Life History Parameters and Fishing Aspects of the Alien Nimble Spray Crab Percnon gibbesi in a Native Area of the Central-East Atlantic
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
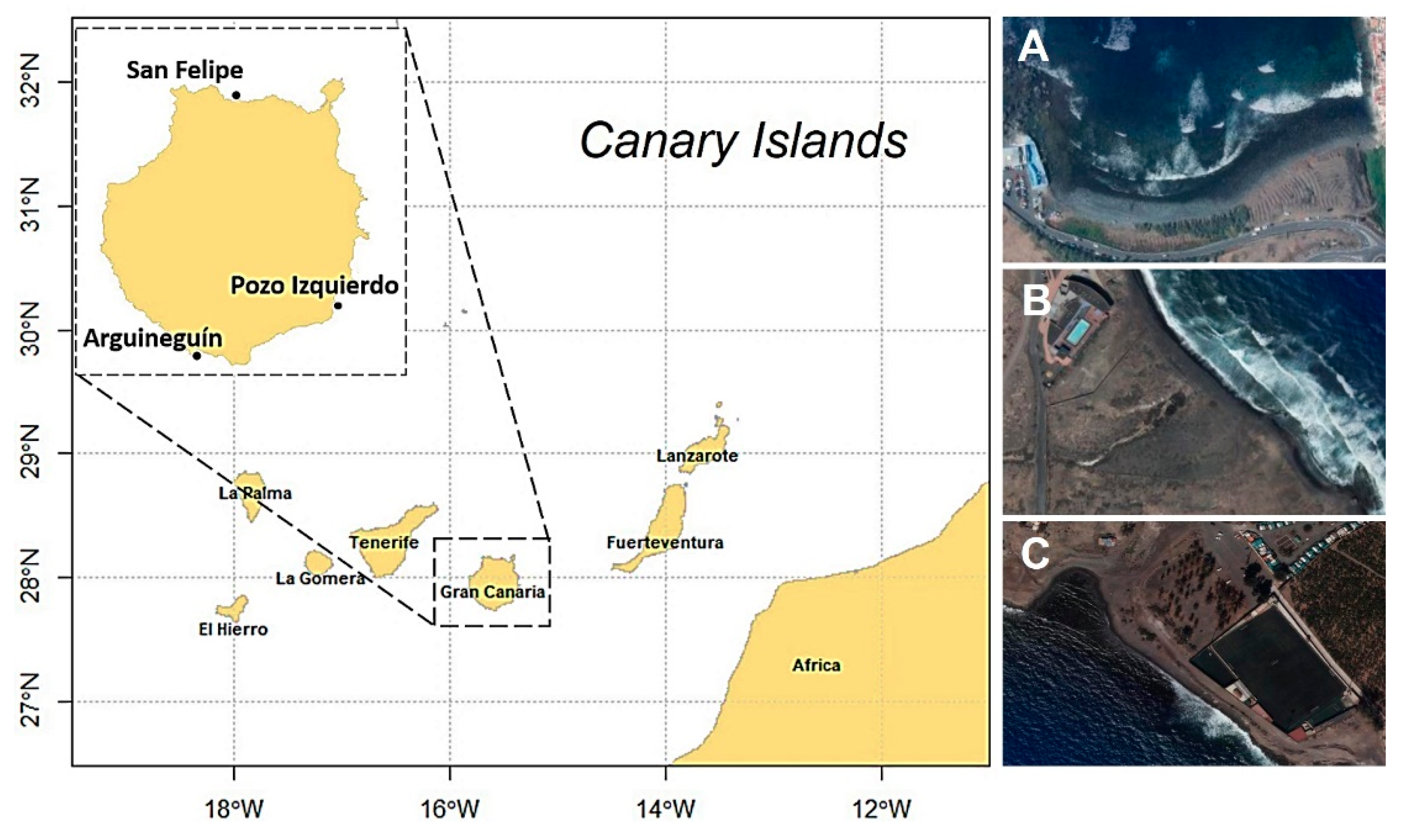
2.1. Study Area and Data Collection
2.2. Data Analysis
2.2.1. Size Structure and Sex Ratio
2.2.2. Length–Weight Relationships
2.2.3. Growth and Mortality Parameters
2.2.4. Fishery Aspects
3. Results
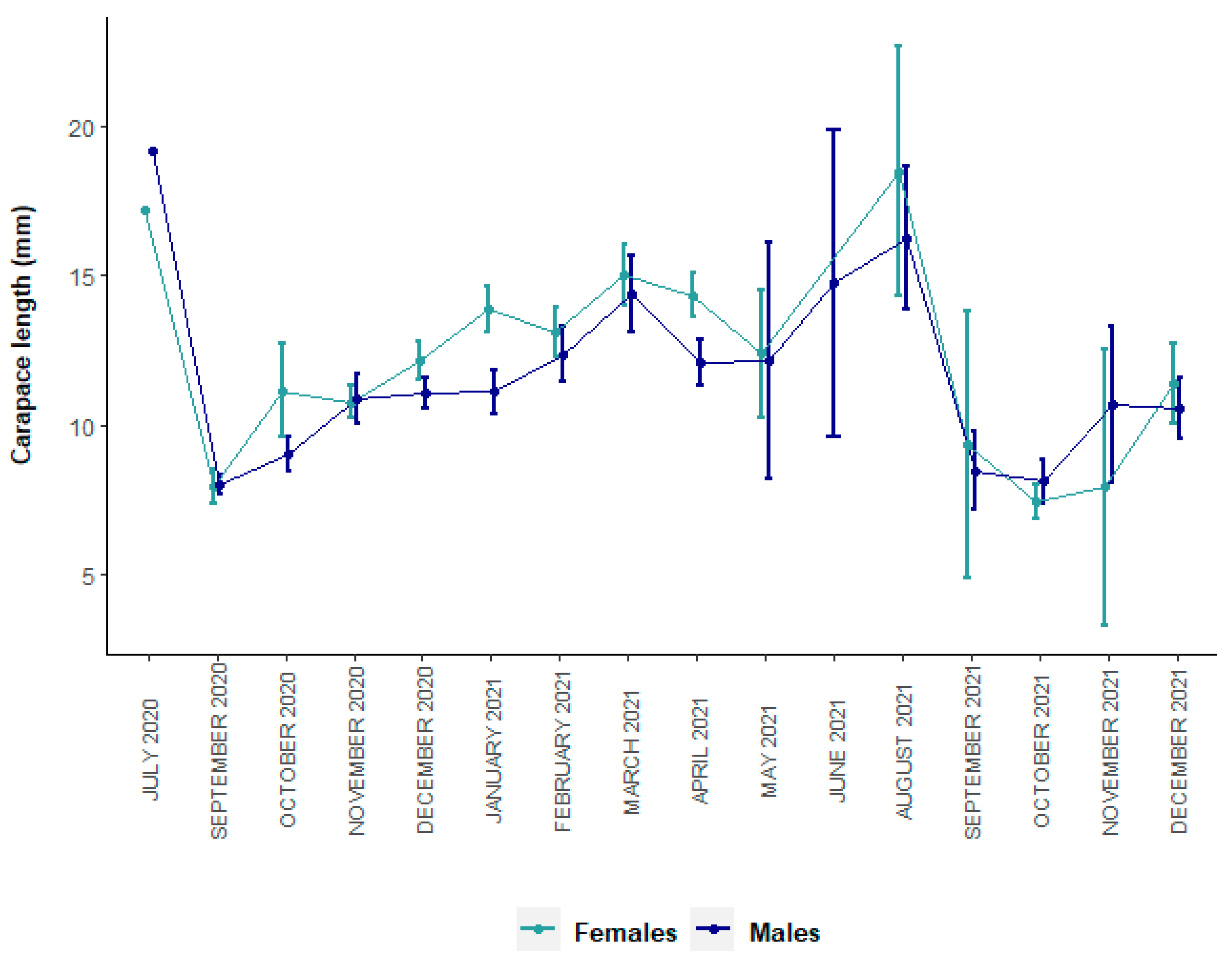
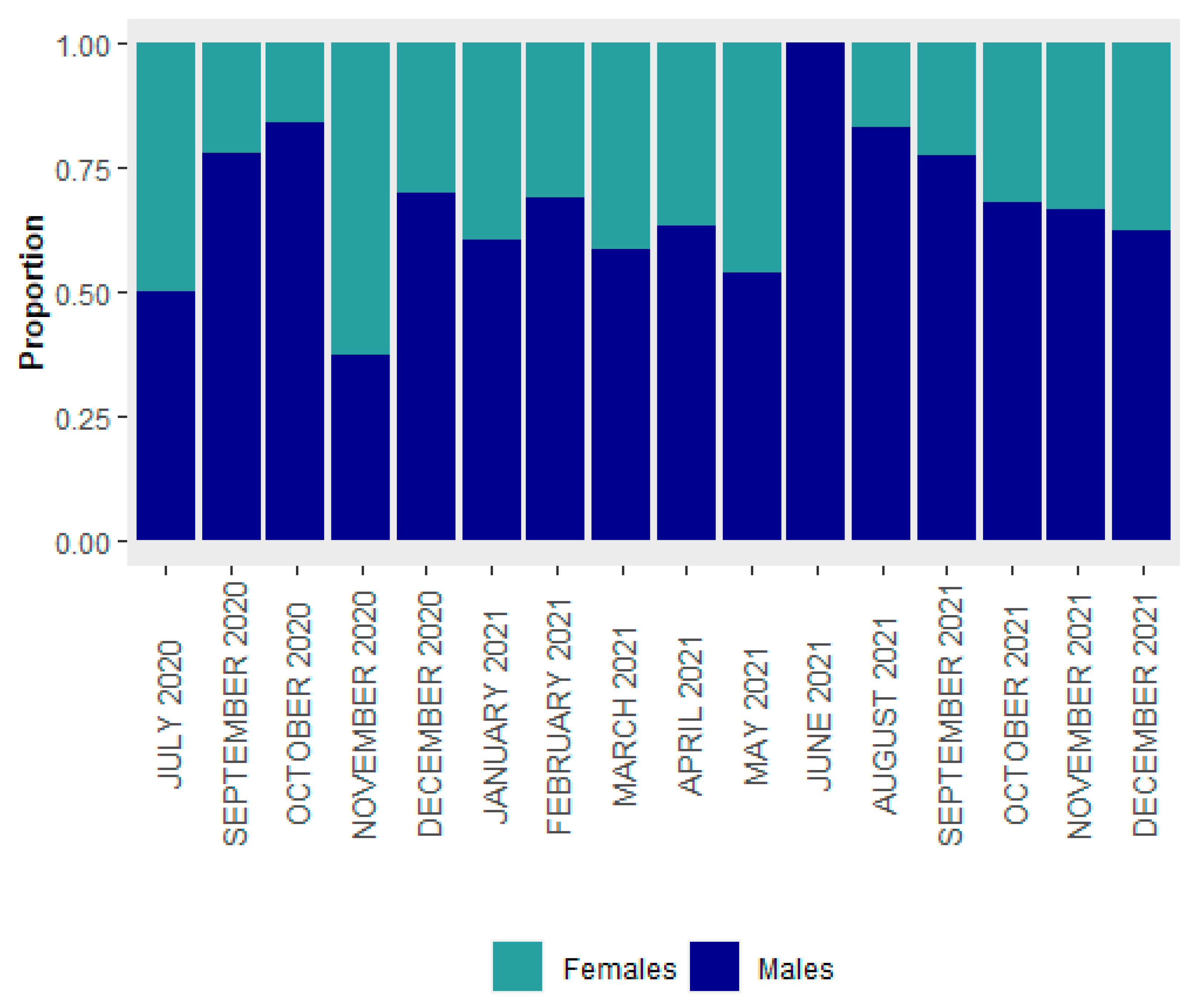
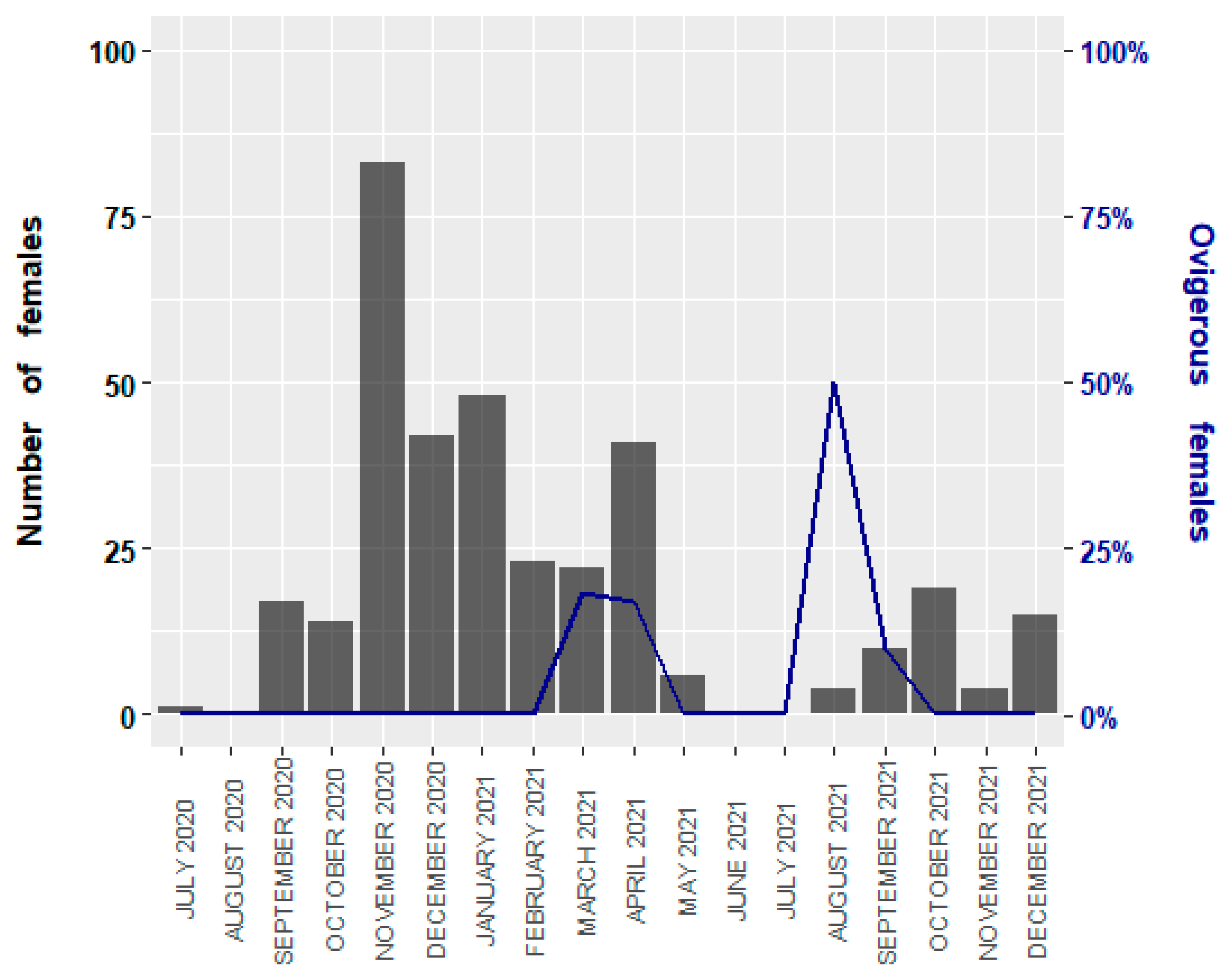
3.1. Size Structure and Sex Ratio
3.2. Length–Weight Relationships
3.3. Growth and Mortality Parameters
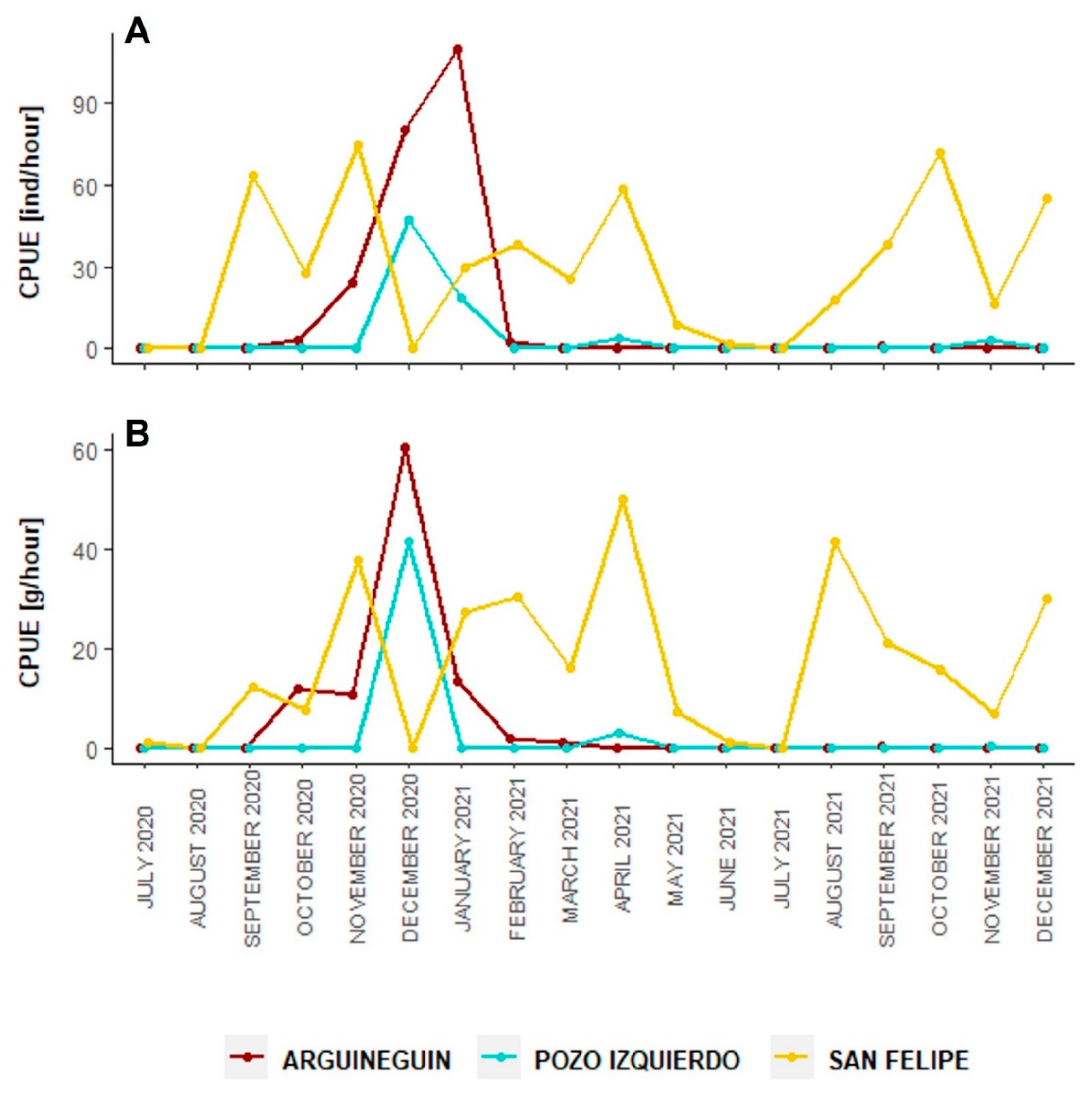
3.4. Fishery Aspects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClanahan, T.R.; Castilla, J.C.; White, A.T.; Defeo, O. Healing small-scale fisheries by facilitating complex socio-ecological systems. Rev. Fish Biol Fish. 2009, 19, 33–47. [Google Scholar] [CrossRef]
- Dowling, N.A.; Smith, A.D.; Smith, D.C.; Parma, A.M.; Dichmont, C.M.; Sainsbury, K.; Cope, J.M. Generic solutions for data-limited fishery assessments are not so simple. Fish Fish. 2019, 20, 174–188. [Google Scholar] [CrossRef]
- BOC. Boletín Oficial de Canarias, Orden de 2 de mayo de 2011, por la que se fijan determinados aspectos del marisqueo a pie para la recolección de algunas especies de mariscos de Canarias. Cons. De Agric. Ganad. Y Pesca 2011, 93, 11235–11239. [Google Scholar]
- González, J.A. Brachyuran crabs (Crustacea: Decapoda) from the Canary Islands (eastern Atlantic): Checklist, zoogeographic considerations and conservation. Sci. Mar. 2016, 80, 89–102. [Google Scholar] [CrossRef]
- Pezzuto, P.R.; Alvarez-Perez, J.A.; Wahrlich, R. Deep-sea shrimps (Decapoda: Aristeidae): New target of the deep-water trawling fishery in Brazil. Braz. J. Oceanogr. 2006, 54, 123–134. [Google Scholar] [CrossRef]
- Svane, I.; Hammett, Z.; Lauer, P. Impacts of trawling on benthic macro-fauna and flora of the Spencer Gulf prawn fishing grounds. Estuar. Coast. Shelfish Sci. 2009, 82, 621–631. [Google Scholar] [CrossRef]
- Rasmuson, L.K. The biology, ecology and fishery of the Dungeness crab, Cancer magister. Adv. Mar. Biol. 2013, 65, 95–148. [Google Scholar]
- Hirose, G.L.; Souza, L.S.; Silva, S.L.R.; Alves, D.F.R.; Negreiros-Fransozo, M.L. Estructura de la población de cangrejo rojo de manglar, Goniopsis cruentata (Decapoda: Grapsidae) bajo diferentes impactos de pesca: Implicaciones para la gestión de los recursos. Rev. De Biol. Trop. 2015, 63, 443–457. [Google Scholar] [CrossRef]
- Vedavyasa, R.P.; Thomas, M.M.; Sudhakara, R.G. The crab fishery resources of India. In Proceedings of the Symposium on Living Resources of the Seas around India, Mandapam Camp, India, 1973. [Google Scholar]
- Freire, J.; Bernárdez, C.; Corgos, A.; Fernández, L.; González-Gurriarán, E.; Sampedro, M.P.; Verísimo, P. Management strategies for sustainable invertebrate fisheries in coastal ecosystems of Galicia (NW Spain). Aquat. Ecol. 2002, 36, 41–50. [Google Scholar] [CrossRef]
- Ingles, J.A. Status of the blue crab fisheries in the Philippines. In Turbulent Seas: The Status of Philippines Marine Fisheries; Department of Agriculture, Bureau of Fisheries and Aquatic Resources: Cebu City, Philippines, 2004; pp. 47–52. [Google Scholar]
- Harlıoğlu, M.M.; Farhadi, A.; Ates, A.S. A Review of the Marine Crab Fisheries in the Turkish Seas. Croat. J. Fish. 2018, 76, 125–134. [Google Scholar] [CrossRef]
- Pavlowich, T.; Kapuscinski, A.R. Understanding spearfishing in a coral reef fishery: Fisher’s opportunities, constraints, and decision-making. PLoS ONE 2017, 12, e0181617. [Google Scholar] [CrossRef]
- Alves, R.R.; Nishida, A.K.; Hernández, M.I. Environmental perception of gatherers of the crab’caranguejo-uçá’(Ucides cordatus, Decapoda, Brachyura) affecting their collection attitudes. J. Ethnobiol. Ethnomed. 2005, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Noguera, R.; Riera, R. Dinámica de las poblaciones de Xantho spp (cangrejilla) (Decapoda, Xanthidae) en la franja costera de Arrecife (Lanzarote, islas Canarias). Vieraea 2011, 39, 97–104. [Google Scholar] [CrossRef]
- Trussell, G.C.; Ewanchuk, P.J.; Bertness, M.D.; Silliman, B.R. Trophic cascades in rocky shore tide pools: Distinguishing lethal and nonlethal effects. Oecologia 2004, 139, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Hull, W.W.; Bourdeau, P.E. Can crabs kill like a keystone predator? A field-test of the effects of crab predation on mussel mortality on a northeast Pacific rocky shore. PLoS ONE 2017, 12, e0183064. [Google Scholar] [CrossRef]
- Boudreau, S.A.; Worm, B. Ecological role of large benthic decapods in marine ecosystems: A review. Mar. Ecol. Prog. Ser. 2012, 469, 195–213. [Google Scholar] [CrossRef]
- Cogo, G.B.; Biasi, C.; Santos, S. The effect of the macrocosumer Aegla longirostri (Crustacea, Decapoda) on the invertebrate community in a subtropical stream. Acta Limnol. Bras. 2014, 26, 143–153. [Google Scholar] [CrossRef]
- Pombo, M. The Atlantic Ghost Crab Ocypode Quadrata (Decapoda: Ocypodidae) as Bioindicator of Sandy Beaches: Assessment of the Influence of Environmental, Bahavioral and Population Factors. Doctoral Thesis, Instituto Oceanográfico, University of São Paulo, São Paulo, Brazil, 2015. [Google Scholar]
- Freire, J. Feeding ecology of Liocarcinus depurator (Decapoda: Portunidae) in the Ria de Arousa (Galicia, north-west Spain): Effects of habitat, season and life history. Mar. Biol. 1996, 126, 297–311. [Google Scholar] [CrossRef]
- Al-Wazzan, Z.; Giménez, L.; Behbahani, M.; Le Vay, L. Trophic niche separation in sympatric rocky shore crabs. J. Mar. Biol. Assoc. UK 2019, 99, 1171–1180. [Google Scholar] [CrossRef]
- Spivak, E.D.; Arévalo, E.; Cuesta, J.A.; González-Gordillo, J.I. Population structure and reproductive biology of the stone crab Xantho poressa (Crustacea: Decapoda: Xanthidae) in the “Corrales de Rota” (south-western Spain) a human-modified intertidal fishing area. J. Mar. Biol. Assoc. UK 2009, 90, 323–334. [Google Scholar] [CrossRef]
- Milne Edwards, H. Notions Preliminaires D’histoire Naturelle Pour Servir D’introduction au Cours Elementaire D’histoire Naturelle; Notions preliminaire de zoologie: Vienna, Austria, 1853. [Google Scholar]
- Tiralongo, F.; Messina, G.; Lombardo, B.M. Invasive species control: Predation on the alien crab Percnon gibbesi (H. Milne Edwards, 1853) (Malacostraca: Percnidae) by the rock goby, Gobius paganellus Linnaeus, 1758 (Actinopterygii: Gobiidae). J. Mar. Sci. Eng. 2021, 9, 393. [Google Scholar] [CrossRef]
- Hernández, J.E.; Palazón-Fernández, J.L.; Hernández, G.; Bolaños, J. The effect of temperature and salinity on the larval development of Stenorhynchus seticornis (Brachyura: Inachidae) reared in the laboratory. J. Mar. Biol. Assoc. UK 2012, 92, 505–513. [Google Scholar] [CrossRef]
- Deudero, S.; Frau, A.; Cerda, M.; Hampel, H. Distribution and densities of the decapod crab Percnon gibbesi, an invasive Grapsidae, in western Mediterranean waters. Mar. Ecol. Prog. Ser. 2005, 285, 151–156. [Google Scholar] [CrossRef]
- Nizinski, M.S. Annotated checklist of decapod crustaceans of Atlantic coastal and continental shelf waters of the United States. Proc. Biol. Soc. Wash. 2003, 116, 96–157. [Google Scholar]
- Relini, M.; Orsi, L.; Puccio, V.; Azzurro, E. The exotic crab Percnon gibbesi (H Milne Edwards, 1853) (Decapoda, Grapsidae) in the central Mediterranean. Sci. Mar. 2000, 64, 337–340. [Google Scholar] [CrossRef]
- Garcia, L.; Reviriego, B. Presència del cranc subtropical Percnon gibbesi a les Illes Balears. Primera cita a la Mediterrànea occidental. Boletín De La Soc. De Hist. Nat. De Balear. 2000, 43, 81–89. [Google Scholar]
- Müller, C. First record of Percnon gibbesi (Crustacea: Brachyura: Grapsidae) for the Balearic Islands. Senckenberg. Marit. 2001, 31, 83–89. [Google Scholar] [CrossRef]
- Mori, M.; Vacchi, M. On a new occurrence of the alien flat crab, Percnon gibbesi (H Milne Edwards), in the southern Sicily (Central Mediterranean Sea) (Crustacea, Brachyura, Grapsidae). Ann. Del Mus. Civ. Stor. Nat. Giancomo Doria 2002, 114, 295–302. [Google Scholar]
- Katsanevakis, S.; Poursanidis, D.; Yokes, M.B.; Macic, V.; Beqiraj, S.; Kashta, L.; Sghaeir, Y.R.; Zakhama-Sraieb, R.; Benamer, I.; Bitar, G.; et al. Twelve years after the first report of the crab Percnon gibbesi (H Milne Edwards, 1853) in the Mediterranean: Current distribution and invasion rates. J. Biol. Res. 2011, 16, 224–236. [Google Scholar]
- MITECO. Fichas del Catálogo Español de Especies Exóticas Invasoras RD 630/2013; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2015.
- FAO. Code of Conduct for Responsible Fisheries; Food and Agriculture Organization of the United Nations: Rome, Italy, 1995. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing. 2023. Available online: https://wwwR-projectorg/ (accessed on 20 March 2023).
- Sudjana, J. Statistics Method Bandung; Tarsito Press: Bandung, Indonesia, 1989; p. 508. [Google Scholar]
- Tiralongo, F.; Arculeo, M.; Yeo, M.D.; Kakou, B.I.; Adepo-Gourene, A.B. First data on population structure and growth parameters of Ocypode cursor (Linnaeus, 1758) along the Mediterranean and Atlantic coast. Cah. Biol. Mar. 2020, 61, 405–413. [Google Scholar]
- Von Bertalanffy, L.A. Quantitative Theory of Organic Growth (Inquiries on Growth Laws II). Hum. Biol. 1938, 10, 183–213. [Google Scholar]
- Mildenberger, T.K.; Taylor, M.H.; Wolff, M. TropFishR an R Package for Fisheries Analysis with Length-frequency data. Methods Ecol. Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef]
- Taylor, M.H.; Mildenberger, T.K. Extending Electronic Length Frequency Analysis in R. Fish Manag. Ecol. 2017, 24, 330–338. [Google Scholar] [CrossRef]
- Wetherall, J.A.; Polovina, J.J.; Ralston, S. Estimating growth and mortality in steady-state fish stocks from length frequency data. ICLARM Confer. Proc. 1987, 13, 53–74. [Google Scholar]
- Pauly, D. Some Simple Methods for Assessment of Tropical Fish Stock; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1983; p. 52. [Google Scholar]
- Sparre, P.; Venema, S. Introduction to Tropical Fish Stock Assessment Part 1: Manual; FAO: Rome, Italy, 1998. [Google Scholar]
- Then, A.Y.; Hoenig, J.M.; Norman, G.H.; Hewitt, D.A. Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J. Mar. Sci. 2015, 72, 82–92. [Google Scholar] [CrossRef]
- Welch, B.L. On the comparison of several mean values—An alternative approach. Biometrika 1951, 38, 330–336. [Google Scholar] [CrossRef]
- Yanagida, T. Package “Misty”: Miscellaneous Functions, ver.047, 2023. Available online: https://pbil.univ-lyon1.fr/CRAN/web/packages/misty/misty.pdf (accessed on 20 March 2023).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2018. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. J. Math. Methods Biosci. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Pérez-Sanchez, J.M.; Moreno-Batet, E. Invertebrados Marinos en Canarias; Cabildo Insular de Gran Canaria: Las Palmas, Spain, 1991; p. 335. [Google Scholar]
- Zeneto, A.; Gofas, S.; Verlaque, M.; Çinar, M.E.; García-Raso, J.E.; Bianchi, C.N.; Morris, C.; Azzurro, E.; Bilecenoglu, M.; Froglia, C.; et al. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I: Spatial distribution Mediterranean. Mar. Sci. 2010, 11, 381–493. [Google Scholar] [CrossRef]
- González-Pérez, J.A. Catálogo de los Crustáceos Decápodos de las Islas Canarias: Gambas, Langostas, Cangrejos; Publicaciones Turquesa: Santa Cruz de Tenerife, Spain, 1995; p. 282. [Google Scholar]
- Espino-Rodríguez, F.; Boyra-López, A.; Tuya-Cortés, F.; Haroun-Trabaue, R.J. Guía Visual de Especies Marinas de Canarias; Oceanográfica Divulgación, Educación y Ciencia: Las Palmas, Spain, 2006; p. 48. [Google Scholar]
- Forner, A.; Bas-Silvestre, M.; Martín-Hernández, A.; Álvarez-Canali, D.; Collazo, N. Estudio de las poblaciones de cangrejo utilizadas como carnada en las islas canarias: Situación actual, influencia del marisqueo y tipo de hábitat. Sci. Insul. 2018, 1, 23–36. [Google Scholar] [CrossRef]
- Pipitone, C.; Badalamenti, F.; Sparrow, A. Contribution to the knowledge of Percnon gibbesi (Decapoda, Grapsidae), an exotic species spreading rapidly in Sicilian waters. Crustaceana 2001, 74, 1009–1017. [Google Scholar]
- Puccio, V.; Relini, M.; Azzurro, E. Osservazioni sulla riproduzione di Percnon gibbesi (H Milne Edwards, 1853) nelle isole pelagie (Sicilia). Biol. Mar. Mediterr. 2003, 10, 267–272. [Google Scholar]
- Díaz, H.; Conde, J.E. Population dynamics and life history of the mangrove crab Aratus pisonii (Brachyura, Grapsidae) in a marine environment. Bull. Mar. Sci. 1989, 45, 148–163. [Google Scholar]
- Abelló, P.; Pertierra, J.P.; Reid, D.G. Sexual size dimorphism, relative growth and handedness in Liocarcinus depurator and Macropipus tuberculatus (Brachyura, Portunidade). Sci. Mar. 1990, 54, 195–202. [Google Scholar]
- Castiglioni, D.S.; Fransozo, M.L. Ciclo reproductivo do caranguejo violinista Uca rapax (Smith) (Crustacea, Brachyura, Ocypodidae) habitante de um estuário degradado em Paraty, Rio de Janeiro, Brasil. Rev. Bras. Zool. 2006, 23, 331–339. [Google Scholar] [CrossRef]
- Marochi, M.Z.; Costa, M.; Leite, E.D.; Da Cruz, I.; Masunari, S. To grow or to reproduce? Sexual dimorphism and ontogenetic allometry in two Sesarmidae species (Crustacea: Brachyura). J. Mar. Biol. Assoc. UK 2018, 99, 473–486. [Google Scholar] [CrossRef]
- Rufino, M.; Abelló, P.; Yule, B. Male and female carapace shape differences in Liocarcinus depurator (Decapoda, Brachyura): An application of geometric morphometric analysis to crustaceans. Ital. J. Zool. 2009, 71, 79–83. [Google Scholar] [CrossRef]
- Ju, S.J.; Secor, D.H.; Harvey, H.R. Growth rate variability and lipofucin accumulation rates in the blue crab Callinectes sapidus. Mar. Ecol. Prog. Ser. 2001, 224, 197–205. [Google Scholar] [CrossRef]
- Green, B.S.; Gardner, C.; Hochmuth, J.D.; Linnane, A. Environmental effects on fished lobsters and crabs. Rev. Fish Biol. Fish. 2014, 24, 613–638. [Google Scholar] [CrossRef]
- Lestang, S.; Hall, N.; Potter, I.C. Changes in density, age composition, and growth rate of Portunus pelagicus in a large embayment in which fishing pressures and environmental conditions have been altered. J. Crustacean Biol. 2003, 23, 908–919. [Google Scholar] [CrossRef]
- Sciberras, M.; Schembri, P.J. Observations on the alien crab Percnon gibbesi (Decapoda, Brachyura, Grapsidae) from the Maltese Islands. Rapp. Comm. Inter. Mer. Medit. 2007, 38, 594. [Google Scholar]
- Sciberras, M.; Schembri, P.J. Biology and interspecific interactions of the alien crab Percnon gibbesi in the Maltese islands. Mar. Biol. Res. 2008, 4, 321–332. [Google Scholar] [CrossRef]
- Paula, J.; Hartnoll, R.G. The larval and postlarval development of Percnon gibbesi (Crustacea, Brachyura, Grapsidae) and the identity of the larval genus -Pluteocaris. J. Zool. 1989, 218, 17–37. [Google Scholar] [CrossRef]
- Landeira, J.M.; Lozano-Soldevilla, F. Seasonal of planktonic crustacean decapod larvae in the subtropical waters of Gran Canaria Island, NE Atlantic. Sci. Mar. 2018, 82, 119–134. [Google Scholar] [CrossRef]
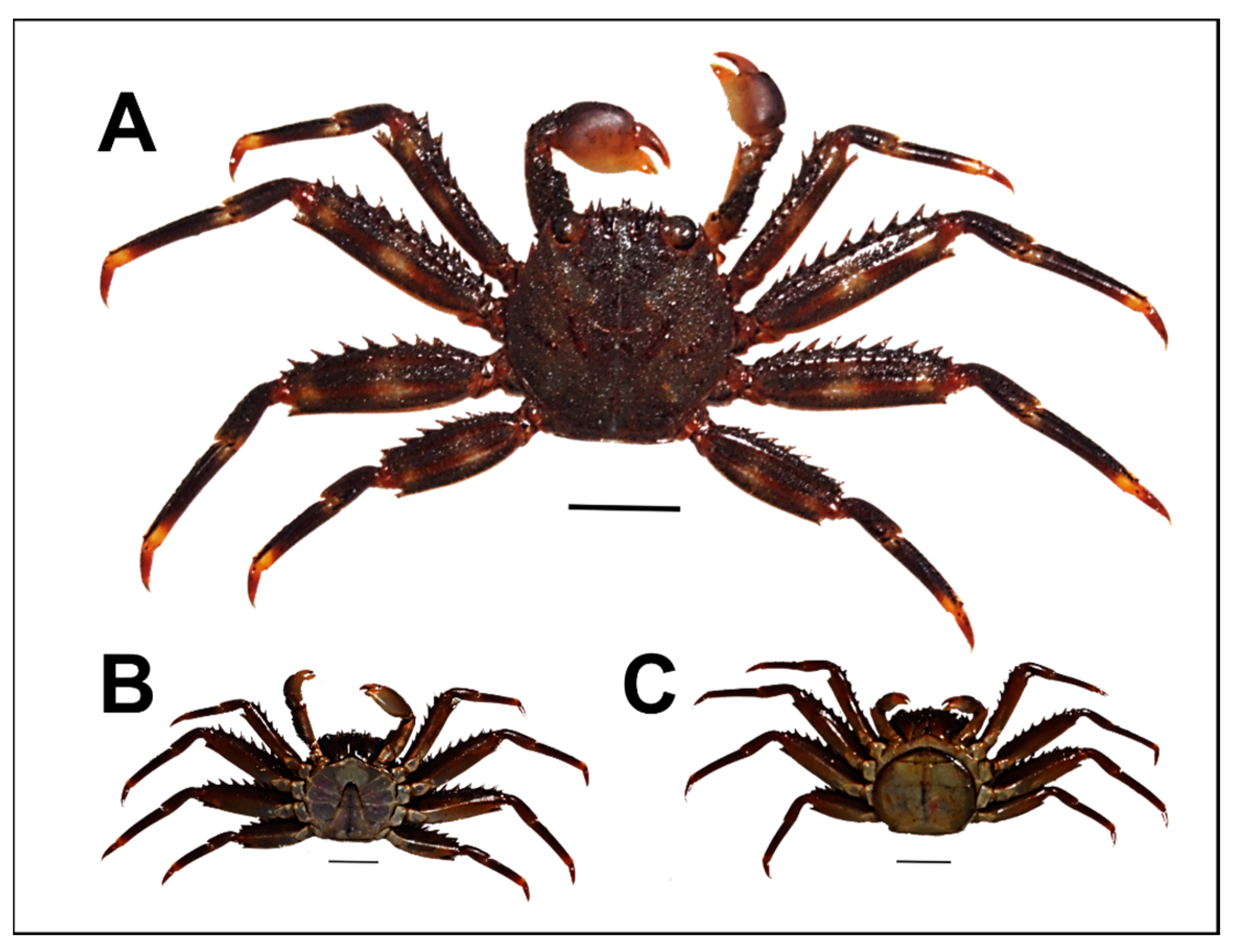

| CW | CL | BW | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | ||
| Arguineguín | Males | 101 | 5.48 | 17.56 | 9.99 | 2.86 | 6.24 | 18.01 | 10.70 | 2.99 | 0.07 | 2.55 | 0.56 | 0.52 |
| Females | 54 | 5.71 | 17.28 | 11.55 | 2.94 | 6.61 | 17.87 | 12.34 | 3.05 | 0.07 | 1.70 | 0.73 | 0.45 | |
| All | 155 | 5.48 | 17.56 | 10.53 | 2.97 | 6.24 | 18.01 | 11.27 | 3.10 | 0.07 | 2.55 | 0.62 | 0.50 | |
| Pozo Izquierdo | Males | 37 | 6.65 | 14.76 | 10.38 | 2.16 | 6.88 | 15.36 | 10.98 | 2.24 | 0.15 | 2.19 | 0.58 | 0.42 |
| Females | 11 | 5.92 | 14.95 | 10.74 | 2.43 | 6.85 | 15.45 | 11.47 | 2.21 | 0.12 | 2.01 | 0.67 | 0.50 | |
| All | 48 | 5.92 | 14.95 | 10.46 | 2.20 | 6.85 | 15.45 | 11.10 | 2.22 | 0.12 | 2.19 | 0.60 | 0.44 | |
| San Felipe | Males | 512 | 3.15 | 21.08 | 9.94 | 3.57 | 4.15 | 22.73 | 10.78 | 3.70 | 0.01 | 5.19 | 0.64 | 0.79 |
| Females | 284 | 5.19 | 19.96 | 11.04 | 3.23 | 5.69 | 22.30 | 19.95 | 3.39 | 0.06 | 3.55 | 0.74 | 0.60 | |
| All | 796 | 3.15 | 21.08 | 10.33 | 3.49 | 4.15 | 22.73 | 11.19 | 3.63 | 0.01 | 5.19 | 0.68 | 0.73 | |
| All areas | Males | 650 | 3.15 | 21.08 | 9.97 | 3.40 | 4.15 | 22.73 | 10.78 | 3.53 | 0.01 | 5.19 | 0.63 | 0.74 |
| Females | 349 | 5.19 | 19.96 | 11.11 | 3.16 | 5.69 | 22.30 | 11.99 | 3.30 | 0.06 | 3.55 | 0.74 | 0.58 | |
| All | 999 | 3.15 | 21.08 | 10.37 | 3.36 | 4.15 | 22.73 | 11.20 | 3.50 | 0.01 | 5.19 | 0.66 | 0.69 | |
| Sex-Ratio | Chi-Square | p-Value | |
|---|---|---|---|
| Arguineguín | 1:0.54 | 0.49 | 0.484 |
| Pozo Izquierdo | 1:0.27 | 6.25 | 0.012 |
| San Felipe | 1:0.60 | 17.94 | <0.001 |
| All areas | 1:0.57 | 10.82 | <0.001 |
| Relationships | Sex | a | b | CI 95% | CI 2.5% | R | p-Value | Growth Model |
|---|---|---|---|---|---|---|---|---|
| CL–BW | Females | 0.0003 | 3.0042 | 2.9181 | 3.0903 | 0.9408 | * | i |
| Males | 0.0002 | 3.1685 | 3.1103 | 3.2267 | 0.9566 | * | a+ | |
| All | 0.0003 | 3.1085 | 3.0590 | 3.1570 | 0.9563 | * | a+ | |
| CW–BW | Females | 0.0005 | 2.9032 | 2.8169 | 2.9894 | 0.9368 | * | a− |
| Males | 0.0004 | 3.0285 | 2.9681 | 3.0889 | 0.9492 | * | i | |
| All | 0.0005 | 2.9810 | 2.9300 | 3.3100 | 0.9501 | * | i |
| Parameters | All Population | Females | Males | |||
|---|---|---|---|---|---|---|
| ELEFAN SA | ELEFAN GA | ELEFAN SA | ELEFAN GA | ELEFAN SA | ELEFAN GA | |
| Asymptotic carapace length (mm) | 29.61 | 26.76 | 28.11 | 25.17 | 28.56 | 25.36 |
| Growth coefficient K (year−1) | 0.24 | 0.34 | 0.22 | 0.33 | 0.19 | 0.28 |
| Summer point oscillation (ts) | 0.04 | 0.28 | 0.11 | 0.51 | 0.09 | 0.32 |
| Amplitude of growth oscillation (C) | 0.07 | 0.60 | 0.23 | 0.50 | 0.04 | 0.50 |
| Growth performance index (Φ′) | 2.03 | 2.21 | 2.24 | 2.26 | 2.19 | 2.14 |
| t_anchor | 0.56 | 0.74 | 0.26 | 0.71 | 0.41 | 0.51 |
| Goodness of fit (Rn_max) score | 0.34 | 0.31 | 0.48 | 0.35 | 0.38 | 0.36 |
| Total Area (Ha) | Biomass Captured (g m−2) | Estimated Biomass Area (kg m−2) | |
|---|---|---|---|
| Arguineguín | 0.59 | 8.16 ± 5.06 | 48.14 ± 29.85 |
| Pozo Izquierdo | 0.45 | 10.65 ± 5.83 | 47.94 ± 26.25 |
| San Felipe | 0.63 | 15.06 ± 7.49 | 94.88 ± 47.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Marrero, A.; Bonino-Pérez, A.; Espino-Ruano, A.; Couce-Montero, L.; Jiménez-Alvarado, D.; Castro, J.J. Life History Parameters and Fishing Aspects of the Alien Nimble Spray Crab Percnon gibbesi in a Native Area of the Central-East Atlantic. Animals 2023, 13, 1427. https://doi.org/10.3390/ani13081427
Guerra-Marrero A, Bonino-Pérez A, Espino-Ruano A, Couce-Montero L, Jiménez-Alvarado D, Castro JJ. Life History Parameters and Fishing Aspects of the Alien Nimble Spray Crab Percnon gibbesi in a Native Area of the Central-East Atlantic. Animals. 2023; 13(8):1427. https://doi.org/10.3390/ani13081427
Chicago/Turabian StyleGuerra-Marrero, Airam, Antonio Bonino-Pérez, Ana Espino-Ruano, Lorena Couce-Montero, David Jiménez-Alvarado, and José J. Castro. 2023. "Life History Parameters and Fishing Aspects of the Alien Nimble Spray Crab Percnon gibbesi in a Native Area of the Central-East Atlantic" Animals 13, no. 8: 1427. https://doi.org/10.3390/ani13081427
APA StyleGuerra-Marrero, A., Bonino-Pérez, A., Espino-Ruano, A., Couce-Montero, L., Jiménez-Alvarado, D., & Castro, J. J. (2023). Life History Parameters and Fishing Aspects of the Alien Nimble Spray Crab Percnon gibbesi in a Native Area of the Central-East Atlantic. Animals, 13(8), 1427. https://doi.org/10.3390/ani13081427





