A Comparison of Small Rodent Assemblages after a 20 Year Interval in the Alps
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Sites
2.2. Small Rodents Live-Trapping
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogora, M.; Frate, L.; Carranza, M.L.; Freppaz, M.; Stanisci, A.; Bertani, I.; Bottarin, R.; Brambilla, A.; Canullo, R.; Carbognani, M.; et al. Assessment of climate change effects on mountain ecosystems through a cross-site analysis in the Alps and Apennines. Sci. Total Environ. 2018, 624, 1429–1442. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability, Sixth Assessment Report; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Chemini, C.; Rizzoli, A. Land use change and biodiversity conservation in the Alps. J. Mt. Ecol. 2003, 7, 1–7. [Google Scholar] [CrossRef]
- Bebi, P.; Seidl, R.; Motta, R.; Fuhr, M.; Firm, D.; Krumm, F.; Conedera, M.; Ginzler, C.; Wohlgemuth, T.; Kulakowski, D. Changes of forest cover and disturbance regimes in the mountain forests of the Alps. For. Ecol. Manag. 2017, 388, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Gobiet, A.; Kotlarski, S.; Beniston, M.; Heinrich, G.; Rajczak, J.; Stoffel, M. 21st century climate change in the European Alps—A review. Sci. Total Environ. 2014, 493, 1138–1151. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, S.C.; Wüthrich, C.; Croci-Maspoli, M.; Weingartner, R.; Appenzeller, C. Snow variability in the Swiss Alps 1864–2009. Int. J. Climatol. 2013, 33, 3162–3173. [Google Scholar] [CrossRef]
- Oddi, L.; Galvagno, M.; Cremonese, E.; Filippa, G.; Migliavacca, M.; Bassignana, M.; Morra di Cella, U.; Siniscalco, C. Heat waves and droughts strongly impact productivity and ecosystem functioning in an abandoned subalpine grassland. In Proceedings of the EGU General Assembly Conference Abstracts, Copernicus Meetings, Virtual, 4–8 May 2020; p. 19266. [Google Scholar]
- Gellrich, M.; Baur, P.; Koch, B.; Zimmermann, N.E. Agricultural land abandonment and natural forest re-growth in the Swiss mountains: A spatially explicit economic analysis. Agric. Ecosyst. Environ. 2007, 118, 93–108. [Google Scholar] [CrossRef]
- Kulakowski, D.; Bebi, P.; Rixen, C. The interacting effects of land use change, climate change and suppression of natural disturbances on landscape forest structure in the Swiss Alps. Oikos 2011, 120, 216–225. [Google Scholar] [CrossRef]
- Williams, J.W.; Jackson, S.T.; Kutzbach, J.E. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl. Acad. Sci. USA 2007, 104, 5738–5742. [Google Scholar] [CrossRef]
- Watson, J.E.M.; Iwamura, T.; Butt, N. Mapping vulnerability and conservation adaptation strategies under climate change. Nat. Clim. Chang. 2013, 3, 989–994. [Google Scholar] [CrossRef]
- Greenberg, R. Ecological plasticity, neophobia, and resource use in birds. Stud. Avian Biol. 1990, 13, 431–437. [Google Scholar]
- Thurman, L.L.; Stein, B.A.; Beever, E.A.; Foden, W.; Geange, S.R.; Green, N.; Gross, J.E.; Lawrence, D.J.; LeDee, O.; Olden, J.D.; et al. Persist in place or shift in space? Evaluating the adaptive capacity of species to climate change. Front. Ecol. Environ. 2020, 18, 520–528. [Google Scholar] [CrossRef]
- Colles, A.; Liow, L.H.; Prinzing, A. Are specialists at risk under environmental change? Neoecological, paleoecological and phylogenetic approaches. Ecol. Lett. 2009, 12, 849–863. [Google Scholar] [CrossRef]
- Burrows, M.T.; Schoeman, D.S.; Richardson, A.J.; Molinos, J.G.; Hoffmann, A.; Buckley, L.B.; Moore, P.J.; Brown, C.J.; Bruno, J.F.; Duarte, C.M.; et al. Geographical limits to species-range shifts are suggested by climate velocity. Nature 2014, 507, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Mawdsley, J.R.; O’Malley, R.; Ojima, D.S. A review of climate-change adaptation strategies for wildlife management and biodiversity conservation. Conserv. Biol. 2009, 23, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Bright Ross, J.G.; Peters, W.; Ossi, F.; Moorcroft, P.R.; Cordano, E.; Eccel, E.; Bianchini, F.; Ramanzin, M.; Cagnacci, F. Climate change and anthropogenic food manipulation interact in shifting the distribution of a large herbivore at its altitudinal range limit. Sci. Rep. 2021, 11, 7600. [Google Scholar] [CrossRef]
- Myers, P.; Lundrigan, B.L.; Hoffman, S.M.G.; Haraminac, A.P.; Seto, S.H. Climate-induced changes in the small mammal communities of the Northern Great Lakes Region. Glob. Chang. Biol. 2009, 15, 1434–1454. [Google Scholar] [CrossRef]
- Angert, A.L.; Crozier, L.G.; Rissler, L.J.; Gilman, S.E.; Tewksbury, J.J.; Chunco, A.J. Do species’ traits predict recent shifts at expanding range edges? Ecol. Lett. 2011, 14, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.; Patton, J.L.; Conroy, C.J.; Parra, J.L.; White, G.C.; Beissinger, S.R. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science 2008, 322, 261–264. [Google Scholar] [CrossRef]
- Büchi, L.; Vuilleumier, S. Coexistence of Specialist and Generalist Species Is Shaped by Dispersal and Environmental Factors. Am. Nat. 2014, 183, 612–624. [Google Scholar] [CrossRef]
- Pernollet, C.A.; Korner-Nievergelt, F.; Jenni, L. Regional changes in the elevational distribution of the Alpine Rock Ptarmigan Lagopus muta helvetica in Switzerland. Ibis 2015, 157, 823–836. [Google Scholar] [CrossRef]
- Channell, R.; Lomolino, M.V. Trajectories to extinction: Spatial dynamics of the contraction of geographical ranges. J. Biogeogr. 2000, 27, 169–179. [Google Scholar] [CrossRef]
- Neff, F.; Korner-Nievergelt, F.; Rey, E.; Albrecht, M.; Bollmann, K.; Cahenzli, F.; Chittaro, Y.; Gossner, M.M.; Martínez-Núñez, C.; Meier, E.S.; et al. Different roles of concurring climate and regional land-use changes in past 40 years’ insect trends. Nat. Commun. 2022, 13, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Rehnus, M.; Bollmann, K.; Schmatz, D.R.; Hackländer, K.; Braunisch, V. Alpine glacial relict species losing out to climate change: The case of the fragmented mountain hare population (Lepus timidus) in the Alps. Glob. Chang. Biol. 2018, 24, 3236–3253. [Google Scholar] [CrossRef]
- Hof, A.R.; Jansson, R.; Nilsson, C. Future Climate Change Will Favour Non-Specialist Mammals in the (Sub)Arctics. PLoS ONE 2012, 7, e52574. [Google Scholar] [CrossRef]
- Mills, C.G.; Allen, R.J.; Blythe, R.A. Resource spectrum engineering by specialist species can shift the specialist-generalist balance. Theor. Ecol. 2020, 13, 149–163. [Google Scholar] [CrossRef]
- Devictor, V.; Julliard, R.; Jiguet, F. Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 2008, 117, 507–514. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- García-Navas, V.; Sattler, T.; Schmid, H.; Ozgul, A. Temporal homogenization of functional and beta diversity in bird communities of the Swiss Alps. Divers. Distrib. 2020, 26, 900–911. [Google Scholar] [CrossRef]
- Rowe, R.J.; Finarelli, J.A.; Rickart, E.A. Range dynamics of small mammals along an elevational gradient over an 80-year interval. Glob. Chang. Biol. 2010, 16, 2930–2943. [Google Scholar] [CrossRef]
- Krebs, C.J.; Boonstra, R.; Gilbert, B.S.; Kenney, A.J.; Boutin, S. Impact of climate change on the small mammal community of the Yukon boreal forest. Integr. Zool. 2019, 14, 528–541. [Google Scholar] [CrossRef]
- Walther, G.-R.; Beißner, S.; Burga, C.A. Trends in the upward shift of alpine plants. J. Veg. Sci. 2005, 16, 541–548. [Google Scholar] [CrossRef]
- La Morgia, V.; Balbo, C.; Memoli, S.; Isaia, M. Rodents in grassland habitats: Does livestock grazing matter? A comparison of two Alpine sites with different grazing histories. Zoosystema 2015, 37, 571–580. [Google Scholar] [CrossRef]
- Negro, M.; Novara, C.; Bertolino, S.; Rolando, A. Ski-pistes are ecological barriers to forest small mammals. Eur. J. Wildl. Res. 2013, 59, 57–67. [Google Scholar] [CrossRef]
- Bertoldi, G.; Bozzoli, M.; Crespi, A.; Matiu, M.; Giovannini, L.; Zardi, D.; Majone, B. Diverging snowfall trends across months and elevation in the northeastern Italian Alps. Int. J. Climatol. 2023, 43, 1–26. [Google Scholar] [CrossRef]
- Zibordi, F. Indagine Sull’ecologia Trofica di una Popolazione di Ermellino (Mustela erminea) in alta Val Nambrone (Parco Naturale Adamello Brenta). Master’s Thesis, Milan University, Milan, Italy, 1998. [Google Scholar]
- Andreassen, H.P.; Sundell, J.; Ecke, F.; Halle, S.; Haapakoski, M.; Henttonen, H.; Huitu, O.; Jacob, J.; Johnsen, K.; Koskela, E.; et al. Population cycles and outbreaks of small rodents: Ten essential questions we still need to solve. Oecologia 2021, 195, 601–622. [Google Scholar] [CrossRef]
- Kamenišťák, J.; Baláž, I.; Tulis, F.; Jakab, I.; Ševčík, M.; Poláčiková, Z.; Klimant, P.; Ambros, M.; Rychlik, L. Changes of small mammal communities with the altitude gradient. Biologia 2020, 75, 713–722. [Google Scholar] [CrossRef]
- Benedek, A.M.; Sîrbu, I.; Lazăr, A. Responses of small mammals to habitat characteristics in Southern Carpathian forests. Sci. Rep. 2021, 11, 12031. [Google Scholar] [CrossRef] [PubMed]
- Butet, A.; Delettre, Y.R. Diet differentiation between European arvicoline and murine rodents. Acta Theriol. 2011, 56, 297–304. [Google Scholar] [CrossRef]
- Mazurkiewicz, M. Factors Influencing the Distribution of the Bank Vole in Forest Habitats. Acta Theriol. 1994, 39, 113–126. [Google Scholar] [CrossRef]
- Torre, I.; Arrizabalaga, A. Habitat preferences of the bank vole Myodes glareolus in a Mediterranean mountain range. Acta Theriol. 2008, 53, 241–250. [Google Scholar] [CrossRef]
- Luque-Larena, J.J.; López, P.; Gosálbez, J.; Lopez, P.; López, P.; Gosálbez, J. Microhabitat use by the snow vole Chionomys nivalis in alpine environments reflects rock-dwelling preferences. Can. J. Zool. 2002, 80, 36–41. [Google Scholar] [CrossRef]
- Rubel, F.; Brugger, K.; Haslinger, K.; Auer, I. The climate of the European Alps: Shift of very high resolution Köppen-Geiger climate zones 1800–2100. Meteorol. Zeitschrift 2017, 26, 115–125. [Google Scholar] [CrossRef]
- Bertolino, S.; Vieceli, S.; Tontini, L.; Giacoma, C. Roditori e Soricomorfi nelle valli Ferret e Veni. Rev. Vald. Hist. Nat. 2008, 61–62, 293–306. [Google Scholar]
- Pollock, K.H.; Nichols, J.D.; Brownie, C.; Hines, J.H. Statistical Inference for Capture-Recapture Experiments. Wildl. Monogr. 1990, 107, 3–97. [Google Scholar]
- Amstrup, S.C.; McDonald, T.L.; Manly, B.F.J. Handbook of Capture-Recapture Analysis; Princeton University Press: Princeton, NJ, USA, 2005; ISBN 1400837715. [Google Scholar]
- McCleery, R.; Monadjem, A.; Conner, L.M.; Austin, J.D.; Taylor, P.J. Methods for Ecological Research on Terrestrial Small Mammals; Johns Hopkins University Press: Baltimore, MD, USA, 2021; ISBN 9781421442112. [Google Scholar]
- Wever, K.E.; Geessink, F.J.; Brouwer, M.A.E.; Tillema, A.; Ritskes-Hoitinga, M. A systematic review of discomfort due to toe or ear clipping in laboratory rodents. Lab. Anim. 2017, 51, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, G.C.; Rozenfeld, F.M. A permanent marking method to identify individual small rodents from birth to sexual maturity. J. Zool. 2001, 254, 203–206. [Google Scholar] [CrossRef]
- Prevot-Julliard, A.-C.; Henttonen, H.; Yoccoz, N.G.; Stenseth, N.C. Delayed maturation in female bank voles: Optimal decision or social constraint? J. Anim. Ecol. 1999, 68, 684–697. [Google Scholar] [CrossRef]
- Hoffmann, A.; Decher, J.; Rovero, F.; Schaer, J.; Voigt, C.; Wibbelt, G. Field Methods and Techniques for Monitoring Mammals; Museum für Naturkunde: Berlin, Germany, 2010; pp. 482–529. ISBN 1784-1283. [Google Scholar]
- Legge Provinciale 9 Dicembre 1991, n. 24. Available online: https://www.consiglio.provincia.tn.it/leggi-e-archivi/codice-provinciale/Pages/legge.aspx?uid=933 (accessed on 24 March 2023).
- Hill, M.O. Correspondence Analysis: A Neglected Multivariate Method. J. R. Stat. Soc. Ser. C Appl. Stat. 1974, 23, 340–354. [Google Scholar] [CrossRef]
- Hill, M.O.; Gauch, H.G. Detrended Correspondence Analysis: An Improved Ordination Technique. In Classification and Ordination; Springer: Berlin/Heidelberg, Germany, 1980; pp. 47–58. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Prentice, I.C. A Theory of Gradient Analysis. Adv. Ecol. Res. 1988, 18, 271–317. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Ordination. In Data Analysis in Community and Landscape Ecology; Cambridge University Press: Cambridge, UK, 1995; pp. 91–274. ISBN 0521475740. [Google Scholar]
- Rao, C.R. The Use and Interpretation of Principal Component Analysis in Applied Research. Indian J. Stat. Ser. A 1961, 26, 329–358. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Simone, I.; Cagnacci, F.; Provensal, C.; Polop, J. Environmental determinants of the small mammal assemblage in an agroecosystem of central Argentina: The role of Calomys musculinus. Mamm. Biol. 2010, 75, 496–509. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Permutation Versus Bootstrap Significance Tests in Multiple Regression and Anova. In Bootstrapping and Related Techniques; Springer: Berlin/Heidelberg, Germany, 1992; pp. 79–85. [Google Scholar]
- Peters, W.; Hebblewhite, M.; Decesare, N.; Cagnacci, F.; Musiani, M. Resource separation analysis with moose indicates threats to caribou in human altered landscapes. Ecography 2013, 36, 487–498. [Google Scholar] [CrossRef]
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation: Indianapolis, IN, USA, 2021. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package “Vegan”, Community Ecology Package, version 2.5-7; CRAN: Indianapolis, IN, USA, 2020. [Google Scholar]
- Wickham, H. Tidyverse: Easily Install and Load “Tidyverse”; RStudio: Boston, MA, USA, 2017. [Google Scholar]
- Kryštufek, B.; Shenbrot, G. Voles and Lemmings (Arvicolinae) of the Palaearctic Region; University of Maribor, University Press: Maribor, Slovenia, 2022; ISBN 9789612866112. [Google Scholar]
- Werner, J.R.; Krebs, C.J.; Donker, S.A.; Sheriff, M.J. Forest or meadow: The consequences of habitat for the condition of female arctic ground squirrels (Urocitellus parryii plesius). Can. J. Zool. 2015, 93, 791–797. [Google Scholar] [CrossRef]
- Gilman, S.E.; Urban, M.C.; Tewksbury, J.; Gilchrist, G.W.; Holt, R.D. A framework for community interactions under climate change. Trends Ecol. Evol. 2010, 25, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Deffontaine, V.; Libois, R.; Kotlík, P.; Sommer, R.; Nieberding, C.; Paradis, E.; Searle, J.B.; Michaux, J.R. Beyond the Mediterranean peninsulas: Evidence of central European glacial refugia for a temperate forest mammal species, the bank vole (Clethrionomys glareolus). Mol. Ecol. 2005, 14, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Fløjgaard, C.; Morueta-Holme, N.; Skov, F.; Madsen, A.B.; Svenning, J.-C. Potential 21st century changes to the mammal fauna of Denmark—implications of climate change, land-use, and invasive species. IOP Conf. Ser. Earth Environ. Sci. 2009, 8, 012016. [Google Scholar] [CrossRef]
- Imholt, C.; Reil, D.; Eccard, J.A.; Jacob, D.; Hempelmann, N.; Jacob, J. Quantifying the past and future impact of climate on outbreak patterns of bank voles (Myodes glareolus). Pest. Manag. Sci. 2015, 71, 166–172. [Google Scholar] [CrossRef]
- Calhoun, J.B.; Casby, J.U. Calculation of home range and density of small mammals. United States Public Health Monogr. 1958, 55, 1–24. [Google Scholar]
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Dormann, C.F.; Forchhammer, M.C.; Grytnes, J.A.; Guisan, A.; et al. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. Camb. Philos. Soc. 2013, 88, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Delattre, P.; Giraudoux, P.; Baudry, J.; Quéré, J.P.; Fichet, E. Effect of landscape structure on Common Vole (Microtus arvalis) distribution and abundance at several space scales. Landsc. Ecol. 1996, 11, 279–288. [Google Scholar] [CrossRef]
- Janeau, G.; Aulagnier, S. Snow vole Chionomys nivalis (Martins, 1842). J. Mt. Ecol. 1997, 4, 1–11. [Google Scholar]
- Semenzato, P.; Cagnacci, F.; Ossi, F.; Eccel, E.; Morellet, N.; Hewison, A.J.M.; Sturaro, E.; Ramanzin, M. Behavioural heat-stress compensation in a cold-adapted ungulate: Forage-mediated responses to warming Alpine summers. Ecol. Lett. 2021, 24, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Ergon, T.; Cavanagh, R.; Reid, K.; Scantlebury, D.M.; Lambin, X. Resting and daily energy expenditures of free-living field voles are positively correlated but reflect extrinsic rather than intrinsic effects. Proc. Natl. Acad. Sci. USA 2003, 100, 14057–14062. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, J.A.; Lawler, J.J.; Schumaker, N.H. Intrinsic and extrinsic drivers of source-sink dynamics. Ecol. Evol. 2016, 6, 892–904. [Google Scholar] [CrossRef]
- Huitu, O.; Norrdahl, K.; Korpimäki, E. Competition, predation and interspecific synchrony in cyclic small mammal communities. Ecography 2004, 27, 197–206. [Google Scholar] [CrossRef]
- Beever, E.A.; Hall, L.E.; Varner, J.; Loosen, A.E.; Dunham, J.B.; Gahl, M.K.; Smith, F.A.; Lawler, J.J. Behavioral flexibility as a mechanism for coping with climate change. Front. Ecol. Environ. 2017, 15, 299–308. [Google Scholar] [CrossRef]
- Elsen, P.R.; Tingley, M.W. Global mountain topography and the fate of montane species under climate change. Nat. Clim. Chang. 2015, 5, 772–776. [Google Scholar] [CrossRef]
- Tafani, M.; Cohas, A.; Bonenfant, C.; Gaillard, J.-M.; Allainé, D. Decreasing litter size of marmots over time: A life history response to climate change? Ecology 2013, 94, 580–586. [Google Scholar] [CrossRef]
- Bertolino, S.; Büchner, F.; Mori, E.; Büchner, S. Presence of the hazel dormouse muscardinus avellanarius at the limit of its altitudinal range. Hystrix 2016, 27, 215–218. [Google Scholar] [CrossRef]
- McCain, C.M. Elevational gradients in diversity of small mammals. Ecology 2005, 86, 366–372. [Google Scholar] [CrossRef]
- Körner, C. The use of ‘altitude’ in ecological research. Trends Ecol. Evol. 2007, 22, 569–574. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, M.J.; Clark, W.A.; Emmerson, F.H.; Juarez, S.M.; Kay, F.R.; O’Farrell, T.M.; Goodlett, T.Y. Use of a Mesh Live Trap for Small Mammals: Are Results from Sherman Live Traps Deceptive? J. Mammal. 1994, 75, 692–699. [Google Scholar] [CrossRef]
- Ylönen, H.; Jacob, J.; Kotler, B.P. Trappability of rodents in single-capture and multiple-capture traps in arid and open environments: Why don’t Ugglan traps work? Ann. Zool. Fennici 2003, 40, 537–541. [Google Scholar]
- Jung, T.S. Comparative efficacy of Longworth, Sherman, and Ugglan live-traps for capturing small mammals in the Nearctic boreal forest. Mammal Res. 2016, 61, 57–64. [Google Scholar] [CrossRef]

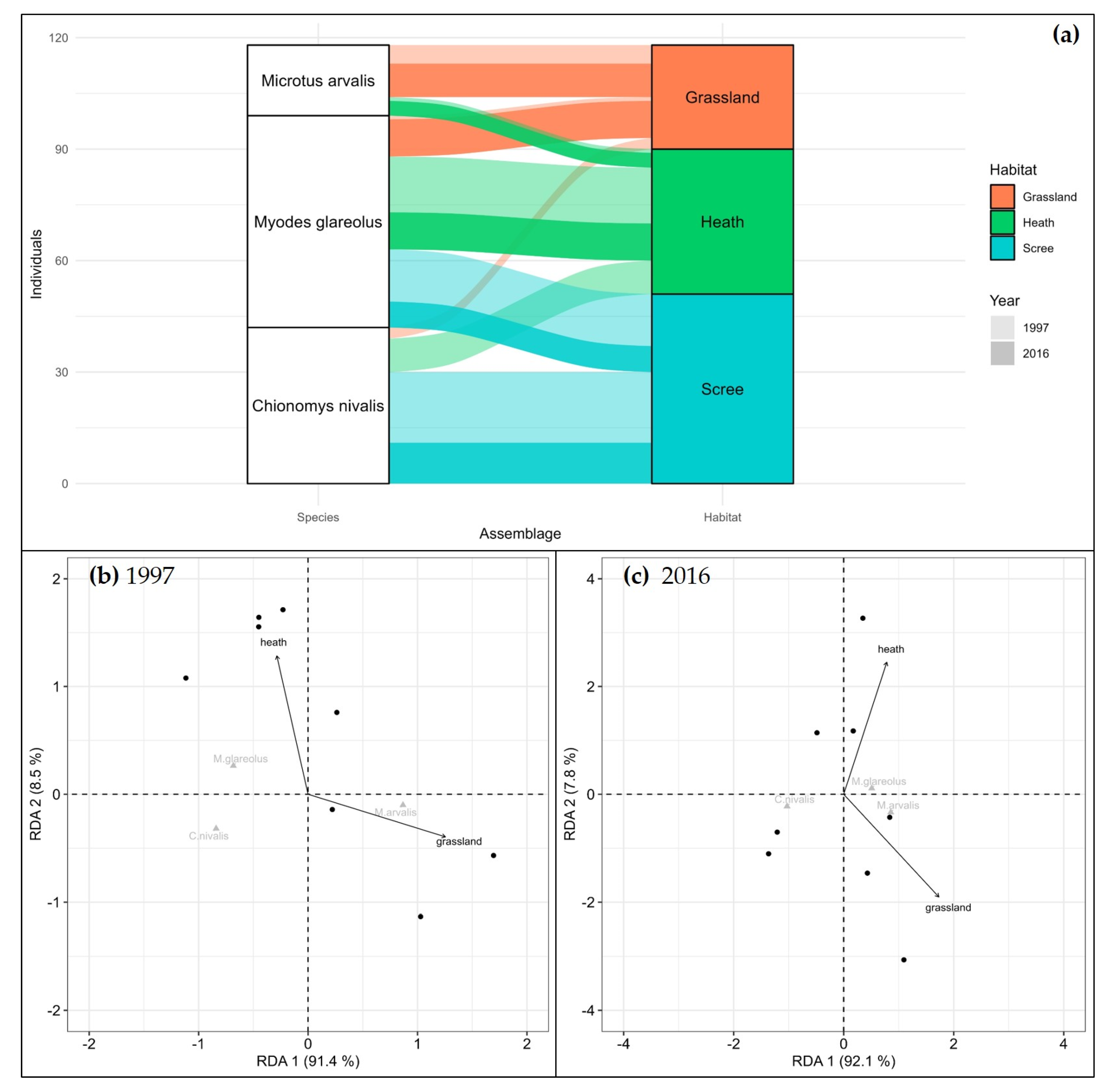
| Species | 1997 | 2016 | ||||
|---|---|---|---|---|---|---|
| Grassland | Heath | Scree | Grassland | Heath | Scree | |
| Chionomys nivalis | 3 | 9 | 19 | - | - | 11 |
| Myodes glareolus | 1 | 15 | 14 | 10 | 10 | 7 |
| Microtus arvalis | 5 | 1 | - | 9 | 4 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, G.; Scaravelli, D.; Mustoni, A.; Armanini, M.; Zibordi, F.; Devineau, O.; Cagnacci, F.; Grasso, D.A.; Ossi, F. A Comparison of Small Rodent Assemblages after a 20 Year Interval in the Alps. Animals 2023, 13, 1407. https://doi.org/10.3390/ani13081407
Ferrari G, Scaravelli D, Mustoni A, Armanini M, Zibordi F, Devineau O, Cagnacci F, Grasso DA, Ossi F. A Comparison of Small Rodent Assemblages after a 20 Year Interval in the Alps. Animals. 2023; 13(8):1407. https://doi.org/10.3390/ani13081407
Chicago/Turabian StyleFerrari, Giulia, Dino Scaravelli, Andrea Mustoni, Marco Armanini, Filippo Zibordi, Olivier Devineau, Francesca Cagnacci, Donato A. Grasso, and Federico Ossi. 2023. "A Comparison of Small Rodent Assemblages after a 20 Year Interval in the Alps" Animals 13, no. 8: 1407. https://doi.org/10.3390/ani13081407
APA StyleFerrari, G., Scaravelli, D., Mustoni, A., Armanini, M., Zibordi, F., Devineau, O., Cagnacci, F., Grasso, D. A., & Ossi, F. (2023). A Comparison of Small Rodent Assemblages after a 20 Year Interval in the Alps. Animals, 13(8), 1407. https://doi.org/10.3390/ani13081407







