pituitary-adrenal axis. J. Neurosci. 1995, 15, 7417–7426. [Google Scholar] [CrossRef]
- Klasing, K.C.; Korver, D.R. Leukocytic cytokines regulate growth rate and composition following activation of the immune system. J. Anim. Sci. 1997, 75, 58–67. [Google Scholar]
- Johnson, J.D.; O’Connor, K.A.; Watkins, L.R.; Maier, S.F. The role of IL-1β in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience 2004, 127, 569–577. [Google Scholar] [CrossRef]
- Lodjak, J.; Tilgar, V.; Mägi, M. Does the interaction between glucocorticoids and insulin-like growth factor 1 predict nestling fitness in a wild passerine? Gen. Comp. Endocrinol. 2016, 225, 149–154. [Google Scholar] [CrossRef]
- Verhulst, S.; Tinbergen, J.M.; Daan, S. Multiple breeding in the Great Tit. A trade-off between successive reproductive attempts? Funct. Ecol. 2010, 11, 714–722. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, L.; Yang, W.; Xu, X.; Wang, W.; Liang, W.; Zhang, S. Do migrant and resident species differ in the timing of increases in reproductive and thyroid hormone secretion and body mass? A case study in the comparison of pre-breeding physiological rhythms in the Eurasian Skylark and Asian Short-toed Lark. Avian Res. 2017, 8, 101–109. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Wang, W.; Yang, W.; Liang, W. Clock gene is associated with individual variation in the activation of reproductive endocrine and behavior of Asian short toed lark. Sci. Rep. 2017, 7, 15002. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, Y.; Osmond, H.L.; Cockburn, A.; Kruuk, L.E.B. When to start and when to stop: Effects of climate on breeding in a multi-brooded songbird. Glob. Chang. Biol. 2019, 26, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.; Presley, S.J. Niche overlap and temporal activity patterns of social wasps (Hymenoptera: Vespidae) in a Brazilian cashew orchard. Sociobiology 2010, 56, 121–131. [Google Scholar]
- Mckinnon, L.; Picotin, M.; Bolduc, E.; Juillet, C.; Bêty, J. Timing of breeding, peak food availability, and effects of mismatch on chick growth in birds nesting in the High Arctic. Can. J. Zool. 2012, 90, 961–971. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, L.; Zhang, X.; Liang, W. Predicting the vulnerability of birds to trophic threat posed by phenological mismatch based on nutritional and physiological status of nestlings. Conserv. Physiol. 2019, 7, coz096. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, X.; Wang, W.; Zhao, L.; Gao, L.; Yang, W. Annual variation in the reproductive hormone and behavior rhythm in a population of the Asian short-toed lark: Can spring temperature influence activation of the HPG axis of wild birds? Horm. Behav. 2017, 95, 76–84. [Google Scholar] [CrossRef]
- Durant, S.E.; Hopkins, W.A.; Hepp, G.R.; Walters, J.R. Ecological, evolutionary, and conservation implications of incubation temperature-dependent phenotypes in birds. Biol. Rev. 2013, 88, 499–509. [Google Scholar] [CrossRef]
- Sparkman, A.M.; Vleck, C.M.; Bronikowski, A.M. Evolutionary ecology of endocrine-mediated life-history variation in the garter snake Thamnophis elegans. Ecology 2009, 90, 720–728. [Google Scholar] [CrossRef]
- Thapa, B.; Lee, K. Metabolic influence on macrophage polarization and pathogenesis. BMB Rep. 2019, 52, 360–372. [Google Scholar] [CrossRef]
- Semba, H.; Takeda, N.; Isagawa, T.; Sugiura, Y.; Honda, K.; Wake, M.; Miyazawa, H. HIF-1α-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat. Commun. 2016, 7, 11635. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, Q.; Rong, P.; Wang, H.Y.; Chen, S. The energy sensing LKB1-AMPKα1 pathway regulates IGF1 secretion and consequent activation of the IGF1R-PKB pathway in primary hepatocytes. FEBS J. 2017, 284, 2096–2109. [Google Scholar] [CrossRef] [PubMed]
- Hasselquist, D.; Nilsson, J.A. Physiological mechanisms mediating costs of immune responses: What can we learn from studies of birds? Anim. Behav. 2012, 83, 1303–1312. [Google Scholar] [CrossRef]
- Iseri, V.J.; Klasing, K.C. Dynamics of the systemic components of the chicken (Gallus gallus domesticus) immune system following activation by Escherichia coli: Implications for the costs of immunity. Dev. Comp. Immunol. 2013, 40, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Saino, N.; Calza, S.; Møller, A.P. Immunocompetence of nestling Barn swallows in relation to brood size and parental effort. J. Anim. Ecol. 1997, 66, 827–836. [Google Scholar] [CrossRef]
- Straile, D.; Kerimoglu, O.; Peeters, F. Trophic mismatch requires seasonal heterogeneity of warming. Ecology 2015, 96, 2794–2805. [Google Scholar] [CrossRef]
- Shipley, J.R.; Twining, C.W.; Taff, C.C.; Vitousek, M.N.; Flack, A.; Winkler, D.W. Birds advancing lay dates with warming springs face greater risk of chick mortality. Proc. Natl. Acad. Sci. USA 2020, 117, 25590–25594. [Google Scholar] [CrossRef]
- Kitaysky, A.S.; Wingfield, J.C.; Piatt, J.F. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav. Ecol. 2001, 12, 619–625. [Google Scholar] [CrossRef]
- Brzek, P.; Konarzewski, M. Effect of food shortage on the physiology and competitive abilities of sand martin (Riparia riparia) nestlings. J. Exp. Biol. 2001, 204, 3065–3074. [Google Scholar] [CrossRef]
- Gil, D.; Bulmer, E.; Celis, P.; Lo’pez-Rull, I. Adaptive developmental plasticity in growing nestlings: Sibling competition induces differential gape growth. Proc. R. Soc. B-Biol. Sci. 2008, 275, 549–554. [Google Scholar] [CrossRef]
- Lodjak, J.; Mägi, M.; Rooni, U.; Tilgar, V. Context-dependent effects of feather corticosterone on growth rate and fledging success of wild passerine nestlings in heterogeneous habitat. Oecologia 2015, 179, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Saino, N.; Incagli, M.; Martinelli, R.; Ambrosini, R.; Møller, A.P. Immunity, growth and begging behavior of nestling Barn Swallows Hirundo rustica in relation to hatching order. J. Avian Biol. 2001, 32, 263–270. [Google Scholar] [CrossRef]
- Sternalski, A.; Mougeot, F.; Bretagnolle, V. Phenotypic variation in nestlings of a bird of prey under contrasting breeding and diet conditions. Biol. J. Linn. Soc. 2012, 107, 799–812. [Google Scholar] [CrossRef]
- Loiseau, C.; Sorci, G.; Dano, S.; Chastel, O. Effects of experimental increase of corticosterone levels on begging behavior, immunity and parental provisioning rate in house sparrows. Gen. Comp. Endocr. 2008, 155, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ancona, S.; Drummond, H. Life history plasticity of a tropical seabird in response to El Niño anomalies during early life. PLoS ONE 2013, 8, e72665. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer | Size (bp) | Accession No. |
|---|---|---|---|
| TNF-α | F:5-CCGCCCAGTTCAGATGAGTT-3 R:5-GCAACAACCAGCTATGCACC-3 | 130 | MF000729.1 |
| IFN-γ | F:5-TGAGCCAGATTGTTTCGATG-3 R: 5-CTTGGCCAGGTCCATGATA-3 | 248 | NM_205149.1 |
| IL-1β | F:5-ACTGGGCATCAAGGGCTACA-3 R:5-GCTGTCCAGGCGGTAGAAGA-3 | 142 | NM_204524.1 |
| GAPDH | F:5-CACTGTCAAGGCTGAGAACG-3 R:5-GATAACACGCTTAGCACCA-3 | 187 | NM_204305.1 |
| Response Variable | Explanatory Variable | F | p |
|---|---|---|---|
| IFN-γ | NBP | 64.015 | <0.001 |
| Nest temperature | 1.004 | 0.323 | |
| NBP × Nest temperature | 0.262 | 0.611 | |
| TNF-α | NBP | 59.585 | <0.001 |
| Nest temperature | 3.343 | 0.075 | |
| NBP × Nest temperature | 0.049 | 0.826 | |
| IL-1β | NBP | 53.372 | <0.001 |
| Nest temperature | 3.584 | 0.066 | |
| NBP × Nest temperature | 2.578 | 0.117 | |
| IGF-1 | NBP | 20.675 | <0.001 |
| Nest temperature | 2.342 | 0.134 | |
| NBP × Nest temperature | 0.001 | 0.970 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, G.; Zhang, X.; Li, X.; Zhang, S. Immunity and Growth Plasticity of Asian Short-Toed Lark Nestlings in Response to Changes in Food Conditions: Can It Buffer the Challenge of Climate Change-Induced Trophic Mismatch? Animals 2023, 13, 860. https://doi.org/10.3390/ani13050860
Lu G, Zhang X, Li X, Zhang S. Immunity and Growth Plasticity of Asian Short-Toed Lark Nestlings in Response to Changes in Food Conditions: Can It Buffer the Challenge of Climate Change-Induced Trophic Mismatch? Animals. 2023; 13(5):860. https://doi.org/10.3390/ani13050860
Chicago/Turabian StyleLu, Guang, Xinjie Zhang, Xinyu Li, and Shuping Zhang. 2023. "Immunity and Growth Plasticity of Asian Short-Toed Lark Nestlings in Response to Changes in Food Conditions: Can It Buffer the Challenge of Climate Change-Induced Trophic Mismatch?" Animals 13, no. 5: 860. https://doi.org/10.3390/ani13050860
APA StyleLu, G., Zhang, X., Li, X., & Zhang, S. (2023). Immunity and Growth Plasticity of Asian Short-Toed Lark Nestlings in Response to Changes in Food Conditions: Can It Buffer the Challenge of Climate Change-Induced Trophic Mismatch? Animals, 13(5), 860. https://doi.org/10.3390/ani13050860







