Diversity of Underwater Vocalizations in Chinese Soft-Shelled Turtle (Pelodiscus sinensis)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials & Methods
2.1. Data Collection
2.2. Data Analysis
2.3. Statistical Analyses
3. Results
3.1. Description of Call Types
3.2. Similarity Calculation
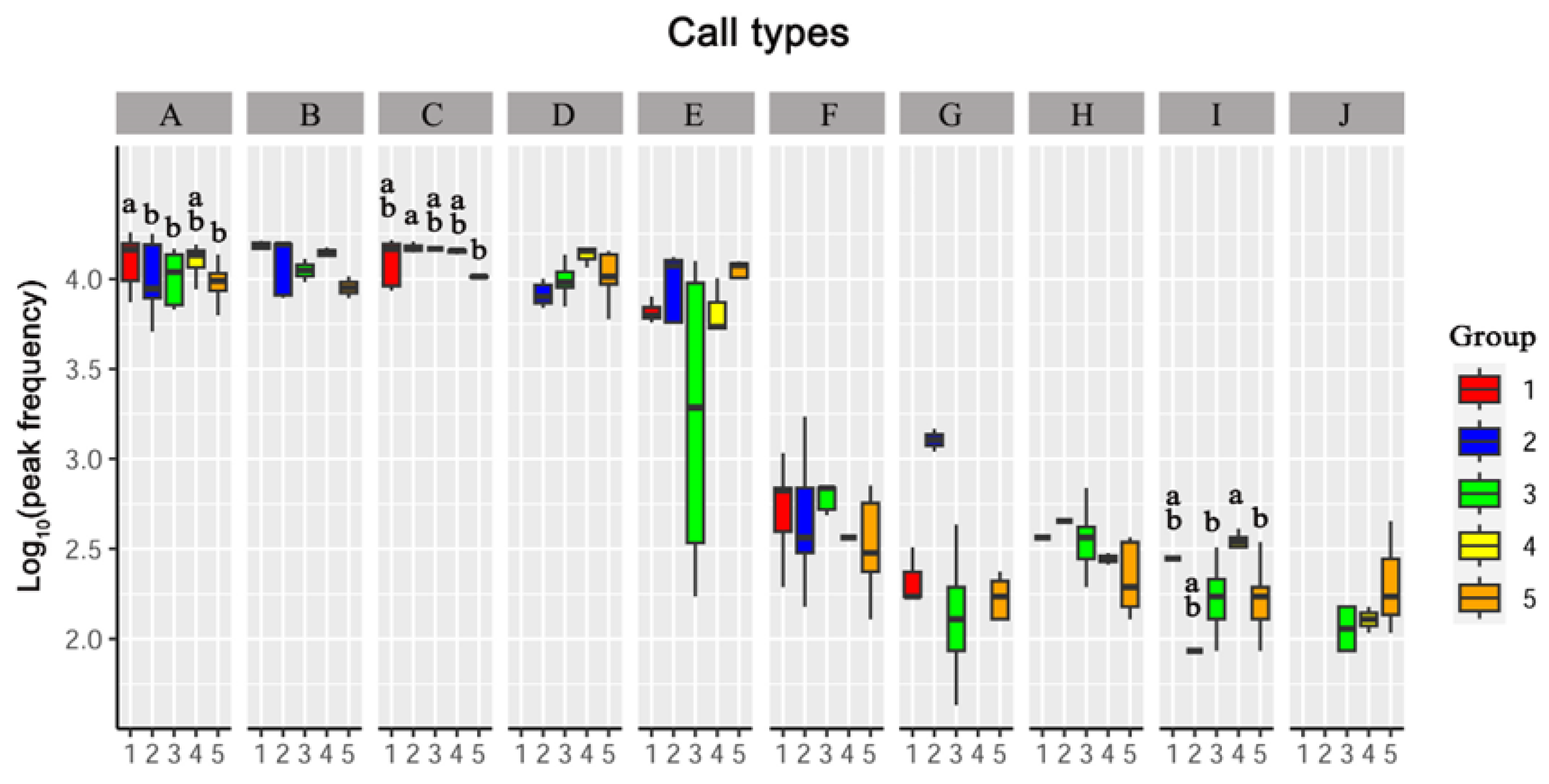
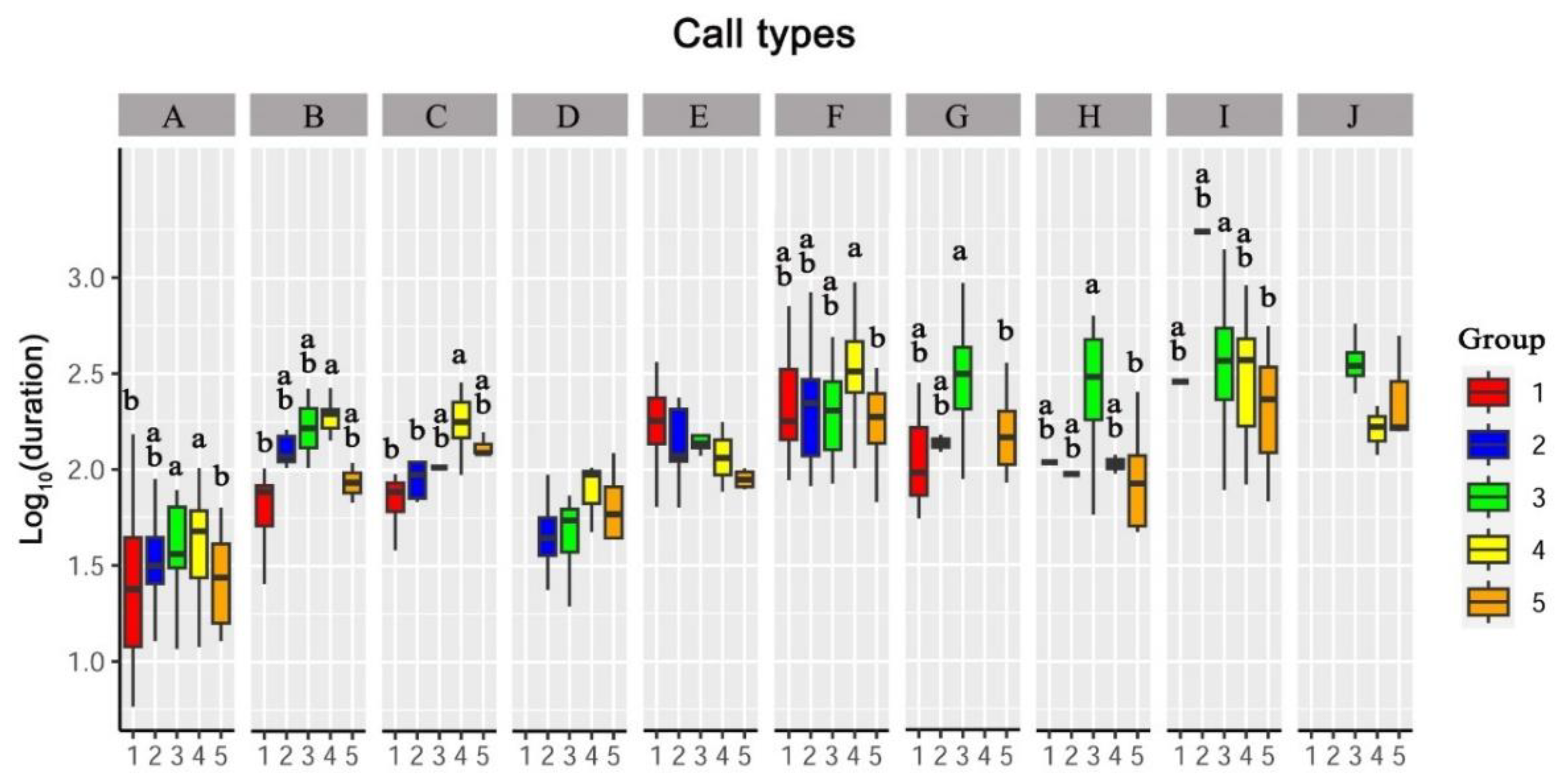
3.3. Differences in Vocalizations between Sexes and Age Groups
3.3.1. Peak Frequency
3.3.2. Duration
4. Discussion
4.1. Vocalization Diversity
4.2. Sex and Age Differences in Vocalization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shang, Y.C. Animal Behavior, 2nd ed.; Peking University Press: Beijing, China, 2014; pp. 256–267. [Google Scholar]
- Sacchi, R.; Galeotti, P.; Fasola, M.; Ballasina, D. Vocalizations and courtship intensity correlate with mounting success in marginated tortoises Testudo marginata. Behav. Ecol. Sociobiol. 2003, 55, 95–102. [Google Scholar] [CrossRef]
- Galeotti, P.; Sacchi, R.; Fasola, M.; Ballasina, D. Do mounting vocalisations in tortoises have a communication function? A comparative analysis. Br. J. Herpetol. 2005, 15, 61–71. [Google Scholar]
- Ferrara, C.R.; Mortimer, J.A.; Vogt, R.C. First evidence that hatchlings of Chelonia mydas emit sounds. Copeia 2014, 2, 245–247. [Google Scholar] [CrossRef]
- Galeotti, P.; Sacchi, R.; Rosa, D.P.; Fasola, M. Female preference for fast-rate, high-pitched calls in Hermann’s tortoises (Testudo hermanni). Behav. Ecol. 2005, 16, 301–308. [Google Scholar] [CrossRef]
- Monteiro, C.C.; Carmo, H.M.; Santos, A.J.; Corso, G.; Sousa-Lima, R.S. First record of bioacoustic emission in embryos and hatchlings of hawksbill sea turtles (Eretmochelys imbricata). Chelonian Conserv. Biol. 2019, 18, 273–278. [Google Scholar] [CrossRef]
- Ferrara, C.R.; Vogt, R.C.; Giles, J.C.; Kuchling, G. Chelonian vocal communication. In Biocommunication of Animals; Witzany, G., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 261–274. [Google Scholar]
- Chen, Z.; Wiens, J.J. The origins of acoustic communication in vertebrates. Nat. Commun. 2020, 11, 369. [Google Scholar] [CrossRef] [PubMed]
- Jorgewich-Cohen, G.; Townsend, S.W.; Padovese, L.R.; Klein, N.; Praschag, P.; Ferrara, C.R.; Ettmar, S.; Menezes, S.; Varani, A.P.; Serano, J.; et al. Common evolutionary origin of acoustic communication in choanate vertebrates. Nat. Commun. 2022, 13, 6089. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.C.; Davis, J.A.; Mccauley, R.D.; Kuchling, G. Voice of the turtle: The underwater acoustic repertoire of the long-necked freshwater turtle, Chelodina oblonga. J. Acoust. Soc. Am. 2009, 126, 434–443. [Google Scholar] [CrossRef]
- Ferrara, C.R.; Vogt, R.C.; Sousa-Lima, R.S.; Lenz, A.; Morales-Mávil, J.E. Sound communication in embryos and hatchlings of Lepidochelys kempii. Chelonian Conserv. Biol. 2019, 18, 279–283. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, L.; Li, H.; Wang, T.; Shi, H.; Wang, J. Underwater vocalizations of Trachemys scripta elegans and their differences among sex–age groups. Front. Ecol. Evol. 2022, 10, 1022052. [Google Scholar] [CrossRef]
- Carr, A.F. Handbook of Turtles; Comstock Publishing Associates: New York, NY, USA, 1995; pp. 122–134. [Google Scholar]
- Lutcavage, M.E.; Lutz, P.L. Diving physiology. In The Biology of Sea Turtles; Lutz, P.L., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 1997; pp. 277–296. [Google Scholar]
- Cook, S.L.; Forrest, T.G. Sounds produced by nesting leatherback sea turtles (Dermochelys coriacea). Herpetol. Rev. 2005, 36, 387–390. [Google Scholar]
- Ferrara, C.R.; Vogt, R.C.; Eisemberg, C.C.; Doody, J.S. First evidence of the pig-nosed turtle (Carettochelys insculpta) vocalizing underwater. Copeia 2017, 105, 29–32. [Google Scholar] [CrossRef]
- Ferrara, C.R.; Vogt, R.; Sousa-Lima, R.; Tardio, B.M.R.; Bernardes, V.G.D. Sound Communication and Social Behavior in an Amazonian River Turtle (Podocnemis expansa). Herpetologica 2014, 70, 149–156. [Google Scholar] [CrossRef]
- Zhou, L.; Lei, J.; Zhai, X.; Shi, H.; Wang, J. Chinese striped-neck turtles vocalize underwater and show differences in peak frequency among different age and sex groups. PeerJ 2022, 10, e14628. [Google Scholar] [CrossRef]
- Charrier, I.; Jeantet, L.; Maucourt, L.; Régis, S.; Lecerf, N.; Benhalilou, A.; Chevallier, D. First evidence of underwater vocalizations in green sea turtles Chelonia mydas. Endanger. Species Res. 2022, 48, 31–41. [Google Scholar] [CrossRef]
- Gans, C.; Maderson, P.F.A. Sound producing mechanisms in recent reptiles: Review and comment. Integr. Comp. Biol. 1973, 13, 1195–1203. [Google Scholar] [CrossRef]
- Sacchi, R.; Galeotti, P.; Fasola, M.; Gerzeli, G. Larynx morphology and sound production in three species of testudinidae. J. Morphol. 2004, 261, 175–183. [Google Scholar] [CrossRef]
- Manolakis, D.G.; Ingle, V.K.; Kogon, S.M. Statistical and Adaptive Signal Processing: Spectral Estimation, Signal Modeling, Adaptive Filtering and Array Processing (Artech House Signal Processing Library); Artech House: Norwood, UK, 2005; pp. 111–113. [Google Scholar]
- Zheng, J. Signals and Systems (Volume 1), 3rd ed.; Higher Education Press: Beijing, China, 2011; pp. 165–176. [Google Scholar]
- Wang, K.; Ren, J.; Chen, H.; Lyu, Z.; Guo, X.; Jiang, K.; Chen, J.; Li, J.; Guo, P.; Wang, Y.; et al. The updated checklists of amphibians and reptiles of China. Biodivers. Sci. 2020, 28, 189–218. [Google Scholar]
- IUCN Red List. Available online: https://www.iucnredlist.org/species/39620/97401140 (accessed on 22 December 2022).
- Yamada, K.; Nishida-Umehar, C.; Matsuda, Y. Molecular and cytogenetic characterization of site-specific repetitive DNA sequences in the Chinese soft-shelled turtle (Pelodiscus sinensis, Trionychidae). Chromosome Res. 2005, 13, 33–46. [Google Scholar] [CrossRef]
- Li, H.; Liu, J.; Xiong, L.; Zhang, H.; Zhou, H.; Yin, H.; Jing, W.; Li, J.; Shi, Q.; Wang, Y.; et al. Phylogenetic relationships and divergence dates of softshell turtles (Testudines: Trionychidae) inferred from complete mitochondrial genomes. J. Evol. Biol. 2017, 30, 1011–1023. [Google Scholar] [CrossRef]
- Zhu, Q.; Kong, F.; Shi, H. Nesting activity of the Chinese softshell turtle, Pelodiscus sinensis, on the Yellow River, North western China. Chelonian Conserv. Biol. 2021, 20, 290–295. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, S.; Niu, C. Effects of Extreme Light Cycle and Density on Melatonin, Appetite, and Energy Metabolism of the Soft-Shelled Turtle (Pelodiscus sinensis). Biology 2022, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, J.D. Nonparametric Statistical Inference, 2nd ed.; Marcel Dekker: New York, NY, USA, 1985; pp. 47–54. [Google Scholar]
- Dallal, G.E.; Wilkinson, L. An analytic approximation to the distribution of Lilliefor’s test statistic for normality. Am Stat. 1986, 40, 294–296. [Google Scholar]
- Siegel, S. Nonparametric Statistics for the Behavioral Sciences; McGraw-Hill: New York, NY, USA, 1956; pp. 122–134. [Google Scholar]
- Ferrara, C.R.; Vogt, R.C.; Pappas, M. Emydoidea Blandingii (blanding’s Turtle) vocalizations. Herpetol. Rev. 2018, 49, 526–527. [Google Scholar]
- Russell, A.P.; Bauer, A.M. Vocalization by extant non-avian reptiles: A synthetic overview of phonation and the vocal apparatus. Anat. Rec. 2021, 304, 1478–1528. [Google Scholar] [CrossRef]
- Göppert, E. Larynx and trachea. In Handbook of Comparative Anatomy of Vertebrates (Vol. 3); Bolk, L., Göppert, E., Kallius, E., Lubosch, W., Eds.; Urban and Schwarzenberg: Berlin, Germany, 1937; pp. 797–866. [Google Scholar]
- Ferrara, C.R.; Vogt, R.C.; Sousa-Lima, R.S. Turtle Vocalizations as the First Evidence of Posthatching Parental Care in Chelonians. J. Comp. Psychol. 2013, 127, 24–32. [Google Scholar] [CrossRef]
- Capranica, R.R.; Moffat, A.J.M. Neurobehavioral correlates of sound communication in anurans. In Advances in Vertebrate Neuroethology; Ewert, J.P., Ed.; Springer: New York, NY, USA, 1983; pp. 701–730. [Google Scholar]
- Gerhardt, H.C.; Huber, F. Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions; University of Chicago Press: Chicago, IL, USA, 2002; pp. 122–130. [Google Scholar]
- Rogers, L.J.; Kaplan, G.T. Songs, Roars, and Rituals: Communication in Birds, Mammals, and Other Animals; Harvard University Press: Boston, MA, USA, 2000; pp. 103–112. [Google Scholar]
- Ryan, M.J.; Perrill, S.A.; Wilczynski, W. Auditory tuning and call frequency predict population- based mating preferences in the cricket frog, Acris crepitans. Am. Nat. 1992, 139, 1370–1383. [Google Scholar] [CrossRef]
- Wilczynski, W.; Rand, A.S.; Ryan, M.J. Evolution of calls and auditory tuning in the Physalaemus pustulosus species group. Brain Behav. Evol. 2001, 58, 137–151. [Google Scholar] [CrossRef]
- Christensen-Dalsgaard, J.; Brandt, C.; Willis, K.L.; Christensen, C.B.; Ketten, D.; Edds-Walton, P.; Fay, R.R.; Madsen, P.T.; Carr, C.E. Specialization for underwater hearing by the tympanic middle ear of the turtle, Trachemys scripta elegans. Proc. Royal Soc. B 2012, 279, 2816–2824. [Google Scholar] [CrossRef]
- Piniak, W.E.D.; Eckert, S.A.; Harms, C.A.; Stringer, E.M. Underwater Hearing Sensitivity of the Leatherback Sea Turtle (Dermochelys coriacea): Assessing the Potential Effect of Anthropogenic Noise; U.S. Department of the Interior and Bureau of Ocean Energy Management: Herndon, VA, USA, 2012; pp. 8–13. [Google Scholar]
- Piniak, W.E.D.; Mann, D.A.; Eckert, S.A.; Harms, C.A. Amphibious hearing in sea turtles. In The Effects of Noise on Aquatic Life; Popper, A.N., Hawkins, A., Eds.; Springer: New York, NY, USA, 2012; pp. 83–87. [Google Scholar]
- Wang, T.; Li, H.; Cui, J.; Zhai, X.; Shi, H.; Wang, J. Auditory brainstem responses in the red-eared slider Trachemys scripta elegans (Testudoformes: Emydidae) reveal sexually dimorphic hearing sensitivity. J. Comp. Physiol. A 2019, 205, 847–854. [Google Scholar] [CrossRef]
- McKenna, L.N.; Paladino, F.V.; Tomillo, P.S.; Robinson, N.J. Do Sea Turtles Vocalize to Synchronize Hatching or Nest Emergence? Copeia 2019, 107, 120–123. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, X.; Duan, P.; Wang, D.; Wang, Z.; Wang, K. Spatial–temporal variations in biosonar activity of Yangtze finless porpoise in the lower reaches of the Yangtze River and its correlation with underwater noise: Are quieter non-shipping branches the remaining shelters? Aquat. Conserv. 2021, 31, 964–978. [Google Scholar] [CrossRef]
- Feng, A.S.; Narins, P.M.; Xu, C.H.; Lin, W.Y.; Yu, Z.L.; Qiu, Q.; Xu, Z.; Shen, J. Ultrasonic communication in frogs. Nature 2006, 440, 333–336. [Google Scholar] [CrossRef]
- Narins, P.M.; Feng, A.S.; Lin, W.; Schnitzler, H.; Denzinger, A.; Suthers, R.A.; Xu, C. Old World frog and bird vocalizations contain prominent ultrasonic harmonics. J. Acoust. Soc. Am. 2004, 115, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.X.; Xu, Z.M.; Feng, A.S.; Narins, P.M. Large odorous frogs (Odorrana graminea) produce ultrasonic calls. J. Comp. Physiol. A 2011, 119, 1027–1030. [Google Scholar] [CrossRef]
- Young, B.A.; Nejman, N.; Meltzer, K.; Marvin, J. The mechanics of sound production in the puff adder Bitis arietans (Serpentes: Viperidae) and the information content of the snake hiss. J. Exp. Biol. 1999, 202, 2281–2289. [Google Scholar] [CrossRef]
- Young, B.A. Snake bioacoustics: Toward a richer understanding of the behavioral ecology of snakes. Q. Rev. Biol. 2003, 78, 303–325. [Google Scholar] [CrossRef] [PubMed]
- Todd, N.P.M. Estimated source intensity and active space of the American alligator (Alligator Mississippiensis) vocal display. J. Acoust. Soc. Am. 2007, 122, 2906. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, D.; Wu, X.; Wang, C.; Wang, R.; Xia, T. Response Specificity to Advertisement Vocalization in the Chinese Alligator (Alligator sinensis). Ethology 2009, 115, 832–839. [Google Scholar] [CrossRef]
- Yamasaki, M.; Matsumura, S. Development of sounds and mother-infant communication in the Natterer’s bat, Myotis nattereri bombinus. Bull. Akiyoshi-Dai Mus. Nat. Hist. 2004, 39, 23–36. [Google Scholar]
- Liu, Y.; Feng, J.; Jiang, Y.L.; Wu, L.; Sun, K.P. Vocalization development of greater horseshoe bat, Rhinolophus ferrumequinum (Rhinolophidae, Chiroptera). Folia Zool. 2007, 56, 126–136. [Google Scholar]
- Zuo, M.; Zeng, S.; Peng, W.; Zhang, X. Neural development of vocal behavior in striated mannikin (Lonchura striata swinhoei). Acta Zool. Sin. 2002, 48, 50–57. [Google Scholar]
- Vasconcelos, R.O.; Ladich, F. Development of vocalization, auditory sensitivity and acoustic communication in the Lusitanian toadfish Halobatrachus didactylus. J. Exp. Biol. 2008, 211, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dong, Y. The Study on Acoustic Behavior in the Melopsitiacus Undelafusp of Different ages. J. Shanxi Norm. Univ. Sci. Ed. 2007, 21, 93–95. [Google Scholar]
- Cosens, S.E. Development of vocalizations in the American Coot. Can. J. Zool. 1981, 59, 1921–1928. [Google Scholar] [CrossRef]
- Funakoshi, K.; Nomura, E.; Matsukubo, M.; Wakita, Y. Postnatal Growth and Vocalization Development of the Lesser Horseshoe Bat, Rhinolophus cornutus, in the Kyushu District, Japan. Mamm. Study 2010, 35, 65–78. [Google Scholar] [CrossRef]
- Hammerschmidt, K.; Newman, J.D.; Champoux, M.; Suomi, S.J. Changes in rhesus macaque “Coo” vocalizations during Early Development. Ethology 2000, 106, 873–886. [Google Scholar] [CrossRef]
- Tobias, M.L.; Kelley, D.B. Vocalizations by a sexually dimorphic isolated larynx: Peripheral constraints on behavioral expression. J. Neurosci. 1987, 7, 3191–3197. [Google Scholar] [CrossRef]
- Sathyan, R.; Engelbrecht, A.; Couldridge, V.C.K. Morphological, acoustic and genetic divergence in the bladder grasshopper Bullacris unicolor. Ecol. Evol. 2017, 29, 552–573. [Google Scholar]
- Narins, P.M.; Feng, A.S.; Fay, R.R. Hearing and Sound Communication in Amphibians (Vol. 28); Springer Science and Business Media: New York, NY, USA, 2006; pp. 233–236. [Google Scholar]
- Garcia, M.; Charlton, B.D.; Wyman, M.T.; Fitch, W.T.; Reby, D. Do Red Deer Stags (Cervus elaphus) Use Roar Fundamental Frequency (F0) to Assess Rivals? PLoS ONE 2013, 8, e83946. [Google Scholar] [CrossRef]
- Bowman, R.I. The evolution of song in Darwin’s finches. In Patterns of Evolution in Galapagos Organisms; Bowman, R.I., Berson, M., Leviton, A.E., Eds.; Association for the Advancement of Science: San Francisco, CA, USA, 1983; pp. 237–537. [Google Scholar]
- Ryan, M.J. The Tungara Frog; University of Chicago Press: Chicago, IL, USA, 1985; pp. 34–43. [Google Scholar]
- Buck, E.J.; Monroe, T.; Zwicky, G.; Derryberry, E.P.; Lipshutz, S.E. Species and sex differences in vocalizations between sex-role reversed shorebirds, Northern Jacana (Jacana spinosa) and Wattled Jacana (J. jacana). Wilson J. Ornithol. 2020, 132, 343–351. [Google Scholar] [CrossRef]
- Miller, A.H. The vocal apparatus of North American owls. Condor 1934, 36, 204–213. [Google Scholar] [CrossRef]
- Fischer, J.; Kitchen, D.M.; Seyfarth, R.M.; Cheney, D.L. Baboon loud calls advertise male quality: Acoustic features and their relation to rank, age, and exhaustion. Behav. Ecol. Sociobiol. 2004, 56, 140–148. [Google Scholar] [CrossRef]
- Barelli, C.; Mundry, R.; Heistermann, M.; Hammerschmidt, K. Cues to androgens and quality in male gibbon songs. PLoS ONE 2013, 8, e82748. [Google Scholar] [CrossRef]
- Ammie, K.K. Evidence for sexual dimorphism in chimpanzee vocalizations: A comparison of male and female call production and acoustic parameters. In The Chimpanzees of the Taï Forest 40 Years of Research; Boesch, C., Wittig, R., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 410–421. [Google Scholar]
- Lu, N.; Chen, B.; Qing, J.; Lei, J.; Wang, T.; Shi, H.; Wang, J. Transcriptome analyses provide insights into the auditory function in Trachemys scripta elegans. Animals 2022, 12, 2410. [Google Scholar] [CrossRef] [PubMed]
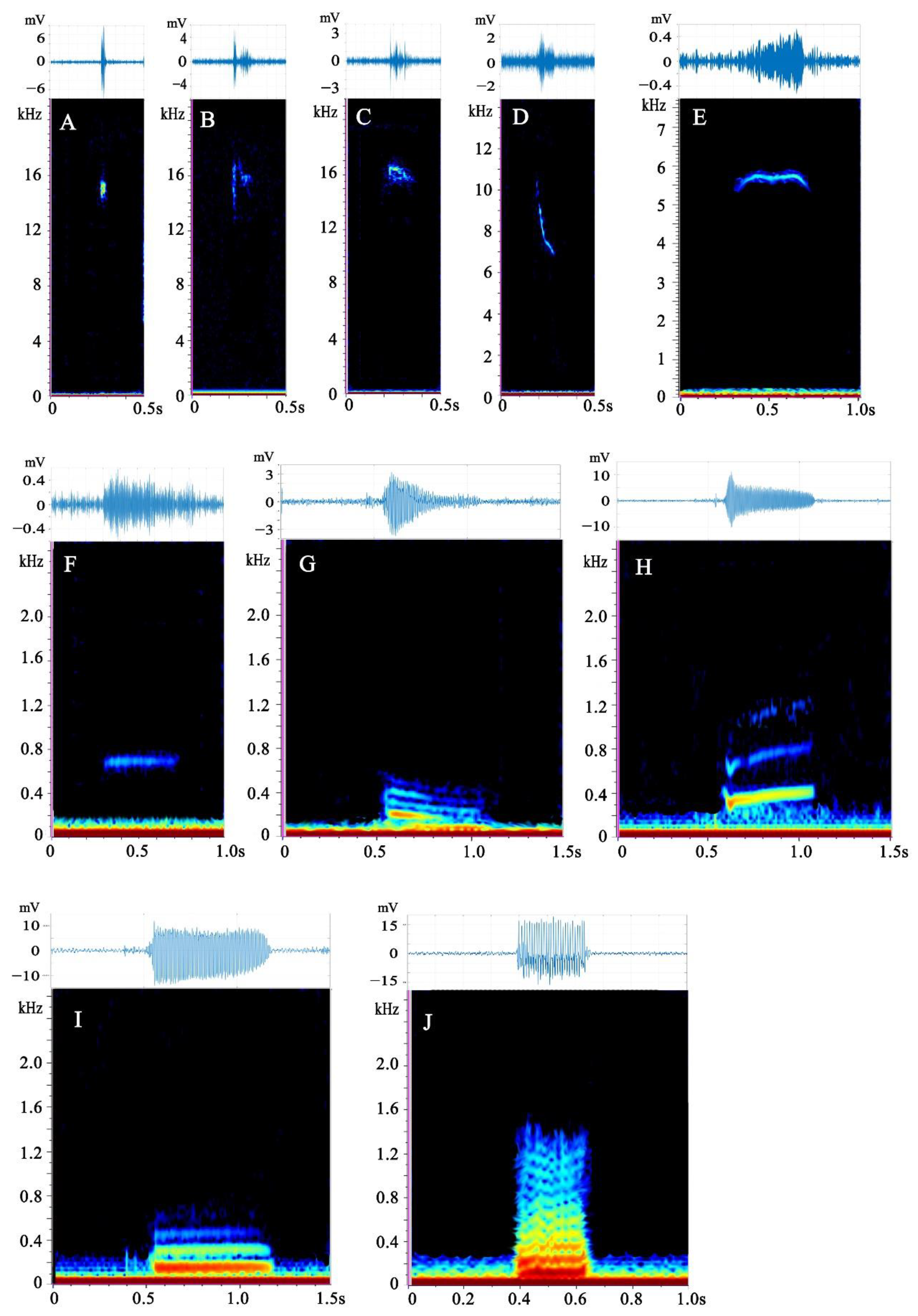
| Type | Adult Females | Ratio (%) | Adult Males | Ratio (%) | Subadult Females | Ratio (%) | Subadult Males | Ratio (%) | Mixed-Sex Adults | Ratio (%) | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 27 | 11.49 | 21 | 8.94 | 65 | 27.66 | 69 | 29.36 | 53 | 22.55 | 235 |
| B | 8 | 22.22 | 2 | 5.56 | 5 | 13.89 | 19 | 52.78 | 2 | 5.56 | 36 |
| C | 26 | 49.06 | 1 | 1.89 | 6 | 11.32 | 15 | 28.30 | 5 | 9.43 | 53 |
| D | 3 | 10.00 | 6 | 20.00 | 16 | 53.33 | 5 | 16.67 | 30 | ||
| E | 3 | 9.38 | 4 | 12.50 | 5 | 15.63 | 16 | 50.00 | 4 | 12.50 | 32 |
| F | 27 | 30.68 | 14 | 15.91 | 16 | 18.18 | 19 | 21.59 | 12 | 13.64 | 88 |
| G | 0.00 | 69 | 77.53 | 2 | 2.25 | 3 | 3.37 | 15 | 16.85 | 89 | |
| H | 2 | 6.25 | 19 | 59.38 | 1 | 3.13 | 1 | 3.13 | 9 | 28.13 | 32 |
| I | 34 | 29.57 | 52 | 45.22 | 1 | 0.87 | 1 | 0.87 | 27 | 23.48 | 115 |
| J | 3 | 30.00 | 4 | 40.00 | 3 | 30.00 | 10 | ||||
| Total | 133 | 192 | 117 | 143 | 135 | 720 |
| Type | Low Frequency Median ± IQR (Hz) | High Frequency Median ± IQR (Hz) | Peak Frequency Median ± IQR (Hz) | Duration Median ± IQR (ms) | Bandwidth (Hz) | No. of Harmonics |
|---|---|---|---|---|---|---|
| A | 10,656 ± 6218 | 11,567 ± 6324 | 10,745 ± 6180 | 31.57 ± 30.45 | 689 ± 947 | |
| B | 13,230 ± 5811 | 15,749 ± 1929 | 14,729 ± 3553 | 95.58 ± 92.17 | 1497 ± 1975 | |
| C | 13,714 ± 2248 | 14,539 ± 1811 | 14,320 ± 2068 | 122.86 ± 108.89 | 538 ± 527 | |
| D | 8572 ± 6323 | 10,262 ± 5093 | 9055 ± 6013 | 49.15 ± 32.49 | 722 ± 1098 | |
| E | 6506 ± 5939 | 7086 ± 5836 | 6406 ± 3726 | 137.92 ± 102.55 | 367 ± 333 | |
| F | 331 ± 303.25 | 426 ± 334 | 366 ± 367 | 244.76 ± 186.78 | 86 ± 43 | |
| G | 86 ± 56 | 385 ± 228 | 129 ± 108 | 284.08 ± 240.57 | 129 ± 86 | 1~11 |
| H | 275 ± 162 | 721 ± 503 | 345 ± 210 | 182.43 ± 264.17 | 129 ± 183 | 1~7 |
| I | 151 ± 158 | 424 ± 405 | 215 ± 172 | 327.62 ± 275.51 | 86 ± 21 | 1~7 |
| J | 84 ± 45 | 1058 ± 1039 | 140 ± 54 | 231.95 ± 232.79 | 216 ± 215 | 2~12 |
| Call Types | A | B | C | D | E | F | G | H | I | J | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average value of correlation coefficients between call types | A | 0.71 | |||||||||
| B | 0.16 | 0.77 | |||||||||
| C | 0.15 | 0.21 | 0.78 | ||||||||
| D | 0.15 | 0.22 | 0.21 | 0.71 | |||||||
| E | 0.12 | 0.15 | 0.17 | 0.17 | 0.80 | ||||||
| F | 0.14 | 0.18 | 0.20 | 0.20 | 0.16 | 0.77 | |||||
| G | 0.11 | 0.15 | 0.15 | 0.16 | 0.12 | 0.14 | 0.77 | ||||
| H | 0.10 | 0.14 | 0.15 | 0.14 | 0.11 | 0.13 | 0.11 | 0.60 | |||
| I | 0.11 | 0.15 | 0.16 | 0.15 | 0.13 | 0.15 | 0.11 | 0.10 | 0.71 | ||
| J | 0.14 | 0.18 | 0.19 | 0.19 | 0.15 | 0.17 | 0.14 | 0.12 | 0.14 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Lei, J.; Zhai, X.; Lu, N.; Shi, H.; Wang, J. Diversity of Underwater Vocalizations in Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Animals 2023, 13, 812. https://doi.org/10.3390/ani13050812
Zhou L, Lei J, Zhai X, Lu N, Shi H, Wang J. Diversity of Underwater Vocalizations in Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Animals. 2023; 13(5):812. https://doi.org/10.3390/ani13050812
Chicago/Turabian StyleZhou, Lu, Jinhong Lei, Xiaofei Zhai, Ningning Lu, Haitao Shi, and Jichao Wang. 2023. "Diversity of Underwater Vocalizations in Chinese Soft-Shelled Turtle (Pelodiscus sinensis)" Animals 13, no. 5: 812. https://doi.org/10.3390/ani13050812
APA StyleZhou, L., Lei, J., Zhai, X., Lu, N., Shi, H., & Wang, J. (2023). Diversity of Underwater Vocalizations in Chinese Soft-Shelled Turtle (Pelodiscus sinensis). Animals, 13(5), 812. https://doi.org/10.3390/ani13050812





