Going Wild in the City—Animal Feralization and Its Impacts on Biodiversity in Urban Environments
Abstract
Simple Summary
Abstract
1. Introduction
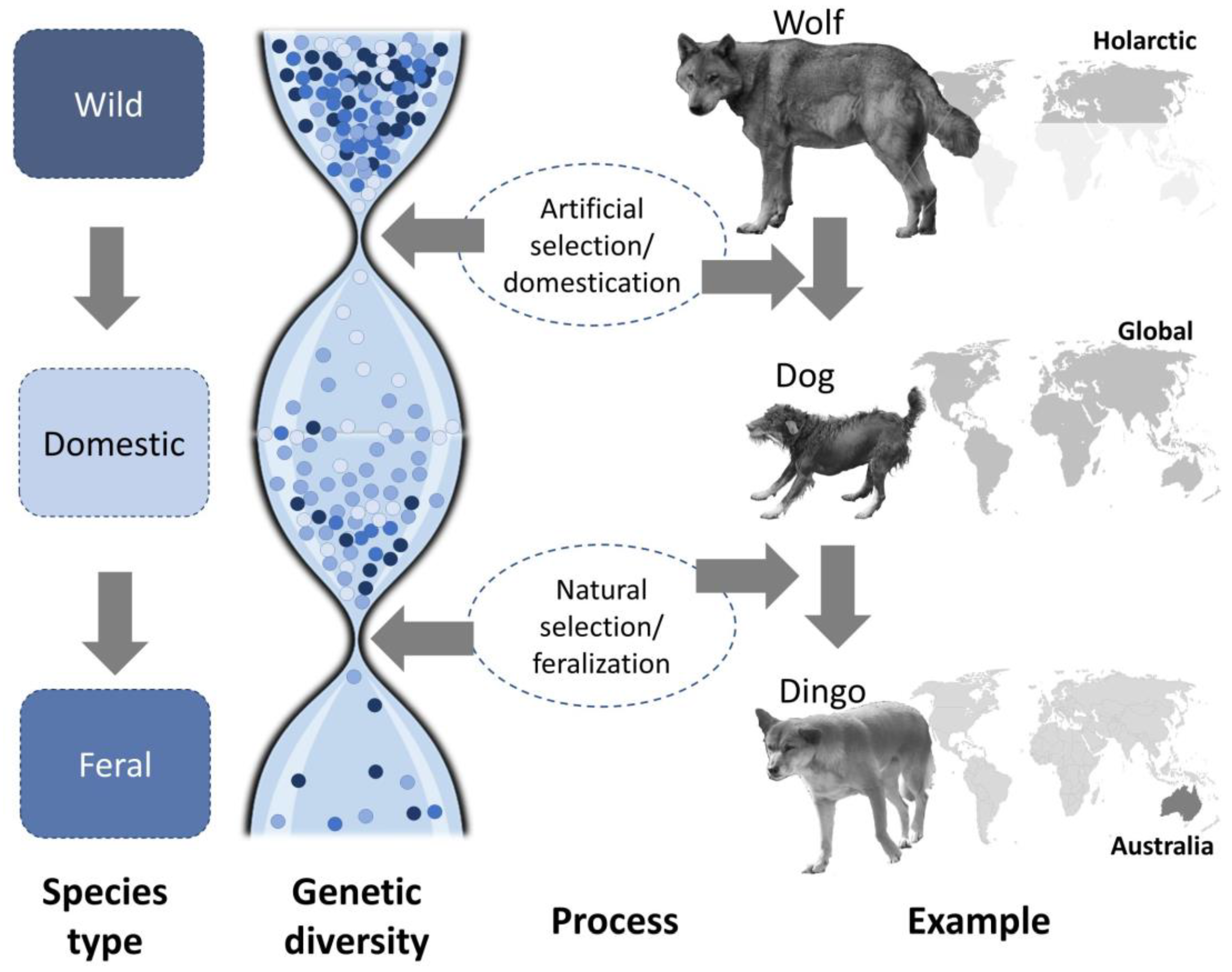
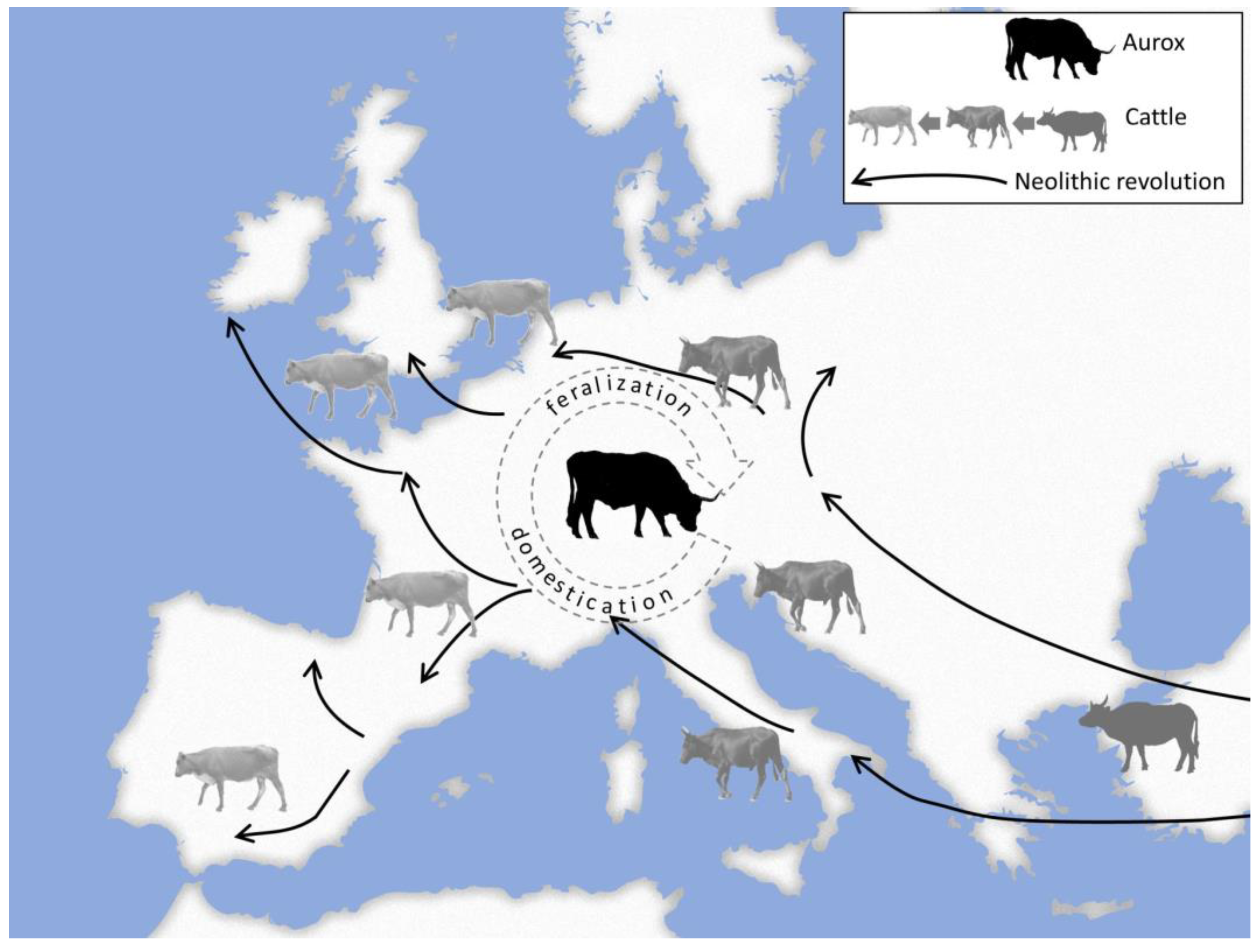
2. Domestication and Feralization
3. Conservation Impacts of Feralization
- Predation on native species. Despite an abundance of literature on the effects of native predators, such as wolves, on domesticated species, we have not found any comprehensive reviews of similar impacts by most feral species. North American bullfrogs (Lithobates catesbeiana), for example, were raised in captivity in many areas in connection with farming for frog leg meat. Once released or escaped, feral populations quickly became major predators of multiple species [26]. Perhaps the most consequential feral predator, however, is the domestic cat (Felis silvestris f. catus) [27].
- Competition with native species. Having been widely introduced from captive stocks, North American bullfrogs also effectively outcompeted many native anurans [26]. Similarly, the presence of feral pigs on Santa Cruz Island (California, USA) led to near extirpation of the native Island fox, Urocyon littoralis [28]. As with the previous category, however, we found species-specific reviews—e.g., for alfalfa (Medicago sativa) [29] and reviews of non-native impacts, which include feral taxa—e.g., for foxes and cats [30] and water hyacinth (Eichhornia crassipes) [31], which also causes eutrophication (Figure 2), but not comprehensive reviews of competition between feral and native species.
- 4.
- 5.
- Disease vectoring occurs when the presence of a feral taxon allows disease-causing organisms to spread to novel native taxa or increases the rate at which such spread occurs. For example, feral pigs have been shown to vector soil-borne plant pathogens in New Zealand [41]. Feral populations of the frog Xenopus laevis in Chile are infected with Batrachochytrium dendrobatidis, a fungal pathogen causing amphibian population declines, and can spread the fungus to native species [42].
- 6.
- Cases of ecological replacement of an extinct ecological equivalent are hard to assess for impact, since the extinction preceded the arrival of the feral taxon. For example, the European mink (Mustela lutreola) disappeared in many parts of its range before the American mink (Neovison vison) was introduced. However, in parts of the range the feral introduction contributed to the disappearance of the native species, and attempts to reintroduce the European mink are also hampered where the American counterpart is present [43]. Somewhat similarly, the wild horse (Equus ferus) had almost completely disappeared and attempts to rewild it are partially based on domesticated animals [44]. Similar issues with genetic mixing were seen in markhors (Capra falconeri) being bred at zoos for reintroduction, over a third of which were found to carry genes from domestic goats (Capra aegagrus f. hircus) [45]. A release of such hybrids might meet with conservation objectives, but could arguably be considered an introduction of a new form. Another interesting example of replacement of long-extinct ecological equivalents is provided by fallow deer (Dama dama) and its important role in transforming cultural landscapes in medieval England between the 11th and 16th century AD [46]. The conservation consequences of this intentional rewilding appear to be consistent with management choices or at least public wishes. For example, feral rabbits on Okunoshima island in Japan [47] and feral horses of Australia [48] are perceived as non-natural but nevertheless regarded as worthy of protection.
- 7.
- Some feral taxa take root in areas where there is no comparable species. Examples are feral parakeet (Psittacula) populations in different cities in Germany and France, [49] or greater rheas (Rhea americana) in northern Germany [50]. Such introduction may have no ecological impact on the kinds discussed above, because the ecological niche they invade was previously unoccupied, but other impacts, such as changes in fruit dispersal on sprouting success, as well as competition for nesting places and food with native urban birds, may arise in the case of the parakeet. Moreover, feral parakeets may pose a threat of spreading pathogens to other birds [49]. Although no negative impacts have so far been reported in terms of greater rheas in Germany, they may pose a threat for ground-nesting species owing to nest-trampling [50].
- 8.
- Finally, the contribution of feral taxa to human-wildlife conflict creates public relations problems eroding support for conservation. For example, feral Canada geese and other waterfowl (Figure 3C,D) have proliferated in urban settings to the point where they create a public nuisance [25]. Although a number of species fall into this category, particularly in urban settings, the most consequential example is the domestic cat. This species, while doubtlessly causing extensive depredation of birds and other species, is also beloved by many [27]. The resulting conflict, most notably between wildlife managers and animal rights advocates, is extremely difficult to resolve and contributes to a divide between pro-nature groups that might otherwise find much to cooperate on [51].
| Feral Taxon | Ecological Impacts | Region (Example) | Start of Feralization (Example) | Reference |
|---|---|---|---|---|
| Lagomorpha | ||||
| Oryctolgus cuniculus f. domestica | Negatively affecting natural vegetation | Okunoshima, Japan | 1970s | [47] |
| Rodentia | ||||
| Mus musculus f. domestica | - | Faray Island | 1940s | [52] |
| Carnivora | ||||
| Canis lupus f. familiaris | Despite the small population have an enormous negative impact on native fauna | Galapagos Islands | 1930s | [53] |
| Felis silvestris f. catus | Intermixing with progenitor taxon, predator of various taxa | Sardinia | 3000yBP | [54] |
| Neovison vison | Predator of various taxa, in competition with native mustelids | Germany | 1950s | [15] |
| Mustela putorius f. furo | Predator of various taxa | New Zealand | 1880s | [55] |
| Artiodactyla | ||||
| Camelus dromedarius | Negatively affecting natural vegetation | Australia | 1908–1911 | [56,57] |
| Lama guanicoe f. glama | Failed to become established | Australia | 1900 | [56] |
| Dama dama | Transforming cultural landscape | UK | 11th–16th century | [46] |
| Bos primigenius f. taurus | Transforming cultural landscape, surrogate taxon → “rewilding” | Oostvaardersplassen, Netherlands | 1980s | [58] |
| Bos gaurus f. frontalis | Intermixing with progenitor taxon | Bangladesh | ? | [59] |
| Bos mutus f. grunniens | Intermixing with progenitor taxon | Helan mountains, China | ? | [35] |
| Bubalus arnee f. bubalis | Natural vegetation | Western Australia | 1850s | [56,60] |
| Capra aegagrus f. hircus | Various taxonomic groups affected, severe impact on vegetation | Galapagos islands | 1813 | [61] |
| Ovis orientalis f. aries | - | Shackleford Banks, island, USA | 1940s | [62] |
| Sus scrofa f. domestica | Significant ecological damage, various taxa | South America | Since 1493 | [63] |
| Perissodactyla | ||||
| Equus ferus f. caballus | Overgrazing, affecting reproductive characteristics of dominant grass species | Assateague Island, USA | 1500s | [64] |
| Equus africanus f. asinus | Declared as “vermin” in some regions | Kimberleys, Australia | 1930s | [56] |
| Feral Taxon | Ecological Impacts | Region (Example) | Start of Feralization (Example) | Reference |
|---|---|---|---|---|
| Galliformes | ||||
| Gallus gallus f. domestica | Possibly reservoirs and vectors of avian diseases | Several Galapagos islands | Late 1990s | [53] |
| Pavo cristatus | - | UK | 1980s | [65] |
| Numida meleagris | Failed to become established | Australia | 1920s | [56] |
| Corturnix japonica | Important gene pool as native population decreasing | Hawaiian islands | 1920s-1950s | [20] |
| Meleagris gallopavo | - | Hawke’s Bay, New Zealand | 1860s | [66] |
| Columbiformes | ||||
| Columba livia f. domestica | Declared as “vermin” in some regions | Western Australia | 1950s | [56] |
| Anseriformes | ||||
| Alopochen aegyptiacus | - | The Hague, Netherlands | 1967 | [67] |
| Anser anser | Co-existing with autochthonous individuals of the same species | Germany | 1980s | [68] |
| Branta canadensis | Competition with native waterfowl | UK | 1660s | [25] |
| Anas platyrhynchos | Hybridization with native Anas gracilis | New Zealand | 1862 | [69] |
| Aix galericulata | - | Madeiran arc hipelago | 2010 | [70] |
| Passeriformes | ||||
| Acridotheres tristis | Predation and aggressive competition with native wildlife | Canberra, Australia | 1968 | [71] |
| Psittaciformes | ||||
| Melopsittacus undulatus | - | Florida, USA | Late 1950s | [72] |
| Psittacula krameri | Potentially becoming a disease vector | Germany, France | 1960 and 1970s | [49] |
| Myiopsitta monachus | - | New York, USA | Late 1960s | [72] |
| Pyrrhura molinae | - | Texas, USA | 1990s | [72] |
| Brotogeris versicolurus | - | California, USA | 1970s | [72] |
| Brotogeris chiriri | - | California, USA | 1970s | [72] |
| Amazona viridigenalis | - | California, USA | 1990s | [72] |
| Rheiformes | ||||
| Rhea americana | Possibly nest-trampling of ground-nesting bird species | Northern Germany | 2000 | [50] |
| Feral Taxon | Ecological Impacts | Region (Example) | Start of Feralization (Example) | Reference |
|---|---|---|---|---|
| “Reptiles” | ||||
| Trachemys scripta | Competition with native freshwater tortoises | Sicily, Italy | 1990s | [73] |
| Lissamphibians | ||||
| Lithobates catesbeiana | Competition with other amphibians, predator of various taxa | Santay Island, Ecuador | 1990s | [74] |
| “Osteichthyes” | ||||
| Cyprinus carpio | Because of early introduction difficult to assess | Central Europe | 1st–2nd century | [75] |
| Oncorhynchus mykiss | Threat to localized biota, various taxa | Italy | Since 1890s | [76] |
| Carassius gibelio f. auratus | Competition with native Carassius carassius | UK | 17th century | [77] |
| Insecta | ||||
| Apis mellifera scutellata | Hybridization with European Apis mellifera | Mexico | 1980s | [78] |
4. Urbanization
5. Conservation Impacts of Urbanization
6. Domestication, Feralization, and Urbanization: A Conservation Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeller, U.; Göttert, T. The relations between evolution and domestication reconsidered-implications for systematics, ecology, and nature conservation. Glob. Ecol. Conserv. 2019, 20, e00756. [Google Scholar] [CrossRef]
- Young, M.S. The evolution of domestic pets and companion animals. Vet. Clin. North Am. Small Anim. Pract. 1985, 15, 297–309. [Google Scholar] [CrossRef]
- Zeuner, F.E. A History of Domesticated Animals; Hutchinson: London, UK, 1963. [Google Scholar]
- Larson, G.; Fuller, D.Q. The evolution of animal domestication. Ann. Rev..Ecol. Evol. Syst. 2014, 45, 115–136. [Google Scholar] [CrossRef]
- Shiboleth, M. The Canaan Dog–Preserving a Biblical Dog in Modern Times. 2016. Available online: https://www.academia.edu/3572141/The_Canaan_Dog_Preserving_a_Biblical_Dog_in_Modern_Times (accessed on 1 February 2023).
- Bourke-Borrowes, D. The dog temple at Peking. J. R. Asiat. Soc. 1931, 18, 256–257. [Google Scholar] [CrossRef]
- Mech, L.D.; Paul, W.J. Wolf body mass cline across Minnesota related to taxonomy? Can. J. Zool. 2008, 86, 933–936. [Google Scholar] [CrossRef]
- Greer, K.A.; Canterberry, S.C.; Murphy, K.E. Statistical analysis regarding the effects of height and weight on life span of the domestic dog. Res. Vet. Sci. 2007, 82, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Trut, L.; Oskina, I.; Kharlamova, A. Animal evolution during domestication: The domesticated fox as a model. Bioessays 2009, 31, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.S.; Purugganan, M.D. Evolution of crop species: Genetics of domestication and diversification. Nat. Rev. Genet. 2013, 14, 840–852. [Google Scholar] [CrossRef]
- Bechtel, H.B.; Bechtel, E. Color mutations in the corn snake (Elaphe guttata guttata): Review and additional breeding data. J. Hered. 1989, 80, 272–276. [Google Scholar] [CrossRef]
- Snyder, N.F.; Derrickson, S.R.; Beissinger, S.R.; Wiley, J.W.; Smith, T.B.; Toone, W.D.; Miller, B. Limitations of captive breeding in endangered species recovery. Conserv. Biol. 1996, 10, 338–348. [Google Scholar] [CrossRef]
- Gering, E.; Incorvaia, D.; Henriksen, R.; Conner, J.; Getty, T.; Wright, D. Getting back to nature: Feralization in animals and plants. Trends Eco. Evol. 2019, 34, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Bonacic, C.; Almuna, R.; Ibarra, J.T. Biodiversity conservation requires management of feral domestic animals. Trends Ecol. Evol. 2019, 34, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, L.; Palazon, S. The American mink in Europe: Status, impacts, and control. Biol. Conserv. 2007, 134, 470–483. [Google Scholar] [CrossRef]
- Greig, K.; Gosling, A.; Collins, C.J.; Boocock, J.; McDonald, K.; Addison, D.J.; Allen, M.S.; David, B.; Gibbs, M.; Higham, C.F.W.; et al. Complex history of dog (Canis familiaris) origins and translocations in the Pacific revealed by ancient mitogenomes. Sci. Rep. 2018, 8, 9130. [Google Scholar] [CrossRef]
- Jousson, A.; Christe, C.; Stauffer, F.; Marazzi, B.; Aberlenc, F.; Maspoli, G.; Naciri, Y. Panmixia and active colonisation of the invasive palm Trachycarpus fortunei (Arecaceae) in Southern Switzerland and Northern Italy as inferred by microsatellites and SNP markers. Biol. Invasions 2022, 24, 3737–3756. [Google Scholar] [CrossRef]
- Kolbe, J.J.; Glor, R.E.; Schettino, L.R.; Lara, A.C.; Larson, A.; Losos, J.B. Multiple sources, admixture, and genetic variation in introduced Anolis lizard populations. Conserv. Biol. 2007, 21, 1612–1625. [Google Scholar] [CrossRef]
- Price, E.O. Behavioral aspects of animal domestication. Q. Rev. Biol. 1984, 59, 1–32. [Google Scholar] [CrossRef]
- Nichols, C.R. A Comparison of the Reproductive and Behavioural Differences in Feral and Domestic Japanese Quail. Doctoral Dissertation, University of British Columbia, Vancouver, BC, Canada, 1991. [Google Scholar]
- Gamborg, C.; Gremmen, B.; Christiansen, S.B.; Sandoe, P. De-domestication: Ethics at the intersection of landscape restoration and animal welfare. Environ. Values 2010, 19, 57–78. [Google Scholar] [CrossRef]
- Hanspach, J.; Kühn, I.; Pyšek, P.; Boos, E.; Klotz, S. Correlates of naturalization and occupancy of introduced ornamentals in Germany. Perspectives in Plant Ecology, Evol. Syst. 2008, 10, 241–250. [Google Scholar] [CrossRef]
- Kowarik, I. Urban ornamentals escaped from cultivation. In Crop Ferality and Volunteerism; CRC Press: Boca Raton, FL, USA, 2005; pp. 97–121. [Google Scholar]
- Anderson, C.B.; Soto, N.; Cabello, J.L.; Pastur, G.M.; Lencinas, M.V.; Wallem, P.K.; Antúnez, D.; Davis, E. Castor canadensis Kuhl (North American Beaver): Building effective alliances between research and management to mitigate the impacts of an invasive ecosystem engineer: Lessons from the study and control of Castor canadensis in the Fuegian archipelago. In A Handbook of Global Freshwater Invasive Species; Francis, R.A., Ed.; Earthscan: London, UK, 2012; pp. 343–355. [Google Scholar]
- Allan, J.R.; Kirby, J.S.; Feare, C.J. The biology of Canada geese Branta canadensis in relation to the management of feral populations. Wildl. Biol. 1995, 1, 129–143. [Google Scholar] [CrossRef]
- D’Amore, A. Rana (Lithobates) catesbeiana shaw (American bullfrog). In A Handbook of Global Freshwater Invasive Species; Francis, R.A., Ed.; Earthscan: New York, NY, USA, 2012; pp. 321–330. [Google Scholar]
- Lepczyk, C.A.; Duffy, D.C.; Bird, D.M.; Calver, M.; Cherkassky, D.; Cherkassky, L.; Dickman, C.R.; Hunter, D.; Jessup, D.; Longcore, T.; et al. A science-based policy for managing free-roaming cats. Biol. Invasions 2022, 24, 3693–3701. [Google Scholar] [CrossRef]
- Melstrom, R.T. Managing apparent competition between the feral pigs and native foxes of Santa Cruz Island. Ecol. Econ. 2014, 107, 157–162. [Google Scholar] [CrossRef]
- Bagavathiannan, M.V.; Van Acker, R.C. The biology and ecology of feral alfalfa (Medicago sativa L.) and its implications for novel trait confinement in North America. Crit. Rev. Plant Sci. 2009, 28, 69–87. [Google Scholar] [CrossRef]
- Dickman, C.R. Impact of exotic generalist predators on the native fauna of Australia. Wildl. Biol. 1996, 2, 185–195. [Google Scholar] [CrossRef]
- Williams, A.E.; Hecky, R.E. Invasive Aquatic Weeds and Eutrophication: The Case of Water Hyacinth in Lake Victoria. In Restoration and Management of Tropical Eutrophic Lakes; Williams, A.E., Hecky, R.E., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 211–250. [Google Scholar]
- Piechocki, R. Wildkatze Felis silvestris SCHREBER. In Buch der Hege Band 1 Haarwild, 5th ed.; Stubbe, M., Ed.; Deutscher Landwirtschaftsverlag: Berlin, Germany, 1989; Volume 1, pp. 429–452. [Google Scholar]
- Hubbard, A.L.; McOris, S.; Jones, T.W.; Boid, R.; Scott, R.; Easterbee, N. Is survival of European wildcats Felis silvestris in Britain threatened by interbreeding with domestic cats? Biol. Conserv. 1992, 61, 203–208. [Google Scholar] [CrossRef]
- Pinto, M.A.; Rubink, W.L.; Patton, J.C.; Coulson, R.N.; Johnston, J.S. Africanization in the United States: Replacement of feral European honeybees (Apis mellifera L.) by an African hybrid swarm. Genetics 2005, 170, 1653–1665. [Google Scholar] [CrossRef]
- Leslie, D.M.; Schaller, G.B. Bos grunniens and Bos mutus (Artiodactyla: Bovidae). Mamm. Species 2009, 2009, 1–17. [Google Scholar] [CrossRef]
- de Jong, J.F.; Iacolina, L.; Prins, H.H.; van Hooft, P.; Crooijmans, R.P.; van Wieren, S.E.; Baños, J.V.; Baubet, E.; Cahill, S.; Ferreira, E.; et al. Spatial genetic structure of European wild boar, with inferences on late-Pleistocene and Holocene demographic history. Heredity 2023, 1–10. [Google Scholar] [CrossRef]
- Wu, M.Y.; Forcina, G.; Low, G.W.; Sadanandan, K.R.; Gwee, C.Y.; van Grouw, H.; Wu, S.; Edwards, S.V.; Baldwin, M.W.; Rheindt, F.E. Historic samples reveal loss of wild genotype through domestic chicken introgression during the Anthropocene. PLoS Genet. 2023, 19, e1010551. [Google Scholar] [CrossRef]
- Rezaei, H.R.; Naderi, S.; Chintauan-Marquier, I.C.; Taberlet, P.; Virk, A.T.; Naghash, H.R.; Rioux, D.; Kaboli, M.; Pompanon, F. Evolution and taxonomy of the wild species of the genus Ovis (Mammalia, Artiodactyla, Bovidae). Mol. Phylogenet. Evol. 2010, 54, 315–326. [Google Scholar] [CrossRef]
- Smith, S.G. Experimental and natural hybrids in north American Typha (Typhaceae). Am. Midl. Nat. 1967, 78, 257–287. [Google Scholar] [CrossRef]
- Parham, J.F.; Papenfuss, T.J.; Sellas, A.B.; Stuart, B.L.; Simison, W.B. Genetic variation and admixture of red-eared sliders (Trachemys scripta elegans) in the USA. Mol. Phylogenet. Evol. 2020, 145, 106722. [Google Scholar] [CrossRef] [PubMed]
- Bassett, I.E.; Horner, I.J.; Hough, E.G.; Wolber, F.M.; Egeter, B.; Stanley, M.C.; Krull, C.R. Ingestion of infected roots by feral pigs provides a minor vector pathway for kauri dieback disease Phytophthora agathidicida. For. Int. J. For. Res. 2017, 90, 640–648. [Google Scholar] [CrossRef]
- Solís, R.; Lobos, G.; Walker, S.F.; Fisher, M.; Bosch, J. Presence of Batrachochytrium dendrobatidis in feral populations of Xenopus laevis in Chile. Biol. Invasions 2010, 12, 1641–1646. [Google Scholar] [CrossRef]
- Põdra, M.; Gómez, A. Rapid expansion of the American mink poses a serious threat to the European mink in Spain. Mammalia 2018, 82, 580–588. [Google Scholar] [CrossRef]
- Göttert, T.; Starik, N. Human–Wildlife Conflicts across Landscapes—General Applicability vs. Case Specificity. Diversity 2022, 14, 380. [Google Scholar] [CrossRef]
- Hammer, S.E.; Schwammer, H.M.; Suchentrunk, F. Evidence for introgressive hybridization of captive markhor (Capra falconeri) with domestic goat: Cautions for reintroduction. Biochem. Genet. 2008, 46, 216–226. [Google Scholar] [CrossRef]
- Sykes, N.; Ayton, G.; Bowen, F.; Baker, K.; Baker, P.; Carden, R.F.; Dicken, C.; Evans, J.; Hoelzel, A.R.; Higham, T.F.G.; et al. Wild to domestic and back again: The dynamics of fallow deer management in medieval England (c. 11th-16th century AD). Sci. Technol. Archaeol. Res. 2016, 2, 113–126. [Google Scholar] [CrossRef]
- Demello, M. The Rabbits of Okunoshima: How Feral Rabbits Alter Space, Create Relationships, and Communicate with People and Each Other. In Texts, Animals, Environments: Zoopoetics and Ecopoetics; Rombach Druck-und Verlagshaus: Breisgau, Germany, 2019. [Google Scholar]
- Nimmo, D.G.; Miller, K.K. Ecological and human dimensions of management of feral horses in Australia: A review. Wildl. Res. 2007, 34, 408–417. [Google Scholar] [CrossRef]
- Kessler, S.; Heenemann, K.; Krause, T.; Twietmeyer, S.; Fuchs, J.; Lierz, M.; Corman, V.M.; Vahlenkamp, T.M.; Rubbenstroth, D. Monitoring of free-ranging and captive Psittacula populations in Western Europe for avian bornaviruses, circoviruses and polyomaviruses. Avian Pathol. 2020, 49, 119–130. [Google Scholar] [CrossRef]
- Lenzen, A.; Milde, L. Relation between Greater Rheas (Rhea americana) and Ground Nesting Birds in Northern Germany. Bachelor’s Thesis, Van Hall Larenstein University of Applied Sciences, Leeuwarden, The Netherlands, 2018. [Google Scholar]
- Perry, G.; Sarge, M.A.; Perry, D. Alternative facts and alternative views: Scientists, managers, and animal rights activists. In Problematic Wildlife II: New Conservation and Management Challenges in the Human-Wildlife Interactions; Angelici, F.M., Rossi, L., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 421–450. [Google Scholar]
- Souquet, L.; Chevret, P.; Ganem, G.; Auffray, J.C.; Ledevin, R.; Agret, S.; Hautier, L.; Renaud, S. Back to the wild: Does feralization affect the mandible of non-commensal house mice (Mus musculus domesticus)? Biol. J. Linn. Soc. 2019, 126, 471–486. [Google Scholar] [CrossRef]
- Phillips, R.B.; Wiedenfeld, D.A.; Snell, H.L. Current status of alien vertebrates in the Galápagos Islands: Invasion history, distribution, and potential impacts. Biol. Invasions 2012, 14, 461–480. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Biro, Z.S.; Herrmann, M.; Hupe, K.; Fernandes, M.; Ragni, B.; Szemethy, L.; Randi, E. Genetic distinction of wildcat (Felis silvestris) populations in Europe, and hybridization with domestic cats in Hungary. Mol. Ecol. 2003, 12, 2585–2598. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Ragg, J.R.; Moller, H.; Waldrup, K.A. Diet of feral ferrets (Mustela furo) from pastoral habitats in Otago and Southland, New Zealand. N. Z. J. Zool. 1995, 22, 363–369. [Google Scholar] [CrossRef]
- Long, J.L. Introduced birds and mammals in Western Australia, 1st ed.; Agriculture Protection Board of Western Australia: South Perth, WA, USA, 1972. [Google Scholar]
- Pople, A.R.; McLeod, S.R. Demography of feral camels in central Australia and its relevance to population control. Rangel. J. 2010, 32, 11–19. [Google Scholar] [CrossRef]
- Lorimer, J.; Driessen, C. Experiments with the wild at the Oostvaardersplassen. Ecosystems 2014, 35, 44–52. [Google Scholar]
- Uzzaman, M.R.; Bhuiyan, M.S.A.; Edea, Z.; Kim, K.S. Semi-domesticated and irreplaceable genetic resource gayal (Bos frontalis) needs effective genetic conservation in Bangladesh: A review. Asian-Australas. J. Anim. Sci. 2014, 27, 1368. [Google Scholar] [CrossRef]
- Werner, P.A. Impact of feral water buffalo and fire on growth and survival of mature savanna trees: An experimental field study in Kakadu National Park, northern Australia. Austral Ecol. 2005, 30, 625–647. [Google Scholar]
- Cruz, F.; Carrion, V.; Campbell, K.J.; Lavoie, C.; Donlan, C.J. Bio-economics of large-scale eradication of feral goats from Santiago Island, Galapagos. J. Wildl. Manag. 2009, 73, 191–200. [Google Scholar] [CrossRef]
- Hess, S.C.; Van Vuren, D.H.; Witmer, G.W. Feral goats and sheep. In Ecology and Management of Terrestrial Vertebrate Invasive Species in the United States; Pitt, N.C., Beasley, J.C., Witmer, G.W., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 289–309. [Google Scholar]
- Hegel, C.G.Z.; Faria, G.M.M.; Ribeiro, B.; Salvador, C.H.; Rosa, C.; Pedrosa, F.; Batista, G.; Sales, L.P.; Wallau, M.; Fornel, R.; et al. Invasion and spatial distribution of wild pigs (Sus scrofa L.) in Brazil. Biol. Invasions 2022, 24, 3681–3692. [Google Scholar] [CrossRef]
- Seliskar, D.M. The response of Ammophila breviligulata and Spartina patens (Poaceae) to grazing by feral horses on a dynamic mid-Atlantic barrier island. Am. J. Bot. 2003, 90, 1038–1044. [Google Scholar] [CrossRef]
- Cheke, A. A long-standing feral Indian peafowl population in Oxfordshire, and a brief survey of the species in Britain. Br. Birds 2019, 112, 337–348. [Google Scholar]
- Beauchamp, A.J. 2013 [updated 2022]. Wild turkey| korukoru. In New Zealand Birds Online; Miskelly, C.M., Ed.; Available online: https://www.nzbirdsonline.org.nz/ (accessed on 13 December 2022).
- Lensink, R. Aspects of the biology of Egyptian Goose Alopochen aegyptiacus colonizing The Netherlands. Bird Study 1999, 46, 195–204. [Google Scholar] [CrossRef]
- Woog, F.; Schmolz, M.; Lachenmaier, K. Die Bestandsentwicklung der Graugans (Anser anser) im Stadtkreis Stuttgart. Ornithol. Jh. Bad.-Württ. 2008, 24, 141–146. [Google Scholar]
- Dyer, J.; Williams, M. An introduction most determined: Mallard (Anas platyrhynchos) to New Zealand. Notornis 2010, 57, 178–195. [Google Scholar]
- Trujillo, D. First data on breeding of Mandarin Duck Aix galericulata in the Madeiran archipelago. Bocagiana Mus. História Nat. Funchal 2012, 235, 1–5. [Google Scholar]
- Tidemann, C.R. Mitigation of the Impact of Mynas on Biodiversity and Public Amenity; Report; The Australian National University: Canberra, ACT, Australia, 2002. [Google Scholar]
- Butler, C.J. Feral parrots in the continental United States and United Kingdom: Past, present, and future. J. Avian Med. Surg. 2005, 19, 142–149. [Google Scholar] [CrossRef]
- Liuzzo, M. First evidence of an egg-laying attempt of feral Trachemys scripta scripta (Schoepff, 1792) in Sicily (Lake Pergusa, Italy). Herpetol. Notes 2020, 13, 365–368. [Google Scholar]
- Cruz Cordovez, C.; Herrera, I.; Espinoza, F.; Rizzo, K. New record of a feral population of Lithobates catesbeianus Shaw, 1802 in a protected area (Santay Island) in the Ecuadorian coast. BioInvasions Rec. 2020, 9, 421–433. [Google Scholar] [CrossRef]
- Füllner, G. Die Domestikation des Karpfens. 1. Teil. Fisch. Angler Sachs. 2002, 9, 82–83. [Google Scholar]
- Candiotto, A.; Bo, T.; Fenoglio, S. Biological and ecological data on an established rainbow trout (Oncorhynchus mykiss) population in an Italian stream. Fundam. Appl. Limnol. 2011, 179, 67–76. [Google Scholar] [CrossRef]
- Tarkan, A.S.; Cucherousset, J.; Zięba, G.; Godard, M.J.; Copp, G.H. Growth and reproduction of introduced goldfish Carassius auratus in small ponds of southeast England with and without native crucian carp Carassius carassius. J. Appl. Ichthyol. 2010, 26, 102–108. [Google Scholar] [CrossRef]
- Quezada-Euán, J.J.G.; Medina, L.M. Hybridization between European and Africanized honeybees (Apis mellifera L.) in tropical Yucatan, Mexico. I. Morphometric changes in feral and managed colonies. Apidologie 1998, 29, 555–568. [Google Scholar] [CrossRef]
- Davis, K. The origin and growth of urbanization in the world. Am. J. Sociol. 1955, 60, 429–437. [Google Scholar] [CrossRef]
- Cohen, M.N. Health and the Rise of Civilization; Yale University Press: New Haven, CT, USA, 1989. [Google Scholar]
- Perry, G.; Stone, L.A.; Obaid, O. Adapting US foreign assistance for a rapidly urbanizing world. Sci. Dipl. 2021, 10, 1–16. [Google Scholar]
- World Bank. World Development Report 2009: Reshaping Economic Geography; World Bank: Washington, DC, USA, 2009. [Google Scholar]
- Hunter, R.F.; Cleland, C.; Cleary, A.; Droomers, M.; Wheeler, B.W.; Sinnett, D.; Nieuwenhuijsen, M.J.; Braubach, M. Environmental, health, wellbeing, social and equity effects of urban green space interventions: A meta-narrative evidence synthesis. Environ. Int. 2019, 130, 104923. [Google Scholar] [CrossRef] [PubMed]
- Flies, E.J.; Lim, J.N.W.; Douglas, I. Editorial: Urban ecology and human health. Front. Ecol. Evol. 2022, 10, 1032022. [Google Scholar] [CrossRef]
- Crompton, J.L. The impact of parks on property values: A review of the empirical evidence. J. Leis. Res. 2001, 33, 1–31. [Google Scholar] [CrossRef]
- Perry, G.; Boal, C.; Verble, R.; Wallace, M. “Good” and “bad” urban wildlife. In Problematic Wildlife II: New Conservation and Management Challenges in the Human-Wildlife Interactions; Angelici, F.M., Rossi, L., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 141–170. [Google Scholar]
- Rigolon, A.; Browning, M.H.; Lee, K.; Shin, S. Access to urban green space in cities of the Global South: A systematic literature review. Urban Sci. 2018, 2, 67. [Google Scholar] [CrossRef]
- Perry, G.; Gebresenbet, F.; DaPra, M.; Branco, P.; Whibesilassie, W.; Jelacic, M.; Eyob, A.E. Why Urban Ecology Matters in Ethiopia. Front. Ecol. Evol. 2022, 10, 843698. [Google Scholar] [CrossRef]
- Perry, G.; Dmi’el, R. Urbanization and sand dunes in Israel: Direct and indirect effects. Isr. J. Ecol. Evol. 1995, 41, 33–41. [Google Scholar]
- McDonald, R.I.; Mansur, A.V.; Ascensão, F.; Crossman, K.; Elmqvist, T.; Gonzalez, A.; Güneralp, B.; Haase, D.; Hamann, M.; Hillel, O.; et al. Research gaps in knowledge of the impact of urban growth on biodiversity. Nat. Sustain. 2020, 3, 16–24. [Google Scholar] [CrossRef]
- Beatley, T. Habitat Conservation Planning: Endangered Species and Urban Growth; University of Texas Press: Austin, TX, USA, 1994. [Google Scholar]
- Li, G.; Fang, C.; Li, Y.; Wang, Z.; Sun, S.; He, S.; Qi, W.; Bao, C.; Ma, H.; Fan, Y.; et al. Global impacts of future urban expansion on terrestrial vertebrate diversity. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Higgs, E.S.; Hall, C.M. Defining novel ecosystems. In Novel Ecosystems: Intervening in the New Ecological World Order; Hobbs, R.J., Higgs, E.S., Hall, C.M., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 58–60. [Google Scholar]
- Adams, L.W.; Leedy, D.L. Integrating Man and Nature in the Metropolitan Environment, Proceedings of a National Symposium on Urban Wildlife; National Institute for Urban Wildlife: Columbia, MD, USA, 1986; pp. 4–7. [Google Scholar]
- Stenberg, K.; Shaw, W.W. Wildlife Conservation and New Residential Developments; University of Arizona Press: Tucson, AZ, USA, 1986. [Google Scholar]
- Carlen, E.; Munshi-South, J. Widespread genetic connectivity of feral pigeons across the Northeastern megacity. Evol. Appl. 2021, 14, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.; Buchanan, B.W.; Fisher, R.N.; Salmon, M.; Wise, S.E. Effects of artificial night lighting on reptiles and amphibians in urban environments. In Urban Herpetology. Herpetological Conservation; Jung, R.E., Mitchell, J.C., Eds.; Society for the Study of Amphibians and Reptiles: Salt Lake City, UT, USA, 2008; Volume 3, pp. 239–256. [Google Scholar]
- Wandeler, P.; Funk, S.M.; Largiader, C.R.; Gloor, S.; Breitenmoser, U. The city-fox phenomenon: Genetic consequences of a recent colonization of urban habitat. Mol. Ecol. 2003, 12, 647–656. [Google Scholar] [CrossRef]
- Fiderer, C.; Göttert, T.; Zeller, U. Movement patterns and habitat preferences of red foxes (Vulpes vulpes) and raccoons (Procyon lotor) in a Special Protection Area in the close vicinity of Berlin. In Biodiversity and the Urban-Rural Iinterface: Conflicts vs. Opportunities-Proceedings of an International Workshop in Linde, Germany; Zeller, U., Perry, G., Göttert, T., Eds.; Humboldt-Universität zu Berlin: Berlin, Germany, 2019; pp. 16–17. [Google Scholar]
- Sosa, J.A. Effects of Urbanization on Movements, Activity, and Translocation Site Fidelity of Ornate Box Turtles (Terrapene ornata ornata) in the Southern High Plains of Texas. Master’s Thesis, Texas Tech University, Lubbock, TX, USA, 2009. [Google Scholar]
- Boal, C.W.; Dykstra, C.R. Urban Raptors: Ecology and Conservation of Birds of Prey in Cities; Island Press: Washington, DC, USA, 2018; pp. 1–302. [Google Scholar]
- Murcia, C.; Aronson, J.; Kattan, G.H.; Moreno-Mateos, D.; Dixon, K.; Simberloff, D. A critique of the ‘novel ecosystem’ concept. Trends Ecol. Evol. 2014, 29, 548–553. [Google Scholar] [CrossRef]
- Valentine, L.E.; Ramalho, C.E.; Mata, L.; Craig, M.D.; Kennedy, P.L.; Hobbs, R.J. Novel resources: Opportunities for and risks to species conservation. Front. Ecol. Environ. 2020, 18, 558–566. [Google Scholar] [CrossRef]
- Simberloff, D. Non-native invasive species and novel ecosystems. F1000prime Rep. 2015, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Siegmund-Schultze, M.; Rischkowsky, B. Relating household characteristics to urban sheep keeping in West Africa. Agric. Syst. 2001, 67, 139–152. [Google Scholar] [CrossRef]
- Gering, E.; Incorvaia, D.; Henriksen, R.; Wright, D.; Getty, T. Maladaptation in feral and domesticated animals. Evol. Appl. 2019, 12, 1274–1286. [Google Scholar] [CrossRef] [PubMed]
- Tomiałojć, L. Human initiation of synurbic populations of waterfowl, raptors, pigeons and cage birds. In Ecology and Conservation of Birds in Urban Environments; Murgui, E., Hedblom, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 271–286. [Google Scholar]
- Jarvis, P.J. Feral animals in the urban environment. In The Routledge Handbook of Urban Ecology; Douglas, I., Anderson, P.M.L., Goode, D., Houck, M.C., Maddox, D., Nagendra, H., Tan, P.Y., Eds.; Routledge: Oxfordshire, UK, 2010; pp. 385–394. [Google Scholar]
- Hunter, M.E.; Johnson, N.A.; Smith, B.J.; Davis, M.C.; Butterfield, J.S.; Snow, R.W.; Hart, K.M. Cytonuclear discordance in the Florida Everglades invasive Burmese python (Python bivittatus) population reveals possible hybridization with the Indian python (P. molurus). Ecol. Evol. 2018, 8, 9034–9047. [Google Scholar] [CrossRef] [PubMed]
- Knox, G.W.; Wilson, S.B. Evaluating north and south Florida landscape performance and fruiting of ten cultivars and a wild-type selection of Nandina domestica, a potentially invasive shrub. J. Environ. Hortic. 2006, 24, 137–142. [Google Scholar] [CrossRef]
- Everard, M.; Pinder, A.C.; Raghavan, R.; Kataria, G. Are well-intended Buddhist practices an under-appreciated threat to global aquatic biodiversity? Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 136–141. [Google Scholar] [CrossRef]
- Borden, J.B.; Flory, S.L. Urban evolution of invasive species. Front. Ecol. Environ. 2021, 19, 184–191. [Google Scholar] [CrossRef]
- Von der Lippe, M.; Kowarik, I. Do cities export biodiversity? Traffic as dispersal vector across urban–rural gradients. Divers. Distrib. 2008, 14, 18–25. [Google Scholar] [CrossRef]
- Kowarik, I.; Von der Lippe, M. Secondary wind dispersal enhances long-distance dispersal of an invasive species in urban road corridors. NeoBiota 2011, 9, 49. [Google Scholar] [CrossRef]
- Padayachee, A.L.; Irlich, U.M.; Faulkner, K.T.; Gaertner, M.; Procheş, Ş.; Wilson, J.R.; Rouget, M. How do invasive species travel to and through urban environments? Biol. Invasions 2017, 19, 3557–3570. [Google Scholar] [CrossRef]
- Adams, C.E.; Lindsey, K.J.; Ash, S.J. Urban Wildlife Management; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Murgui, E.; Hedblom, M. Ecology and Conservation of Birds in Urban Environments; Springer: Cham, Switzerland, 2017; pp. 35–54. [Google Scholar]
- Shochat, E.; Lerman, S.B.; Anderies, J.M.; Warren, P.S.; Faeth, S.H.; Nilon, C.H. Invasion, competition, and biodiversity loss in urban ecosystems. BioScience 2010, 60, 199–208. [Google Scholar] [CrossRef]
- Guillaumet, A.; Russell, I.J. Bird Communities in a Changing World: The Role of Interspecific Competition. Diversity 2022, 14, 857. [Google Scholar] [CrossRef]
- Combs, M.A.; Kache, P.A.; VanAcker, M.C.; Gregory, N.; Plimpton, L.D.; Tufts, D.M.; Fernandez, M.P.; Diuk-Wasser, M.A. Socio-ecological drivers of multiple zoonotic hazards in highly urbanized cities. Glob. Chang. Biol. 2022, 28, 1705–1724. [Google Scholar] [CrossRef]
- Rodriguez, F.; Luna, B.S.; Calderon, O.; Manriquez-Roman, C.; Amezcua-Winter, K.; Cedillo, J.; Garcia-Vazquez, R.; Tejeda, I.A.; Romero, A.; Waldrup, K.; et al. Surveillance of Trypanosoma cruzi infection in Triatomine vectors, feral dogs and cats, and wild animals in and around El Paso county, Texas, and New Mexico. PLoS Negl. Trop. Dis. 2021, 15, e0009147. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Haman, K.H.; Sizemore, G.C.; Farmer, C. Quantifying the presence of feral cat colonies and Toxoplasma gondii in relation to bird conservation areas on O’ahu, Hawai’i. Conserv. Sci. Pract. 2020, 2, e179. [Google Scholar] [CrossRef]
- Serieys, L.E.; Hammond-Aryee, K.; Bishop, J.; Broadfield, J.; O’Riain, M.J.; van Helden, P.D. High seroprevalence of Toxoplasma gondii in an urban caracal (Caracal caracal) population in South Africa. J. Wildl. Dis. 2019, 55, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Yung, L.; Schwarze, S.; Carr, W.; Chapin III, F.S.; Marris, E. Engaging the public in novel ecosystems. In Novel Ecosystems: Intervening in the New Ecological World Order; Hobbs, R.J., Higgs, E.S., Hall, C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 247–256. [Google Scholar]
- Coman, I.A.; Cooper-Norris, C.E.; Longing, S.; Perry, G. It Is a Wild World in the City: Urban Wildlife Conservation and Communication in the Age of COVID-19. Diversity 2022, 14, 539. [Google Scholar] [CrossRef]
- O’Briena, L.E.; Urbaneka, R.E.; Gregory, J.D. Ecological Functions and Human Benefits of Urban Forests. Urban For. Urban Green. 2022, 75, 127707. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Göttert, T.; Perry, G. Going Wild in the City—Animal Feralization and Its Impacts on Biodiversity in Urban Environments. Animals 2023, 13, 747. https://doi.org/10.3390/ani13040747
Göttert T, Perry G. Going Wild in the City—Animal Feralization and Its Impacts on Biodiversity in Urban Environments. Animals. 2023; 13(4):747. https://doi.org/10.3390/ani13040747
Chicago/Turabian StyleGöttert, Thomas, and Gad Perry. 2023. "Going Wild in the City—Animal Feralization and Its Impacts on Biodiversity in Urban Environments" Animals 13, no. 4: 747. https://doi.org/10.3390/ani13040747
APA StyleGöttert, T., & Perry, G. (2023). Going Wild in the City—Animal Feralization and Its Impacts on Biodiversity in Urban Environments. Animals, 13(4), 747. https://doi.org/10.3390/ani13040747







