Simple Summary
Custard apple (Annona squamosa) leaf extract (ASLE) is a phenolic-rich substance and considered a non-conventional feed additive. The present study aimed to evaluate the effect of ASLE dietary supplementation in the diet of Nile tilapia on growth performance, physiological status, and stress resistance. The results revealed that dietary ASLE, up to a 20 g/kg diet, improved growth and feed utilization. ASLE improved the hematological parameters, vital organ functions, and redox status in the fish. Furthermore, increasing ASLE dietary levels improved fish resistance against Aeromonas sobria challenge. Therefore, ASLE could be a potential feed additive in Nile tilapia diets.
Abstract
Plant extracts are a phytochemically-rich alternative to antibiotic and synthetic feed additives, with high systemic bioactivity in animals. The present study aimed to evaluate the effect of a hydroalcoholic extract of custard apple (Annona squamosa) leaf (ASLE) on the growth, hematobiochemical parameters, digestive enzyme activities, redox status, nonspecific immune response, and cold and bacterial infection tolerance in Nile tilapia (Oreochromis niloticus). A total of 300 Nile tilapia fingerlings (11.87 ± 0.48 g) were fed ASLE-supplemented diets at increasing levels of 0, 5, 10, 15, and 20 g/kg for 60 days. At the end of the feeding period, the fish were experimentally challenged with cold water stress or Aeromonas sobria, and mortalities were recorded for 10 days. The results revealed that the growth performance and feed conversion ratio were significantly improved with an increasing level of ASLE supplementation. The hematologic profile and hepato-renal functions were retained within a healthy range in the various groups supplemented with an ASLE diet. Antioxidant status was significantly improved in the serum of fish fed ASLE-supplemented diets, in terms of superoxide dismutase (SOD), catalase (CAT) activities, reduced glutathione, and total antioxidant capacity. Meanwhile, the myeloperoxidase (MPO) and malondialdehyde (MDA) levels decreased significantly. Similarly, there was a noticeable improvement in the hepatic CAT and SOD activities and a reduction of hepatic MDA. Marked improvements in lysozyme activity, nitric oxide production, complement3 level, and phagocytic activity were recorded in groups fed ASLE-supplemented diets, which peaked with the 20 g ASLE/kg diet. Moreover, the serum glucose and cortisol levels significantly declined in groups fed ASLE at levels of 15–20 g/kg compared to the other groups. Supplementation with ASLE increased the activities of protease, lipase, and α-amylase. ASLE supplementation at a concentration of 10–20 g/kg diet enhanced the resistance of Nile tilapia to A. sobria infection. According to this study, ASLE supplementation enhanced the antioxidant balance, non-specific immune response, physiological status, resistance against infection, and growth performance of Nile tilapia at supplementation levels of 10–20 g/kg diet.
1. Introduction
Aquaculture plays, and will continue to play, a crucial role in expanding global fish production to meet the growing demand for aquatic products [1]. The most widely cultivated freshwater aquaculture species is tilapia (Oreochromis sp.), and it is anticipated that production will continue to increase to meet the increasing demand for fish from a population that is expanding [2,3]. Due to its fast growth, high feed utilization, natural spawning, and good edible characteristics, it is the first species raised in Egypt and the third in the world [4]. However, the expansion of aquaculture has created a number of issues, such as low growth, poor health, and increased susceptibility to infectious diseases. These diseases, including bacterial diseases, are opportunistic and can have a significant negative economic impact on freshwater and marine aquaculture [5,6].
In addition, rapid climate change possess a significant threat to ecosystems and living things, notably fish as ecthothermic animals, for which water temperature is an important environmental factor. Moreover, fish are regularly exposed to a variety of stresses in their intense production system [7]. Conditions of temperature stress may result in disruption of the physiological balance of the organism and subsequently hinder growth and survival [8]. Therefore, a significant challenge is faced by fish farmers and the aquaculture industry to enhance culture performance and combat stress.
The use of conventional medications and vaccines for disease prevention and treatment of disease comes with significant drawbacks [9]. In addition, the use of antibiotics in aquaculture to treat and prevent bacterial infections may result in bacteria that are resistant to the antibiotics or the presence of antibiotic residues in fish raised for human consumption [10]. As a result, there is a crucial need to create safe solutions, as affordable alternatives to conventional methods of disease control, to maintain an eco-friendly aquaculture. Over time, there has been significant progress in fish nutrition, which has led to the creation of specialized feed formulations and novel, balanced commercial diets that support optimum growth and the production of high-quality, healthy fish [11]. Various efforts have been made to use medical and aromatic herbs as feed additives, which are innovative approaches to reducing disease risk, as well as boosting the immune system during exposure to stressors such as rough handling, transport, cold temperature, and poor water quality for reared fish [12,13,14]. Among these herbs is Custard Apple, Annona squamosa, which is a member of the Annonaceae family and a species of Annona, which is known mainly for its edible fruits [15]. Various parts of A. squamosa are used in folkloric medicine to treat a variety of diseases [16]. Numerous active chemicals with various pharmacological properties, including anti-inflammatory and anti-tumor actions, were identified in phytochemical analyses [17]. It is used as an anti-inflammatory, anti-diabetic, hepatoprotective, cytotoxic, genetoxic, antitumor, and anti-lice agent [18]. The leaves of Custard apple contain a considerable amount of phenol-based compounds, mainly alkaloids and flavonoids [19,20]. Leaf extracts produced with various solvents were shown to have antibacterial action and they also included sterols, flavonoids, and tannins [21]. Custard apple (Annona squamosa) leaf extract (ASLE) has health-promoting effects and can be used as a potential active ingredient in drugs and functional foods [19]. ASLE had immunostimulatory effects in Catfish [15] and increased resistance to Aeromonas hydrophila [22]. In addition, studies on other animal species and invitro experiments showed anticancer, antidiabetic, antioxidant, antimicrobial, antiobesity, lipid-lowering, and hepatoprotective functions [19,23].
Hence, it appears that ASLE could be beneficial for boosting immune status and lessening the susceptibility of fish to pathogens. However, its use as a dietary supplement in aquaculture has not been fully explored. Therefore, the objective of the current investigation was to evaluate the effect of dietary supplementation with various concentrations of ASLE in O. niloticus diets on growth, and antioxidant and immunological biomarkers, as well as to evaluate its effect on health status indicators using certain haematobiochemical indices, cold stress tolerance, and resistance to A. sobria infection in Nile tilapia.
2. Material and Methods
2.1. Plant Collection and Preparation
The leaves of A. squamosa were collected from local gardens in Sharkia, Egypt, then identified and authenticated by a botanist in the Faculty of Agriculture, Zagazig University, Egypt. The leaves were properly cleaned with distilled water after being washed with tap water, then dried at 45 °C. In an electronic grinder, the plant material was crushed and ground into a fine powder before being stored in an airtight plastic container for further utilization. The leaves extracts were obtained as follows: in a shaking water bath at room temperature for 30 min, 6 g of plant powder was extracted using 100 mL of ethanol:distilled water (1:1). The extract supernatants were collected and filtered using Whatman paper after centrifugation for 15 min at 3000 rpm. The solvent was finally evaporated by vacuum evaporation through a rotary evaporator. The dried extract was maintained in a refrigerator at 4 °C [24]. The bioactive components present in ASLE used in the present study were identified through gas chromatography-mass spectroscopy (GC–MS) analysis [25]. The dominant compounds were sodium benzoate (27.50%), 4,4-Tert-Butylcalix (4) arene (12.34%), 4,4-Dimethylcholesterol (10.30%), Butyloctylpthalate (9.67%), stigmasterol acetate (2.92%), and isoamylacetyate (2.29%).
2.2. Fish Rearing Conditions
Healthy Nile tilapia (O. niloticus) (N = 300, mean weight 11.87 ± 0.48 g) were obtained from nursery ponds at the Central Laboratory for Aquaculture Research (CLAR), Abbassa, Sharkia Province, Egypt. The fish were free of any history of disease or outbreaks. Prior to the experiment, a routine check of the fish’s health was performed [26]. The fish were kept in 80 L capacity glass aquaria and filled with chlorine-free tap water with continuous air supply. The fish were acclimatized to the experimental conditions for 15 days prior to the start of the experiment and fed by hand with a basal diet up to satiation. The light–dark cycle was kept at 12 h/12 h. To maintain water parameters within the optimal recommended levels for growth and survival during the period of the experiment, dissolved oxygen, water temperature, and pH were monitored daily. The water temperature ranged from 25.85 to 26.6 °C, dissolved oxygen was 6.3–6.9 mg/L, pH was 7.2–7.7, and ammonia concentration was 0.20 to 0.25 mg/L. All levels were within the permitted limits for fish aquaculture [27]. Twice a week, three quarters of the water in the aquarium was drained along with settled fish excrement and refilled with fresh and aerated water from a holding tank. The Institutional Animal Care and Use Committee of Egypt’s Zagazig University accepted the experimental protocol (approval no. ZU-IACUC/2/F/284/2022).
2.3. Experimental Design and Diets
Nile tilapia were randomly divided into five quadruple groups (15 fish/replicate, N = 60 fish/group). Five experimental diets were designed by adding the extract of the ASLE to the formula at levels of 0 g/kg diet (control), 5 g/kg diet (0.5%), 10 g/kg diet (1%), 15 g/kg diet (1.5%), and 20 g/kg diet (2%). The ingredients of diets were mixed mechanically, forming pellets of 1.5 mm diameter using a pellet machine. The prepared diets were air-dried for 24 h at room temperature and stored in a refrigerator at 4 °C until use. The chemical composition and ingredients of the basal and experimental diets are shown in Table 1.

Table 1.
Ingredients and proximate chemical analysis of the experimental diets (g/kg).
The experimental diets were offered to the fish at a rate of 3% of their total biomass two times per day (8:00 a.m. and 3:00 p.m.) for 60 days. In accordance with the change in fish weight, the introduced diet % was modified every two weeks, and the uneaten diet was collected, dried, and subtracted from the introduced feed for accurate calculation of feed intake.
2.4. Growth Performance
The fish were sampled every two weeks, to assess growth performance using a sensitive weight balance. The final weight, weight gain (%), specific growth, and feed conversion rate were determined as follows:
where W2 = Weight of fish at time T2 (final), W1 = Weight of fish at time T1 (initial)
Specific growth rate (SGR) = 100 × (Lin W2 − Lin W1)/days
Weight gain (%) = 100 × (weight gain/initial weight)
Feed intake (FI) = Feed consumed/Number of surviving fish
Feed conversion rate (FCR) = Total feed consumed by fish (g)/Weight gain by fish (g)
Protein productive value (PER)= weight gain (g)/protein intake (g)
2.5. Sampling
At the end of the feeding experiment (60 days), blood samples were taken from four randomly chosen fish from each aquarium (16 fish per group), after being anesthetized with 95 mg L–1 clove oil (Oleum Cosmetics, Cairo, Egypt) [28]. From the caudal vessels, blood was drawn in vials containing heparin for hematological parameters. Sterile syringes without anticoagulants were used to collect other blood samples and centrifuged at 1075× g for 20 min, to obtain serum samples and stored in a deep freezer at −20 °C until use. The serum samples were used to perform the biochemical and immunological assays.
Following blood sampling, the fish were necropsied aseptically and samples of the liver and intestines were retrieved. These tissues were preserved on ice-cold dishes, cleansed with ice-cold sterile saline, dried with filter paper, split, and then frozen at −20 °C. Following that, 100 mg of each tissue was added to a tube containing 1 mL of a buffer (10 mM phosphate/20 mM tris-pH 7.0) and homogenized using a Teflon homogenizer, then centrifuged at 10,000× g for 5 min at 4 °C. The supernatants were pooled after centrifugation and kept at −80 °C until use. Intestinal homogenates were used to assess the activity of digesting enzymes, while liver homogenates were employed to identify markers of oxidative stress.
2.6. Evaluation of Health-Related Parameters
2.6.1. Hematological Analyses
Blood parameters assayed were red blood cells (RBC) and differential white blood (WBC) cell counts, using an improved Neubauer hemocytometer with Natt and Herrick diluting fluid. Hemoglobin (Hb), packed cell volume (PCV), mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) were immediately measured after sampling, according to the methods described by Jain [29].
2.6.2. Hepatorenal Function Indicators and Stress Indicators
Serum total proteins and albumin were spectrophotometrically evaluated using techniques described by Henry [30] and Reinhold [31], respectively. Furthermore, serum globulins were calculated using the method of Coles [32]. Aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), creatinine, and urea were evaluated in serum using Spinreact kits (Esteve De Bas, Girona, Spain), according to the protocols established by Wenger et al. [33], Burtis and Ashwood [34], Murray [35], Fossati et al. [36], and Kaplan [37], respectively. The serum glucose was estimated using colorimetric diagnostic kits from spectrum-bioscience (Egyptian Company for Biotechnology, Cairo, Egypt) using the techniques of Trinder [38]. The serum cortisol level was evaluated following the method outlined by Tunn, et al. [39].
2.6.3. Assessment of Oxidant/Antioxidant Status
Antioxidant activities in serum and liver homogenate were evaluated using colorimetric commercial kits purchased from Biodiagnostic Co., Cairo, Egypt. Total antioxidant capacity (TAC) was estimated in serum using colorimetric commercial kits purchased from Bio-Diagnostic Co. (Cairo, Egypt) [40]. Catalase (CAT) activity was monitored using the procedure of Aebi [41]. Superoxide dismutase (SOD) activity was assessed following the protocol of Nishikimi, et al. [42]. Quantitative colorimetric glutathione dehydrogenase (GSH) was performed according to Beutler [43]. Malondialdehyde (MDA) was monitored using the technique of Uchiyama and Mihara [44]. The activity of myeloperoxidase (MPO) in fish serum was measured using the approach of Kumari and Sahoo [45].
2.6.4. Non-Specific Immunological Assessment
Serum lysozyme activity was determined using the turbidimetric method [46] with a suspension of Micrococcus lysodeikticus (Sigma-Aldrich, Burlington, MA, USA). This test is based on the lysis of a Gram-positive bacterium that is sensitive to the lysozyme (M. lysodeikticus). Nitric oxide (NO) and complement3 (C3) levels were determined using ELISA kits (MyBioSource, San Diego, CA, USA), according to the manufacturer’s instructions. The phagocytic activity (%) of leucocytes was assayed in heparinized blood according to Siwicki, et al. [47]. Phagocytic activity = (number of phagocytic cells that phagocytise bacteria/total number of phagocytic cells counted) × 100.
2.6.5. Digestive Enzyme Assays in the Intestine
Following the manufacturer’s instructions, commercial colorimetric kits (Biodiagnostic Co., Cairo, Egypt) were used to measure the activity of protease, α-amylase, and lipase enzymes in intestinal homogenates from the various groups.
2.7. Challenge with Cold Temperature Stress
After the feeding period, fish from each treatment were randomly deposited in triplicates at a rate of five fish per replicate. Using a thermostat attached to the cooling system, the fish were gradually exposed to cold water. The temperature started at 25 °C and was subsequently reduced by one degree every 12 h by regulating the thermostat until reaching 18 °C. Nile tilapia showed severe growth retardation, decreased antioxidant enzyme activities, and immunosuppression at 18 °C, as described by Ibrahim, et al. [14]. The temperature remained at 18 °C for 2 weeks after reaching that level. The daily fish mortality was recorded by keeping the fish under observation.
2.8. Aeromonas Sobria Bacterial Challenge
At the end of the test period (60 days), five fish per replicate (N = 20 fish/group) were challenged with A. sobria (previously isolated from naturally infected Nile tilapia in the Department of Aquatic Animal Medicine, identified and confirmed to be pathogenic). At the Department of Microbiology and Immunology, National Research Center (NRC), Dokki, Giza, Egypt, A. sobria was identified using traditional biochemical assays and an automated VITEK 2-C15 system for bacterial identification (BioMérieux, France), according to the manufacturer’s instructions and as described by Scheidegger, et al. [48] and Zhou, et al. [49]. Lethal dosage (LD50) for A. sobria was first recorded. A variety of doses of live bacteria were intraperitoneally (IP) injected into fish, and three days later, the infected fish mortality was observed. The LD50 that induced 50% fish mortality was 2 × 108 CFU/mL. A sub-lethal dosage was used in the bacterial challenge test. A 0.2 mL dose of suspension cells comprising 1.5 × 107/mL cells was administered intraperitoneally (IP) to the fish using standard MacFarland tubes. A. sobria was isolated from the dead fish to confirm responsibility for the death of the fish. The injected bacteria were re-isolated from moribund and recently dead fish and identified. For ten days, all groups were closely monitored, to note any abnormal findings and daily mortality.
2.9. Statistical Analysis
A one-way analysis of variance (ANOVA) was employed to conduct statistical analysis (SPSS version 16.0, SPSS Inc., Chicago, IL, USA). With statistical significance set at p < 0.05, the differences between groups were compared using Tukey’s multiple comparison post hoc test. The analysis findings are reported as means ± SE (standard error). Additionally, SGR and FCR were used to establish a fit regression model between the levels of ASLE and fish response.
3. Results
3.1. Growth Performance
The effect of ASLE-supplemented diets on growth performance parameters is shown in Table 2. In general, there was a significant influence (p < 0.05) on all growth parameters. ASLE-supplemented diets led to a significant increase (p < 0.05) in the final body weight, weight gain (%), SGR, FI, and PER in ASLE20, followed by ASLE15, ASLE10, ASLE5, and the control group, respectively. The FCR showed a significant reduction in ASLE20, followed by other groups. The dose-response analysis revealed a substantial linear correlation between the SGR and FCR responses to increased dietary supplementation of ASLE (R2 = 0.83) (Figure 1). Fish survival was 100%, with no noticeable differences between the various groups of fish during the feeding period (p > 0.05; Table 2), and the fish in all test groups remained healthy during the feeding period based on their overall activity.

Table 2.
Effect of dietary supplementation with A. squamosa leaves extract (ASLE) on growth performance and feed conversion ratio of O. niloticus for 60 days.
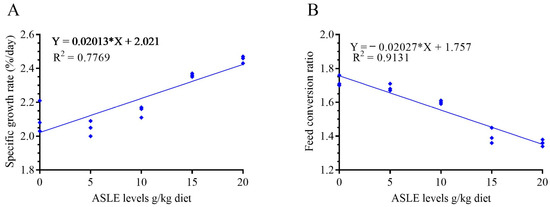
Figure 1.
Fit linear regression model of the specific growth rate (A) and feed conversion ratio (B) in response to increasing dietary supplementation of Annona squamosa leaf extract (g/kg).
3.2. Hematological Indices
The hematological results of Nile tilapia fed with various ASLE levels are shown in Table 3. ASLE-fortified fish diets showed significantly improved hematologic indices (p < 0.05) compared to the control group. In a dose-dependent order, hematological indices, such as RBCs, Hb, PCV%, and WBC levels were markedly increased. Furthermore, the addition of ASLE to Nile tilapia diets considerably increased the values of lymphocytes, heterophils, eosinophils, and monocytes.

Table 3.
Effects of dietary supplementation with A. squamosa leaves extract (ASLE) on hematological indices of O. niloticus for 60 days.
3.3. Hepatorenal Function and Stress Indicators
The serum total protein, albumin, and globulin are given in Table 4, which were enhanced (p < 0.05) in the ASLE20 and ASLE15 groups compared to the other groups. In the same table, the activities of ALT, AST, and ALP activities show a significant reduction (p < 0.05) with increasing ASLE concentration. Furthermore, the serum urea and creatinine levels were significantly decreased by increasing the ASLE level in the diets, and their lowest values were reported in the 20 ASLE g/kg diet, while the highest values were observed with the control diet. The effects of dietary ASLE on the stress indicators of Nile tilapia are illustrated in Table 4. Serum glucose and cortisol levels were significantly (p < 0.05) decreased in fish fed diets enriched with ASLE20 compared with the other groups.

Table 4.
Effect of dietary supplementation with A. squamosa leaf extract (ASLE) on serum hepatic and renal function, as well as stress indicators of O. niloticus for 60 days.
3.4. The Activity of Antioxidant Enzymes
Dietary ASLE20 supplementation resulted in a significant improvement (p < 0.05) in the serum levels of CAT, SOD, GSH, and TAC, followed by ASLE15, ASLE10, ASLE5, and the control, respectively (Table 5). Conversely, the ASLE-supplemented diets reduced oxidative stress, as stipulated by the gradually declining MDA and MPO levels. The ASLE20 groups achieved the best results. The liver CAT and SOD activities were also considerably higher in ASLE5, ASLE10, and ASLE15 than in the other groups, although the concentration of MDA showed the reverse tendency (Table 5).

Table 5.
Effect of dietary supplementation with A. squamosa leaf extract (ASLE) on the serum and liver homogenate oxidative/anti-oxidative status of Oreochromis niloticus for 60 days, as well as its mortality rate after cold challenge for 14 days.
3.5. Nonspecific Immune Parameters
Nile tilapia fed ASLE-based diets had a considerably higher (p < 0.05) non-specific immune response higher than fish fed the control diet (Figure 2). Regarding the lysozyme, and nitric oxide, C3 activities, there were significant enhancements (p < 0.05) observed in fish fed ASLE20, followed by ASLE15. There was a significant increase in phagocytic activity in a progressive manner in ASLE20, ASLE15, ASLE10, ASLE5, and the control.
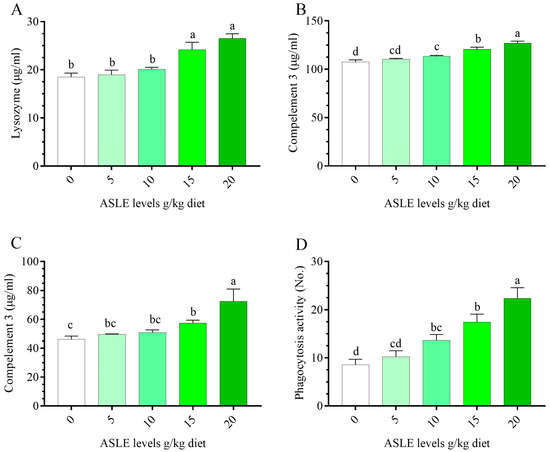
Figure 2.
Effect of dietary supplementation with Annona squamosa leaf extract on innate immune parameters of O. niloticus for 60 days. (A) lysozyme, (B) complement 3, (C) nitric oxide, and (D) phagocytic activity. Columns bearing different letters are significantly different at p < 0.05.
3.6. Activity of Digestive Enzymes in the Intestine
Figure 3 depicts the alterations in the Nile tilapia’s intestinal digestive enzyme activity as a result of 60 days of ASLE dietary supplementation. In comparison to the control group, dietary ASLE improved the release of digestive enzymes (protease, lipase, and α-amylase), and the highest values were recorded with the 10–20 g/kg diet (p < 0.05).
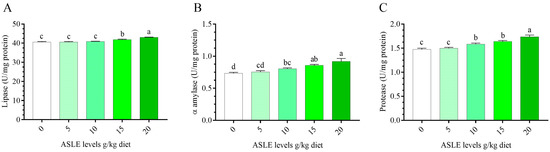
Figure 3.
Effect of dietary supplementation with Annona squamosa leaf extract on intestinal digestive enzymes of O. niloticus for 60 days. (A) lipase, (B) α-amylase, and (C) protease. Columns bearing different letters are significantly different at p < 0.05.
3.7. Cold-Water Stress Tolerance and Challenge with A. sobria
Regarding cold stress, no significant (p > 0.05) mortality was recorded in fish under cold stress in all ASLE-supplemented treatments (Table 5). Resistance of Nile tilapia fed on ASLE-enriched diets for 60 days was recorded against the A. sobria challenge, in terms of percentage cumulative mortality. The control group had the highest mortality rate (60%), while the ASLE20-fed group had the lowest mortality rate (5%). The survival rate was increased in the fish by increasing the level of ASLE20 (95%), where it was 85.00%, 80.00%, and 70.00% in ASLE15, ASLE10, and ASLE5, respectively, as compared with the control group (40.00%), as shown in Figure 4.
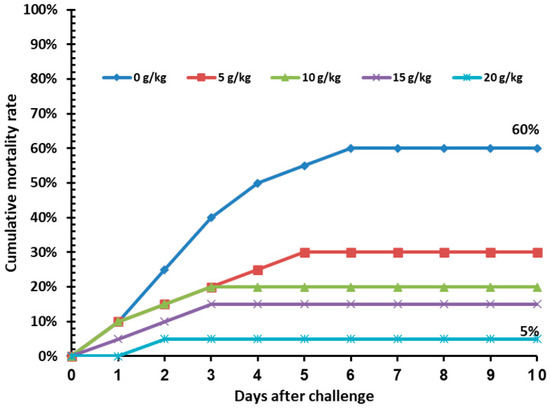
Figure 4.
Cumulative mortality rate (%) in Nile tilapia fed increasing levels of Annona squamosa leaf extract for 60 days and post-challenged by Aeromonas sobria infection for 10 days.
4. Discussion
Aquaculture provides a viable supply of affordable and healthy protein for human consumption and enhances human health [3]. Unfortunately, disease outbreaks continue to be a significant obstacle preventing advanced intensification from achieving sustainable production. Several safe and environmentally friendly compounds for modulating immune state, improve growth performance, and prevent fish disease are being investigated in aquaculture [50,51,52]. They are also used to protect aquaculture animals from external stressors such as contaminated water, cold temperatures, and overcrowding [53,54]. Therefore, our study aimed to address the potential role of ASLE in improving the health, immune, and growth performance of Nile tilapia.
The findings of the present study demonstrated that various health indicators in Nile tilapia could be successfully improved through ASLE dietary replacement. The best results were found in the group of fish fed the 20 g ASLE/kg diet, as indicated by a significant improvement in final body weight, weight gain (%), SGR, FCR, and PER compared to fish fed the control diet. The incorporation of ASLE in the Nile tilapia diet showed statistically significant changes, which were detected in the crude protein and ash in a dose-dependent manner. These observations are consistent with those of Safira et al. [55], who investigated the effect of ASLE on Clarias batrachus growth rate. However, to our knowledge, no study has investigated the impact of A. squamosa on tilapia species. Growth enhancements can be linked with improvement of diet digestion and absorption, leading to improved nutrient utilization. This supposition was verified by the high activity of digestive enzymes observed herein. On the other hand, ASLE may reduce the number of possible pathogens in the gastrointestinal tract (Figure 4), increase the population of beneficial microorganisms, and/or increase the activity of microbial enzymes, all of which would increase feed digestibility and nutrient absorption. In addition, high concentrations of protein, fiber, carbohydrates, essential oils, vitamins, and minerals improved the nutritional status of dietary ASLE [19]. Farag & Paré [56], El-Garhy et al. [57], El-Houseiny, et al. [58]; Toutou, et al. [59] reported superior growth performance following the feeding of certain natural plants or plant extracts to Nile tilapia.
Physiological and stress conditions of fish can be evaluated using hematological parameters [60]. The current study confirmed that ASLE-fed Nile tilapia demonstrated a substantial increase in all blood indices in diets containing 20 g/kg ASLE. This finding suggests that the incorporation of ASLE into the diet could increase the immunity and prevent infection. A. squamosa contains a wide variety of phytochemicals, including proteins, carbohydrates, saponins, alkaloids, flavonoids, phenolics, and glycosides [61]. As ASLE has a higher protein level, both humans and animals can benefit from its nutritional value [62]. Furthermore, A. squamosa contains a variety of minerals, including vitamin A, C, E, B1 (thiamine), B2 (riboflavin), B3 (niacin), B9, and folic acid, that have a hematic impact. Extracts of A. squamosa were found to contain macro and microminerals: Mg, P, Zn, Cu, and Se [63]. To maintain general health, various minerals are necessary [64]. Similar results of enhanced hematological indices were reported with an extract of Mitracarpus scaber leaves being fed to Nile tilapia [65]; as well as Milk thistle and co-enzyme Q10 [58], and A. vulgaris powder [13].
Compared to mammals, fish have a more innate immunity for defense [66]. The phagocytic cells (neutrophils and macrophages), which are an essential part of innate immunity, play a crucial role in eliminating infections through a process known as phagocytosis. Additionally, macrophages emit a potent reactive oxygen called NO to improve their capacity to kill infections through phagocytosis [67]. Another crucial element is lysozyme, which is produced by leucocytes engaged during the start of phagocytosis and has bactericidal effects, by lysing bacterial cell walls [68]. In the current study, dietary ASLE significantly enhanced the non-specific immunological defenses in Nile tilapia compared to those in the control group in a dose-dependent manner. This may have been due to potential biological and pharmaceutical effects, including antioxidant, antibacterial, and antiviral impacts [23,69]. Other elements identified in ASLE that could help to improve fish immunity include acetogenins, alkaloids, flavonoids, phenols, saponins, tannins, glycosides, sesquiterpenes, anthocyanins, steroids, diterpenes, terpenoids, quinones, amino acids, and fatty acids [70,71]. To combat various diseases, polyphenolic chemicals play an important role in the control of a number of physiological and biochemical factors, including enzyme activity, cell differentiation, signal transduction mechanisms, and cellular redox potential [72]. Similar immunomodulatory actions were recorded in Nile tilapia with various herbal dietary additions [13,58].
Numerous crucial biological components, such as DNA and proteins, can be destroyed by oxidative stress. The body has a defense mechanism against oxidative damage to the tissues [73]. CAT and SOD are antioxidant enzymes that can eliminate reactive oxygen free radicals. Glutathione is a non-enzymatic antioxidant that can also counteract these radicals through enzymatic reactions. In the current study, significant increases over the control values were noted in serum antioxidant enzyme activities, including CAT, SOD, GSH, and TAC, as well as a reduction in the MDA content in the serum of fish fed ASLE-supplemented diets. The results also confirmed an improvement in the liver contents of CAT and SOD, with significant decreases in the level of MDA in ASLE-fed groups compared to the control. The leucocyte-produced MPO enzyme is a component of the innate immune system. As a physiological catalyst for lipid peroxidation, this enzyme produces ROS, which in turn affects the inflammatory response [74]. In this investigation, feeding on ASLE-supplied diets resulted in a reduction in serum MPO activity. This result may have been related to the anti-inflammatory effect of A. squamosa, which is related to a number of chemical compounds, including phenolics, annonaceous acetogenins, saponins, flavonoids, alkaloids, glycosides, alkaloids, steroids, and terpenoids, or due to cyclooxygenase enzyme activity inhibition, which is involved in the inflammation process [56]. The antioxidant properties of ASLE may be attributed to A. squamosa containing flavanoids, coumarins, alkaloids, and terpenoids [19,75,76]. Several authors have shown a significant link between fruit phenolic concentrations and antioxidant capability [18,77]. In Nile tilapia fed A. vulgaris, Silybum marianum exerted antioxidant efficacy and consequently protected tissues from oxidative stress, as stated by Mansour, et al. [13]; Khalil, et al. [78], respectively.
In this study, fish fed ASLE-supplemented diets had higher levels of total serum proteins and globulin. This finding suggests that ASLE can boost protective proteins, which can then activate the immune system. High levels of blood protein, particularly globulins, are a good predictor of enhanced liver function and innate immune response [79]. Moreover, the enhancement of serum total protein and globulin may have been due to the fact that ASLE contains a high amount of proteins and amino acids [80]. Similar results were observed in Nile tilapia fed diets supplemented with A. vulgaris [13].
Compounds that serve as antioxidants, lipid peroxidation inhibitors, and have the ability to scavenge free radicals may have hepato-renal protective characteristics. As we mentioned previously, ASLE has high levels of antioxidant enzyme activity. The present study suggested the idea that the incorporation of ASLE in the fish diet led to a reduction of liver functional enzymes (ALT, AST, and ALP) and kidney function indicators. Additionally, ASLE reduced MDA levels in the liver and serum. The current results were supported by Kumar, et al. [19], who mentioned that the effect of ASLE was equivalent to that of a hepatoprotective substance (silymarin), which was attributed to the abundance of coumarins, which may be the main factor causing the hepatoprotective action.
The primary indicators of fish stress are serum glucose and cortisol, which fluctuate depending on changes in the environment or in the diet. Under normal circumstances, cortisol regulates a variety of physiological processes in fish, and it also enables quick physiological changes in response to stress [81]. Several components of intermediary energy metabolism are stimulated by cortisol, which also increases oxygen absorption, boosts gluconeogenesis, and inhibits glycogen synthesis. Cortisol also appears to play a key role in both aerobic and anaerobic metabolism [82]. In the current investigation, blood glucose and cortisol levels were significantly decreased with the dietary addition of ASLE. The richness of ASLE in flavonoids and other essential minerals plays a critical role in controlling glucose uptake and lipid metabolism [19,71,83,84]. Since cortisol plays a significant role in reducing inflammation and improving immunity, as shown by the lower level of MPO in serum, ASLE is therefore believed to be particularly beneficial for reducing stress.
Particularly during the winter months, tilapia in fish farms are occasionally exposed to sudden cold temperatures for a few hours in the early morning. Overall, the greater capacity of the tilapia supplemented with ASLE to withstand cold-water stress was a direct consequence of all of the impacts mentioned. Consistent with this result, Ibrahim, et al. [14] revealed that incorporation of rocket leaves in the diet could ameliorate tilapia fish against cold water stress.
Challenge tests are typically used as the standard assay to assess the overall health of the immune system [85]. A strong indicator of the effectiveness of immunostimulants is the increase in the resistance of fish to pathogenic microorganisms [86]. According to the findings of this study, dietary ASLE had a protective effect against A. sobria infection in the fish. This may have been correlated with the elevation of nonspecific immune parameters, such as phagocytic activity, lysozyme, NO, C3, and antioxidant enzyme activities, in addition to the reduced levels of glucose and cortisol, which could have strengthened the defense of fish against infection. In fact, it has also been reported to possess extraordinary pharmacological capabilities, including antibacterial action [87]. In addition, annotemoyin, an active acetogenin component obtained from chloroform leaf extract, and certain flavonoid compounds purified from the plant’s aqueous leaf extract, both demonstrated notable antibacterial properties [88]. Squamocin, squamostatin, and cholesteryl glucopyranoside are examples of other acetogenins that have been shown to suppress the growth of certain Gram-positive and Gram-negative bacteria [18,89]. The primary components of ASLE’s antimicrobial mechanism of action are the phenolic compounds, which disrupt bacterial metabolic processes, cause cytoplasmic component coagulation and leakage, and have an anti-quorum sensing function [90]. Our findings agree with those of Paul, et al. [55], who investigated the impact of ASLE on the survival rates of C. batrachus. The inclusion of phytochemical components and the antimicrobial characteristics of ASLE contributed to the higher survival rates. The enhancement in the disease resistance of Nile tilapia has been reported when fed various medicinal plants or their extracts [13,91,92].
5. Conclusions
The findings of the current investigation demonstrated that the dietary addition of ASLE at a dose of 20 g/kg could improve the measured blood parameters, demonstrating its hemostatic efficacy on RBCs, Hb, PCV, and WBCs, as well as the immune response, antioxidant status, and tolerance of Nile tilapia to cold water stress and A. sobria infection. Improvements in hepatorenal function, immunological, and antioxidant parameters may strengthen fish’s ability to fight off diseases and tolerate stress, which would ultimately benefit the aquaculture sector. However, more research is required to determine the molecular, immunomodulatory, and other effects brought about by ASLE in other species of fish.
Author Contributions
Conceptualization, W.E.-H.; Data curation, A.A.K.; Formal analysis, A.A.K. and W.E.-H.; Funding acquisition, S.H.A. and A.T.M.; Investigation, A.A.K.; Methodology, A.A.K. and W.E.-H.; Project administration, A.A.K.; Resources, S.H.A. and A.T.M.; Software, S.H.A. and W.E.-H.; Supervision, W.E.-H.; Validation, A.A.K. and W.E.-H.; Visualization, S.H.A. and A.T.M.; Writing—original draft, A.A.K. and W.E.-H.; Writing—review & editing, A.T.M. and W.E.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia GRANT 2701).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Institutional Animal Care and Use Committee, Faculty of Veterinary Medicine, Zagazig University (Approval no. ZU-IACUC/2/F/284/2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
On request, the authors will supply all necessary data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prabu, E.; Rajagopalsamy, C.; Ahilan, B.; Jeevagan, I.J.M.A.; Renuhadevi, M. Tilapia–an excellent candidate species for world aquaculture: A review. Annu. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Haygood, A.M.; Jha, R. Strategies to modulate the intestinal microbiota of Tilapia (Oreochromis sp.) in aquaculture: A review. Rev. Aquacult. 2018, 10, 320–333. [Google Scholar] [CrossRef]
- Mansour, A.T.; Ashour, M.; Alprol, A.E.; Alsaqufi, A.S. Aquatic Plants and Aquatic Animals in the Context of Sustainability: Cultivation Techniques, Integration, and Blue Revolution. Sustainability 2022, 14, 3257. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Tilapia Culture; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Li, Y.; Cai, S.-H. Identification and pathogenicity of Aeromonas sobria on tail-rot disease in juvenile tilapia Oreochromis niloticus. Curr. Microbiol. 2011, 62, 623–627. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Mansour, M.F.; Mohamed, W.A.; Al-Gabri, N.A.; El-Sayed, A.A.; Altohamy, D.E.; Ibrahim, R.E. Silver nanoparticles mitigate Aeromonas hydrophila-induced immune suppression, oxidative stress, and apoptotic and genotoxic effects in Oreochromis niloticus. Aquaculture 2021, 535, 736430. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Liu, Z.; Kang, Y.; Wang, J. Transcriptomic responses to heat stress in rainbow trout Oncorhynchus mykiss head kidney. Fish Shellfish Immunol. 2018, 82, 32–40. [Google Scholar] [CrossRef]
- Baras, E.; Jacobs, B.; Mélard, C. Effect of water temperature on survival, growth and phenotypic sex of mixed (XX–XY) progenies of Nile tilapia Oreochromis niloticus. Aquaculture 2001, 192, 187–199. [Google Scholar] [CrossRef]
- Dadar, M.; Dhama, K.; Vakharia, V.N.; Hoseinifar, S.H.; Karthik, K.; Tiwari, R.; Khandia, R.; Munjal, A.; Salgado-Miranda, C.; Joshi, S.K. Advances in aquaculture vaccines against fish pathogens: Global status and current trends. Rev. Fish. Sci. Aquac. 2017, 25, 184–217. [Google Scholar] [CrossRef]
- Teuber, M. Veterinary use and antibiotic resistance. Curr. Opin. Microbiol. 2001, 4, 493–499. [Google Scholar] [CrossRef]
- Gule, T.T.; Geremew, A. Dietary Strategies for Better Utilization of Aquafeeds in Tilapia Farming. Aquacult. Nutr. 2022, 2022, 9463307. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Khalil, A.A.; Abd-Elhakim, Y.M.; Badr, H.A. The potential role of turmeric and black pepper powder diet supplements in reversing cadmium-induced growth retardation, ATP depletion, hepatorenal damage, and testicular toxicity in Clarias gariepinus. Aquaculture 2019, 510, 109–121. [Google Scholar] [CrossRef]
- Mansour, A.T.; Mahboub, H.H.; Elshopakey, G.E.; Aziz, E.K.; Alhajji, A.H.; Rayan, G.; Ghazzawy, H.S.; El-Houseiny, W. Physiological Performance, Antioxidant and Immune Status, Columnaris Resistance, and Growth of Nile Tilapia That Received Alchemilla vulgaris-Supplemented Diets. Antioxidants 2022, 11, 1494. [Google Scholar] [CrossRef]
- Ibrahim, R.E.; El-Houseiny, W.; Behairy, A.; Abo-Elmaaty, A.; Al-Sagheer, A.A. The palliative role of Eruca sativa leaves dietary supplementation against oxidative stress, immunosuppression, and growth retardation in temperature-stressed Oreochromis niloticus. J. Therm. Biol. 2019, 84, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Khanna, A.; Bhandari, R.; Yadav, S.; Gautam, D. Phytochemical screening of Annona squamosa and haematological studies in clarias batrachus. World J. Pharm. Pharm. Sci. 2016, 5, 1121–1131. [Google Scholar]
- Suresh, K.; Manoharan, S.; Panjamurthy, K.; Kavitha, K. Chemopreventive and antilipidperoxidative efficacy of Annona squamosa bark extracts in experimental oral carcinogenesis. Pak. J. Biol. Sci. 2006, 9, 2600–2605. [Google Scholar] [CrossRef]
- Pardhasaradhi, B.; Reddy, M.; Ali, A.M.; Kumari, A.L.; Khar, A. Antitumour activity of Annona squamosa seed extracts is through the generation of free radicals and induction of apoptosis. Indian J. Biochem. Biophys. 2004, 41, 167–172. [Google Scholar] [PubMed]
- Pandey, N.; Barve, D. Phytochemical and pharmacological review on Annona squamosa Linn. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 1404–1412. [Google Scholar]
- Kumar, M.; Changan, S.; Tomar, M.; Prajapati, U.; Saurabh, V.; Hasan, M.; Sasi, M.; Maheshwari, C.; Singh, S.; Dhumal, S. Custard apple (Annona squamosa L.) leaves: Nutritional composition, phytochemical profile, and health-promoting biological activities. Biomolecules 2021, 11, 614. [Google Scholar] [CrossRef]
- Vanitha, V.; Umadevi, K.; Vijayalakshmi, K. Determination of bioactive components of Annona squamosa L. leaf by GC-MS analysis. Int. J. Pharm. Sci. Drug Res. 2011, 3, 309–312. [Google Scholar]
- Shenoy, C.; Patil, M.; Kumar, R. Antibacterial and wound healing activity of the leaves of Annona squamosa Linn. (Annonaceae). Res. J. Pharmacogn. Phytochem. 2009, 1, 44–50. [Google Scholar]
- Paul, R.; Khanna, A.; Gautam, D.S.; Bhandari, R.; Patel, D.; Nigam, P. Effect of aqueous leaf extract of Annona squamosa on Clarias batrachus fish infected with Aeromonas hydrophila with reference to haematological parameters. World J. Pharm. Res. 2017, 7, 992–1005. [Google Scholar]
- Nguyen, M.; Nguyen, V.; Le, V.; Trieu, L.; Lam, T.; Bui, L.; Nhan, L.; Danh, V. Assessment of preliminary phytochemical screening, polyphenol content, flavonoid content, and antioxidant activity of custard apple leaves (Annona squamosa Linn.). In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK; p. 062012.
- El-Chaghaby, G.A.; Ahmad, A.F.; Ramis, E.S. Evaluation of the antioxidant and antibacterial properties of various solvents extracts of Annona squamosa L. leaves. Arab. J. Chem. 2014, 7, 227–233. [Google Scholar] [CrossRef]
- Abd-Elghany, A.A.; Mohamad, E.A. Ex-vivo transdermal delivery of Annona squamosa entrapped in niosomes by electroporation. J. Radiat. Res. Appl. Sci. 2020, 13, 164–173. [Google Scholar]
- CCoA, C. Canadian Council on Animal Care Guidelines on: The Care and Use of Fish in Research. Teach. Test. 2005. [Google Scholar]
- Boyd, C.E.; Tucker, C.S. Pond Aquaculture Water Quality Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Adeshina, I.; Jenyo-Oni, A.; Emikpe, B. Use of Eugenia cayrophyllata oil as anaesthetic in farm raised african catfish Clarias gariepinus juveniles. Egypt. J. Exp. Biol 2016, 12, 71–76. [Google Scholar]
- Jain, N.C. Essentials of Veterinary Hematology; Lea and Febiger: Philadelphia, PA, USA, 1993; pp. 76–250. [Google Scholar]
- Henry, R.J. Clinical Chemistry, Principles and Technics; Hoeber Medical Division, Harper & Row: New York, NY, USA, 1964. [Google Scholar]
- Reinhold, R. Determination of serum albumin. Clin. Chem 1953, 21, 1370–1372. [Google Scholar]
- Coles, E. Veterinary Clinical Pathology. WB Saunders Company. Phila. Lond. 1986. [Google Scholar]
- Wenger, C.; Kaplan, A.; Rubaltelli, F.; Hammerman, C. Alkaline phosphatase. In Clinical Chemistry; The C. V. Mosby Co.: St. Louis, MO, USA, 1984; pp. 1094–1098. [Google Scholar]
- Burtis, C.A.; Ashwood, E.R. Tietz Textbook Of Clinical Chemistry; American Association for Clinical Chemistry: Washington, DC, USA, 1994. [Google Scholar]
- Murray, R. Aspartate aminotransferase. In Clinical Chemistry; Kaplan, A., Glucose, K., Eds.; The CV Mosby Co.: St. Louis, MO, USA; Toronto, ON, Canada; Princeton, NJ, USA, 1984; pp. 1112–1116. [Google Scholar]
- Fossati, P.; Prencipe, L.; Berti, G. Enzymic creatinine assay: A new colorimetric method based on hydrogen peroxide measurement. Clin. Chem. 1983, 29, 1494–1496. [Google Scholar] [CrossRef]
- Kaplan, A. Urea; In Clinical Chemistry; Kaplan, A., Glucose, K., Eds.; the C. V Mosby Co.St Louis Toronto Princeton. 1984, pp. 418–437.
- Trinder, P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J. Clin. Pathol. 1969, 22, 246. [Google Scholar] [CrossRef]
- Tunn, S.; Möllmann, H.; Barth, J.; Derendorf, H.; Krieg, M. Simultaneous measurement of cortisol in serum and saliva after different forms of cortisol administration. Clin. Chem. 1992, 38, 1491–1494. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods Enzymol; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar]
- Kumari, J.; Sahoo, P. Effects of cyclophosphamide on the immune system and disease resistance of Asian catfish Clarias batrachus. Fish Shellfish Immunol. 2005, 19, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.E. Lysozyme assays. Tech. Fish Immunol. 1990, 1, 101–103. [Google Scholar]
- Siwicki, A.K.; Anderson, D.P.; Rumsey, G.L. Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet. Immunol. Immunopathol. 1994, 41, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Scheidegger, E.; Fracalanzza, S.; Teixeira, L.; Cardarelli-Leite, P. RFLP analysis of a PCR-amplified fragment of the 16S rRNA gene as a tool to identify Enterococcus strains. Mem. Inst. Oswaldo Cruz 2009, 104, 1003–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Song, W.; Shao, Q.; Peng, X.; Xiao, J.; Hua, Y.; Owari, B.N.; Zhang, T.; Ng, W.K. Partial replacement of fish meal by fermented soybean meal in diets for black sea bream, Acanthopagrus schlegelii, juveniles. J. World Aquacult. Soc. 2011, 42, 184–197. [Google Scholar] [CrossRef]
- Mansour, A.T.; Fayed, W.M.; Elkhayat, B.K.; Ali, E.; Omar, M.A.; Nour, A.-A.M.; Morshedy, S.A. Yucca schidigera extract dietary supplementation affects growth performance, hematological and physiological status of European seabass. Ann. Anim. Sci. 2021, 21, 1043–1060. [Google Scholar] [CrossRef]
- García-Beltrán, J.M.; Mansour, A.T.; Alsaqufi, A.S.; Ali, H.M.; Esteban, M.Á. Effects of aqueous and ethanolic leaf extracts from drumstick tree (Moringa oleifera) on gilthead seabream (Sparus aurata L.) leucocytes, and their cytotoxic, antitumor, bactericidal and antioxidant activities. Fish Shellfish Immunol. 2020, 106, 44–55. [Google Scholar] [CrossRef]
- Sallam, A.E.; Mansour, A.T.; Alsaqufi, A.S.; Salem, M.E.-S.; El-Feky, M.M. Growth performance, antioxidative status, innate immunity, and ammonia stress resistance of Siganus rivulatus fed diet supplemented with zinc and zinc nanoparticles. Aquacult. Rep. 2020, 18, 100410. [Google Scholar] [CrossRef]
- Refaey, M.M.; Mehrim, A.I.; Zenhom, O.A.; Mansour, A.T. Effect of fatty acids manipulation on survival and physiological response of hybrid red tilapia under chronic cold stress. Aquaculture 2022, 561, 738663. [Google Scholar] [CrossRef]
- Mansour, A.T.; Hamed, H.S.; El-Beltagi, H.S.; Mohamed, W.F. Modulatory Effect of Papaya Extract against Chlorpyrifos-Induced Oxidative Stress, Immune Suppression, Endocrine Disruption, and DNA Damage in Female Clarias gariepinus. Int. J. Environ. Res. Public 2022, 19, 4640. [Google Scholar] [CrossRef] [PubMed]
- Safira, A.; Widayani, P.; An-Najaaty, D.; Rani, C.A.M.; Septiani, M.; Putra, Y.A.S.; Solikhah, T.I.; Khairullah, A.R.; Raharjo, H.M. A Review of an Important Plants: Annona squamosa Leaf. Pharmacogn. J. 2022, 14, 456–463. [Google Scholar] [CrossRef]
- Farag, M.A.; Paré, P.W. Phytochemical analysis and anti-inflammatory potential of Hyphaene thebaica L. fruit. J. Food Sci. 2013, 78, C1503–C1508. [Google Scholar] [CrossRef]
- El-Garhy, H.A.; Khattab, S.; Moustafa, M.M.; Ali, R.A.; Azeiz, A.Z.A.; Elhalwagi, A.; El Sherif, F. Silybin content and overexpression of chalcone synthase genes in Silybum marianum L. plants under abiotic elicitation. Plant Physiol. Biochem. 2016, 108, 191–202. [Google Scholar] [CrossRef] [PubMed]
- El-Houseiny, W.; Khalil, A.A.; Abd-Elhakim, Y.M.; Arisha, A.H.; Moselhy, A.A.; Dahshan, H.; Saber, T.; Saber, T.M.; Ahmed, M.M. Alleviative effects of dietary Silybum marianum and co-enzyme Q10 on waterborne nickel-induced impaired growth, immunosuppression, tissue damage, immune-related genes dysregulation, and reduced resistance to Pseudomonas aeruginosa in Oreochromis niloticus. Aquacult. Rep. 2022, 26, 101308. [Google Scholar]
- Toutou, M.M.; Soliman, A.A.; Elokaby, M.A.; Abdel-Rahim, M.M.; Abouelwafa, A.E.; Abd Elmoneam, M.Y. The potential antimicrobial effects of dietary supplementation with Arak, Salvadora persica, on growth, health status, and pathogenic bacterial loads in Nile tilapia, Oreochromis niloticus fingerlings. Egypt. J. Aquat. Res. 2019, 45, 251–257. [Google Scholar] [CrossRef]
- Zaragoza, O.D.R.; Rodríguez, M.H.; Bückle Ramirez, L.F. Thermal stress effect on tilapia Oreochromis mossambicus (Pisces: Cichlidae) blood parameters. Mar. Freshwat. Behav. Physiol. 2008, 41, 79–89. [Google Scholar] [CrossRef]
- Kalyani, R.L.; Vijaykumar, P.; Pammi, S.; Rajkumar, M.; Swamy, P.; Murthy, K. Biosynthesis of silver nanoparticles using Annona squamosa leaf extract with synergistic antibacterial activity. Indian J. Pharm. Sci. 2019, 81, 1036–1044. [Google Scholar] [CrossRef]
- Shukry, W.; Galilah, D.; Elrazek, A.; Shapana, H. Mineral composition, nutritional properties, vitamins, and bioactive compounds in Annona squamosa L. grown at different sites of Egypt. Ser. Bot. Environ. Sci 2019, 1, 7–22. [Google Scholar]
- Varadharaj, V.; Janarthanan, U.; Krishnamurthy, V.; Synnah, J. Assessment of Phytonutrients in the Ethanolic Leaf Extract of Annona squamosa (L.). World J. Pharm. Pharm. Sci 2014, 3, 725–732. [Google Scholar]
- Akram, M.; Munir, N.; Daniyal, M.; Egbuna, C.; Găman, M.-A.; Onyekere, P.F.; Olatunde, A. Vitamins and Minerals: Types, sources and their functions. In Functional Foods and Nutraceuticals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 149–172. [Google Scholar]
- Adeshina, I.; Tiamiyu, L.O.; Akpoilih, B.U.; Jenyo-Oni, A.; Ajani, E.K. Dietary Mitracarpus scaber leaves extract improved growth, antioxidants, non-specific immunity, and resistance of Nile tilapia, Oreochromis niloticus to Gyrodactylus malalai infestation. Aquaculture 2021, 535, 736377. [Google Scholar] [CrossRef]
- Uribe, C.; Folch, H.; Enríquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486. [Google Scholar] [CrossRef]
- Neumann, N.F.; Stafford, J.L.; Barreda, D.; Ainsworth, A.J.; Belosevic, M. Antimicrobial mechanisms of fish phagocytes and their role in host defense. Dev. Comp. Immunol. 2001, 25, 807–825. [Google Scholar] [CrossRef]
- Magnadóttir, B.; Jónsdóttir, H.; Helgason, S.; Björnsson, B.; Jørgensen, T.Ø.; Pilström, L. Humoral immune parameters in Atlantic cod (Gadus morhua L.): I. The effects of environmental temperature. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1999, 122, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.H.; Solanki, H.K.; Tripathi, P.; Patel, N.J.; Jani, G.K. Evaluation of antimutagenic potential of Annona squamosa leaf extract. Elixir Hum. Physiol. 2011, 31, 1960–1965. [Google Scholar]
- Kumar, Y. Evaluation of antidiabetic and antioxidant potential of custard apple (Annona squamosa) Leaf extracts: A compositional study. Int. J. Chem. Stud. 2019, 7, 889–895. [Google Scholar]
- Soni, H.; Malik, J.; Yadav, A.P.; Yadav, B. Characterization of rutin isolated by leaves Annona squamosa by modern analytical techniques. Eur. J. Biomed. Pharm. Sci. 2018, 5, 484–489. [Google Scholar]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Saglam, D.; Atli, G.; Dogan, Z.; Baysoy, E.; Gurler, C.; Eroglu, A.; Canli, M. Response of the antioxidant system of freshwater fish (Oreochromis niloticus) exposed to metals (Cd, Cu) in differing hardness. Turk. J. Fish. Aquat. Sci. 2014, 14, 43–52. [Google Scholar]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Vikas, B.; Akhil, B.; Remani, P.; Sujathan, K. Free radical scavenging properties of Annona squamosa. Asian Pac. J. Cancer Prev.: APJCP 2017, 18, 2725. [Google Scholar] [PubMed]
- Lakshmi, S.; Dhanya, G. Phytochemical analysis of Annona squamosa seed extracts. Int. Res. J. Pharm. Appl. Sci. 2013, 3, 29–31. [Google Scholar]
- Abdille, M.H.; Singh, R.; Jayaprakasha, G.; Jena, B. Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem. 2005, 90, 891–896. [Google Scholar] [CrossRef]
- Khalil, A.A.; Abd-Elhakim, Y.M.; Said, E.N.; Moselhy, A.A.; Abu-Elsaoud, A.M.; El-Houseiny, W. Milk thistle and co-enzyme Q10 fortified diets lessen the nickel chloride-induced neurotoxic and neurobehavioral impairments in Oreochromis niloticus via regulating the oxidative stress response, acetylcholinesterase activity, and brain nickel content. Aquaculture 2022, 553, 738102. [Google Scholar] [CrossRef]
- Asadi, M.; Mirvaghefei, A.; Nematollahi, M.; Banaee, M.; Ahmadi, K. Effects of Watercress (Nasturtium nasturtium) extract on selected immunological parameters of rainbow trout (Oncorhynchus mykiss). Open Vet. J. 2012, 2, 32–39. [Google Scholar] [CrossRef]
- Pandey, V.; Giri, I.; Singh, S.; Srivastava, A. Pharmacognostical and physiochemical study on the leaves of Annona squamosa Linn. Int. J. Res. Pharm. Sci. 2014, 4, 8–12. [Google Scholar]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Tort, L.; Koumoundouros, G. Stress in farmed fish. Its consequences in health and performance. In Recent Advances in Aquaculture Research; Springer: Berlin/Heidelberg, Germany, 2010; pp. 55–83. [Google Scholar]
- Tripathi, Y.B. Insulin secreting and α-glucosidase inhibitory activity of hexane extract of Annona squamosa Linn. in streptozotocin (STZ) induced diabetic rats. Indian J. Exp. Biol. 2014, 52, 623–629. [Google Scholar]
- Davis, J.A.; Sharma, S.; Mittra, S.; Sujatha, S.; Kanaujia, A.; Shukla, G.; Katiyar, C.; Lakshmi, B.; Bansal, V.S.; Bhatnagar, P.K. Antihyperglycemic effect of Annona squamosa hexane extract in type 2 diabetes animal model: PTP1B inhibition, a possible mechanism of action? Indian J. Pharmacol. 2012, 44, 326. [Google Scholar] [CrossRef] [PubMed]
- El-Houseiny, W.; Abd El-Hakim, Y.M.; Metwally, M.M.; Ghfar, S.S.A.; Khalil, A.A. The single or combined Silybum marianum and co-enzyme Q10 role in alleviating fluoride-induced impaired growth, immune suppression, oxidative stress, histological alterations, and reduced resistance to Aeromonas sobria in African catfish (Clarias gariepinus). Aquaculture 2022, 548, 737693. [Google Scholar]
- Sakai, M. Current research status of fish immunostimulants. Aquaculture 1999, 172, 63–92. [Google Scholar] [CrossRef]
- Cosentino, S.; Tuberoso, C.I.G.; Pisano, B.; Satta, M.; Mascia, V.; Arzedi, E.; Palmas, F. In-vitro antimicrobial activity and chemical composition of Sardinian thymus essential oils. Lett. Appl. Microbiol. 1999, 29, 130–135. [Google Scholar] [CrossRef]
- Santhoshkumar, R.; Kumar, N.S. Phytochemical analysis and antimicrobial activities of Annona squamosa (L.) leaf extracts. J. Pharmacogn. Phytochem. 2016, 5, 128–131. [Google Scholar]
- Gajalakshmi, S.; Vijayalakshmi, S.; Devi, R.V. Phytochemical and pharmacological properties of Annona muricata: A review. Int. J. Pharm. Pharm. Sci. 2012, 4, 3–6. [Google Scholar]
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials—A review. Plants 2017, 6, 16. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Samir, F.; Abd El-Naby, A.S.; Monier, M.N. Antioxidative and immunostimulatory effect of dietary cinnamon nanoparticles on the performance of Nile tilapia, Oreochromis niloticus (L.) and its susceptibility to hypoxia stress and Aeromonas hydrophila infection. Fish Shellfish Immunol. 2018, 74, 19–25. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; El-Houseiny, W.; Abd Elhakeem, E.-M.; Ebraheim, L.L.; Moustafa, A.A.; Mohamed, A.A.R. Melamine and curcumin enriched diets modulate the haemato-immune response, growth performance, oxidative stress, disease resistance, and cytokine production in Oreochromis niloticus. Aquat. Toxicol. 2020, 220, 105406. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).