Heavy Metal(loid) Accumulation in the Ovarian Tissue of Free-Ranging Queens and Bitches Inhabiting Highly Polluted Urban Environments
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Metal Content Analysis
2.3. Statistical Analysis
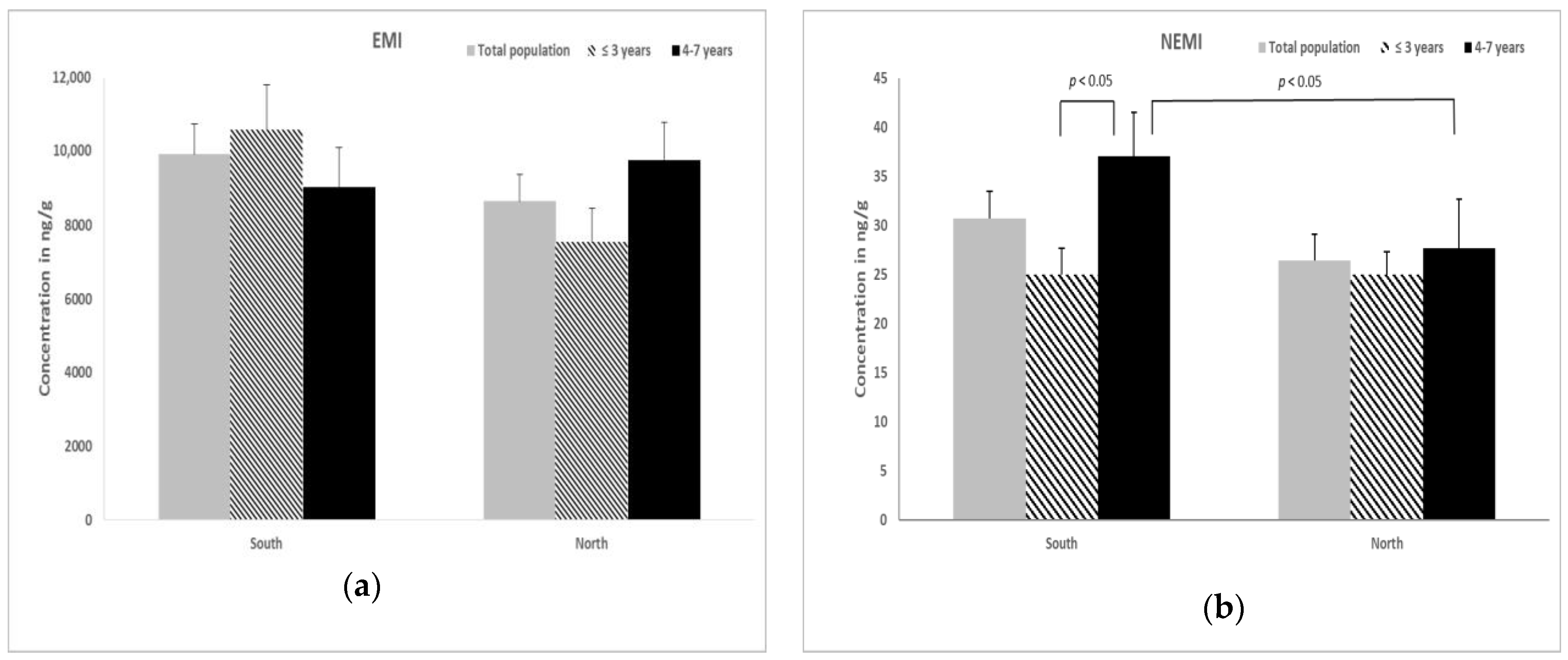
3. Results
3.1. Metal Content in the Ovarian Tissue of Queens
3.2. Metal Content in the Ovarian Tissue of Bitches
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canipari, R.; De Santis, L.; Cecconi, S. Female Fertility and Environmental Pollution. Int. J. Environ. Res. Public Health 2020, 17, 8802. [Google Scholar] [CrossRef]
- Ma, Y.; He, X.; Qi, K.; Wang, T.; Qi, Y.; Cui, L.; Wang, F.; Song, M. Effects of environmental contaminants on fertility and reproductive health. J. Environ. Sci. 2019, 77, 210–217. [Google Scholar] [CrossRef]
- Wrzecińska, M.; Kowalczyk, A.; Cwynar, P.; Czerniawska-Piątkowska, E. Disorders of the Reproductive Health of Cattle as a Response to Exposure to Toxic Metals. Biology 2021, 10, 882. [Google Scholar] [CrossRef]
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedziałek, B.; Opala, T.; Wilczak, M. Impact of heavy metals on the female reproductive system. Ann. Agric. Environ. Med. 2015, 22, 259–264. [Google Scholar] [CrossRef]
- Duffus, J.H. “Heavy Metals”—A meaningless term. Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Hawkes, S.J. What Is a “Heavy Metal”? J. Chem. Educ. 1997, 74, 1374. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Jarsjö, J.; Andersson-Sköld, Y.; Fröberg, M.; Pietroń, J.; Borgström, R.; Löv, Å.; Kleja, D.B. Projecting impacts of climate change on metal mobilization at contaminated sites: Controls by the groundwater level. Sci. Total Environ. 2020, 712, 135560. [Google Scholar] [CrossRef] [PubMed]
- Zaharescu, D.G.; Hooda, P.S.; Burghelea, C.I.; Polyakov, V.; Palanca-Soler, A. Climate change enhances the mobilisation of naturally occurring metals in high altitude environments. Sci. Total Environ. 2016, 560, 73–81. [Google Scholar] [CrossRef]
- Jaiswal, A.; Verma, A.; Jaiswal, P. Detrimental Effects of Heavy Metals in Soil, Plants, and Aquatic Ecosystems and in Humans. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 183–197. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Agarwal, S. Arsenic, cadmium, and lead. In Reproductive and Developmental Toxicology, 2nd ed.; CRC Press: New York, NY, USA, 2017; pp. 537–566. [Google Scholar]
- Gašparík, J.; Binkowski, Ł.J.; Jahnátek, A.; Šmehýl, P.; Dobiaš, M.; Lukáč, N.; Błaszczyk, M.; Semla, M.; Massanyi, P. Levels of Metals in Kidney, Liver, and Muscle Tissue and their Influence on the Fitness for the Consumption of Wild Boar from Western Slovakia. Biol. Trace Elem. Res. 2017, 177, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Keating, A.F. Impact of environmental exposures on ovarian function and role of xenobiotic metabolism during ovotoxicity. Toxicol. Appl. Pharmacol. 2012, 261, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Hirshfield, A.N. Development of Follicles in the Mammalian Ovary. In International Review of Cytology; Jeon, K.W., Friedlander, M., Eds.; Academic Press: Cambridge, MA, USA, 1991; Volume 124, pp. 43–101. [Google Scholar]
- Priya, K.; Setty, M.; Babu, U.V.; Pai, K.S.R. Implications of environmental toxicants on ovarian follicles: How it can adversely affect the female fertility? Environ. Sci. Pollut. Res. Int. 2021, 28, 67925–67939. [Google Scholar] [CrossRef] [PubMed]
- Badr, F.M.; El-Habit, O. Chapter 18—Heavy Metal Toxicity Affecting Fertility and Reproduction of Males. In Bioenvironmental Issues Affecting Men’s Reproductive and Sexual Health; Sikka, S.C., Hellstrom, W.J.G., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 293–304. [Google Scholar]
- Vabre, P.; Gatimel, N.; Moreau, J.; Gayrard, V.; Picard-Hagen, N.; Parinaud, J.; Leandri, R.D. Environmental pollutants, a possible etiology for premature ovarian insufficiency: A narrative review of animal and human data. Environ. Health 2017, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Massányi, P.; Massányi, M.; Madeddu, R.; Stawarz, R.; Lukáč, N. Effects of Cadmium, Lead, and Mercury on the Structure and Function of Reproductive Organs. Toxics 2020, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Banerjee, R.; Nath, S.; Das, S.; Banerjee, S. Metals and female reproductive toxicity. Hum. Exp. Toxicol. 2015, 34, 679–697. [Google Scholar] [CrossRef]
- Kennady, V.; Verma, R.; Chaudhiry, V. Detrimental impacts of heavy metals on animal reproduction: A review. J. Entomol. Zool. Stud. 2018, 06, 27–30. [Google Scholar]
- Dutta, S.; Gorain, B.; Choudhury, H.; Roychoudhury, S.; Sengupta, P. Environmental and occupational exposure of metals and female reproductive health. Environ. Sci. Pollut. Res. 2022, 29, 62067–62092. [Google Scholar] [CrossRef] [PubMed]
- Martino, N.A.; Marzano, G.; Mangiacotti, M.; Miedico, O.; Sardanelli, A.M.; Gnoni, A.; Lacalandra, G.M.; Chiaravalle, A.E.; Ciani, E.; Bogliolo, L.; et al. Exposure to cadmium during in vitro maturation at environmental nanomolar levels impairs oocyte fertilization through oxidative damage: A large animal model study. Reprod. Toxicol. 2017, 69, 132–145. [Google Scholar] [CrossRef]
- Piras, A.R.; Ariu, F.; Maltana, A.; Leoni, G.G.; Martino, N.A.; Mastrorocco, A.; Dell’Aquila, M.E.; Bogliolo, L. Protective effect of resveratrol against cadmium-induced toxicity on ovine oocyte in vitro maturation and fertilization. J. Anim. Sci. Biotechnol. 2022, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xing, X.; Zhang, Y.; Zhang, C.; Wu, Y.; Chen, Y.; Meng, R.; Jia, H.; Cheng, Y.; Zhang, Y.; et al. Lead exposure activates the Nrf2/Keap1 pathway, aggravates oxidative stress, and induces reproductive damage in female mice. Ecotoxicol. Environ. Saf. 2021, 207, 111231. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, Z.; Cao, Y.; Wang, J.; Shen, L.; Jiang, T.; Li, D.; Zou, W.; Zong, K.; Liang, D.; et al. Exposure to multiple toxic metals and polycystic ovary syndrome risk: Endocrine disrupting effect from As, Pb and Ba. Sci. Total Environ. 2022, 849, 157780. [Google Scholar] [CrossRef] [PubMed]
- Serpe, F.P.; Russo, R.; Simone, A.D.; Florio, S.; Esposito, M.; Severino, L. Levels of heavy metals in liver and kidney of dogs from urban environment. Open Vet. J. 2012, 2, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, A.; Lippolis, C.; Vacca, M.; Nardelli, C.; Castegna, A.; Arnesano, F.; Carella, N.; Depalo, R. The Effects of Chronic Lifelong Activation of the AHR Pathway by Industrial Chemical Pollutants on Female Human Reproduction. PLoS ONE 2016, 11, e0152181. [Google Scholar] [CrossRef] [PubMed]
- Ceko, M.J.; Hummitzsch, K.; Hatzirodos, N.; Bonner, W.M.; Aitken, J.B.; Russell, D.L.; Lane, M.; Rodgers, R.J.; Harris, H.H. X-Ray fluorescence imaging and other analyses identify selenium and GPX1 as important in female reproductive function. Metallomics 2015, 7, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Paksy, K.; Gáti, I.; Náray, M.; Rajczy, K. Lead accumulation in human ovarian follicular fluid, and in vitro effect of lead on progesterone producione by cultured human ovarian granulosa cells. J. Toxicol. Environ. Health Part A 2001, 62, 359–366. [Google Scholar] [CrossRef]
- Zenzes, M.T.; Krishnan, S.; Krishnan, B.; Zhang, H.; Casper, R.F. Cadmium accumulation in follicular fluid of women in in vitro fertilization-embryo transfer is higher in smokers. Fertil. Steril. 1995, 64, 599–603. [Google Scholar] [CrossRef]
- Schmalbrock, L.J.; Weiss, G.; Rijntjes, E.; Reinschissler, N.; Sun, Q.; Schenk, M.; Schomburg, L. Pronounced Trace Element Variation in Follicular Fluids of Subfertile Women Undergoing Assisted Reproduction. Nutrients 2021, 13, 4134. [Google Scholar] [CrossRef]
- Backer, L.C.; Grindem, C.B.; Corbett, W.T.; Cullins, L.; Hunter, J.L. Pet dogs as sentinels for environmental contamination. Sci. Total Environ. 2001, 274, 161–169. [Google Scholar] [CrossRef]
- Sumner, R.N.; Harris, I.T.; Van der Mescht, M.; Byers, A.; England, G.C.W.; Lea, R.G. The dog as a sentinel species for environmental effects on human fertility. Reproduction 2020, 159, R265–R276. [Google Scholar] [CrossRef]
- Ceko, M.J.; O’Leary, S.; Harris, H.H.; Hummitzsch, K.; Rodgers, R.J. Trace Elements in Ovaries: Measurement and Physiology1. Biol. Reprod. 2016, 94, 86. [Google Scholar] [CrossRef]
- Grajecki, D.; Zyriax, B.C.; Buhling, K.J. The effect of micronutrient supplements on female fertility: A systematic review. Arch. Gynecol. Obstet. 2012, 285, 1463–1471. [Google Scholar] [CrossRef]
- Buhling, K.J.; Grajecki, D. The effect of micronutrient supplements on female fertility. Curr. Opin. Obstet. Gynecol. 2013, 25, 173–180. [Google Scholar] [CrossRef]
- Michaluk, A.; Kochman, K. Involvement of copper in female reproduction. Reprod. Biol. 2007, 7, 193–205. [Google Scholar]
- Roychoudhury, S.; Nath, S.; Massanyi, P.; Stawarz, R.; Kacaniova, M.; Kolesarova, A. Copper-induced changes in reproductive functions: In vivo and in vitro effects. Physiol. Res. 2016, 65, 11–22. [Google Scholar] [CrossRef]
- Ausili, A.; Bergamin, L.; Romano, E. Environmental Status of Italian Coastal Marine Areas Affected by Long History of Contamination. Front. Environ. Sci. 2020, 8, 34. [Google Scholar] [CrossRef]
- Forte, G.; Bocca, B.; Pisano, A.; Collu, C.; Farace, C.; Sabalic, A.; Senofonte, M.; Fois, A.G.; Mazzarello, V.L.; Pirina, P.; et al. The levels of trace elements in sputum as biomarkers for idiopathic pulmonary fibrosis. Chemosphere 2021, 271, 129514. [Google Scholar] [CrossRef]
- Forte, G.; Fadda, C.; Bocca, B.; Erre, G.L.; Passiu, G.; Madeddu, R. Association Between Exposure to Heavy Metals and Systemic Sclerosis: The Levels of Al, Cd, Hg, and Pb in Blood and Urine of Patients. Biol. Trace Elem. Res. 2019, 190, 1–10. [Google Scholar] [CrossRef]
- Rzymski, P.; Niedzielski, P.; Poniedziałek, B.; Rzymski, P.; Pacyńska, J.; Kozak, L.; Dąbrowski, P. Free-ranging domestic cats are characterized by increased metal content in reproductive tissues. Reprod. Toxicol. 2015, 58, 54–60. [Google Scholar] [CrossRef]
- Neo, J.P.S.; Tan, B.H. The use of animals as a surveillance tool for monitoring environmental health hazards, human health hazards and bioterrorism. Vet. Microbiol. 2017, 203, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Swarup, D.; Patra, R.C.; Dwivedi, S.K.; Dey, S. Blood lead and cadmium in dogs from urban India. Vet. Hum. Toxicol. 2000, 42, 232–233. [Google Scholar] [PubMed]
- López-Alonso, M.; Miranda, M.; García-Partida, P.; Cantero, F.; Hernández, J.; Benedito, J.L. Use of dogs as indicators of metal exposure in rural and urban habitats in NW Spain. Sci. Total Environ. 2007, 372, 668–675. [Google Scholar] [CrossRef]
- Löpez-Alonso, M.; Miranda, M.; García-Partida, P.; Mendez, A.; Castillo, C.; Benedito, J.L. Toxic and trace metal concentrations in liver and kidney of dogs: Influence of diet, sex, age, and pathological lesions. Biol. Trace Elem. Res. 2007, 116, 185–202. [Google Scholar] [CrossRef]
- Esposito, M.; De Roma, A.; Maglio, P.; Sansone, D.; Picazio, G.; Bianco, R.; De Martinis, C.; Rosato, G.; Baldi, L.; Gallo, P. Heavy metals in organs of stray dogs and cats from the city of Naples and its surroundings (Southern Italy). Environ. Sci. Pollut. Res. Int. 2019, 26, 3473–3478. [Google Scholar] [CrossRef]
- Passlack, N.; Mainzer, B.; Lahrssen-Wiederholt, M.; Schafft, H.; Palavinskas, R.; Breithaupt, A.; Neumann, K.; Zentek, J. Concentrations of strontium, barium, cadmium, copper, zinc, manganese, chromium, antimony, selenium and lead in the equine liver and kidneys. SpringerPlus 2014, 3, 343. [Google Scholar] [CrossRef]
- Passlack, N.; Mainzer, B.; Lahrssen-Wiederholt, M.; Schafft, H.; Palavinskas, R.; Breithaupt, A.; Zentek, J. Concentrations of strontium, barium, cadmium, copper, zinc, manganese, chromium, antimony, selenium, and lead in the liver and kidneys of dogs according to age, gender, and the occurrence of chronic kidney disease. J. Vet. Sci. 2015, 16, 57–66. [Google Scholar] [CrossRef]
- Biggeri, A.; Lagazio, C.; Catelan, D.; Pirastu, R.; Casson, F.; Terracini, B. Report on health status of residents in areas with industrial, mining or military sites in Sardinia, Italy. Epidemiol. E Prev. 2006, 30, 5–95. [Google Scholar]
- Pagano, G.; de Biase, A.; Iaccarino, M.; Meriç, S.; Petruzzelli, D.; Tünay, O.; Warnau, M.; Trieff, N.M. Bauxite manufacturing residues from Gardanne (France) and Portovesme (Italy) exert different patterns of pollution and toxicity to sea urchin embryos. Environ. Toxicol. Chem. 2002, 21, 1272–1278. [Google Scholar] [CrossRef]
- Sanna, E.; Floris, G.; Vallascas, E. Town and gender effects on hair lead levels in children from three Sardinian towns (Italy) with different environmental backgrounds. Biol. Trace Elem. Res. 2008, 124, 52–59. [Google Scholar] [CrossRef]
- Sanna, E.; Liguori, A.; Palmas, L.; Soro, M.R.; Floris, G. Blood and hair lead levels in boys and girls living in two Sardinian towns at different risks of lead pollution. Ecotoxicol. Environ. Saf. 2003, 55, 293–299. [Google Scholar] [CrossRef]
- Peluso, M.E.M.; Munnia, A.; Giese, R.W.; Catelan, D.; Rocca, S.; Farigu, S.; Leoni, A.; Bruzzone, M.; Ceppi, M.; Biggeri, A. Exocyclic DNA adducts in sheep with skeletal fluorosis resident in the proximity of the Portoscuso-Portovesme industrial estate on Sardinia Island, Italy. Toxicol. Res. 2015, 4, 986–993. [Google Scholar] [CrossRef]
- Akar, Y.; Ahmad, N.; Khalıd, M. The effect of cadmium on the bovine in vitro oocyte maturation and early embryo development. Int. J. Vet. Sci. Med. 2018, 6, S73–S77. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.; Bogliolo, L.; Deiana, G.; Berlinguer, F.; Rosati, I.; Pintus, P.P.; Ledda, S.; Naitana, S. Influence of cadmium exposure on in vitro ovine gamete dysfunction. Reprod. Toxicol. 2002, 16, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Gupta, P.S.; Selvaraju, S.; Roy, S.C.; Ravindra, J.P. Effects of exposure to heavy metals on viability, maturation, fertilization, and embryonic development of buffalo (Bubalus bubalis) oocytes in vitro. Arch. Environ. Contam. Toxicol. 2010, 58, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Tessaro, I.; Modina, S.C.; Crotti, G.; Franciosi, F.; Colleoni, S.; Lodde, V.; Galli, C.; Lazzari, G.; Luciano, A.M. Transferability and inter-laboratory variability assessment of the in vitro bovine oocyte fertilization test. Reprod. Toxicol. 2015, 51, 106–113. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, X.; Chen, Y.; Liu, X.; Sun, Y.; Xiong, B. Glutathione alleviates the cadmium exposure-caused porcine oocyte meiotic defects via eliminating the excessive ROS. Environ. Pollut. 2019, 255, 113194. [Google Scholar] [CrossRef]

| Metal (ng/g) | Geographical Area | |
|---|---|---|
| South | North | |
| Essential | ||
| Cu | 6220 ± 722 | 5044 ± 757 |
| Fe | 271,574 ± 16,518 a | 401,336 ± 30,907 a |
| Mn | 1239 ± 170 | 1352 ± 152 |
| Zn | 66,503 ± 3784 | 66,107 ± 3192 |
| Se | 1617 ± 59 | 1562 ± 75 |
| Non-essential | ||
| Al | 614 ± 147 | 887 ± 287 |
| As | 139 ± 39 | 107 ± 61 |
| Cd | 52.2 ± 14.0 | 26.4 ± 5.5 |
| Pb | 81.4 ± 16.6 | 51.1 ± 17.9 |
| Metal (ng/g) | Geographical Area | |||
|---|---|---|---|---|
| South | North | |||
| Age | ||||
| ≤1 Year (n = 16) | 2–5 Years (n = 10) | ≤1 Year (n = 6) | 2–5 Years (n = 8) | |
| Essential | ||||
| Cu | 6785 ± 1064 a | 5171 ± 741 | 3472 ± 576 a | 6224 ± 1104 |
| Fe | 272,821 ± 21,783 b | 269,578 ± 26,543 e | 346,739 ± 12,031 b | 442,284 ± 49,679 e |
| Mn | 1275 ± 102 | 1181 ± 88 | 1173 ± 322 | 1444 ± 122 |
| Zn | 67,830 ± 5004 | 64,379 ± 5985 | 68,087 ± 6483 | 64,621 ± 3141 |
| Se | 1652 ± 87 | 1559 ± 65 | 817 ± 259 | 940 ± 481 |
| Non-essential | ||||
| Al | 767 ± 226 | 384 ± 127 | 817 ± 259 | 940 ± 481 |
| As | 114 ± 36 | 180 ± 87 | 192 ± 141 | 43.1 ± 12.2 |
| Cd | 21.6 ± 3.1 c | 101 ± 31 c,f | 27.7 ± 7.7 | 25.4 ± 8.2 f |
| Pb | 36.3 ± 6.3 d | 149 ± 30 d,g | 54.6 ± 31.8 | 48.6 ± 22.4 g |
| Metal (ng/g) | Geographical Area | |
|---|---|---|
| South | North | |
| Essential | ||
| Cu | 3228 ± 334 | 2469 ± 250 |
| Fe | 326,658 ± 35,738 | 277,120 ± 30,525 |
| Mn | 1318 ± 180 | 1142 ± 165 |
| Zn | 59,784 ± 8494 | 58,913 ± 8218 |
| Se | 1497 ± 115 | 1357 ± 88 |
| Non-essential | ||
| Al | 276 ± 47 | 421 ± 69 |
| As | 21.7 ± 4.2 | 21.8 ± 3.9 |
| Cd | 19.7 ± 4.0 a | 12.2 ± 1.8 a |
| Pb | 20.4 ± 3.6 b | 12.2 ± 5.2 b |
| Metal (ng/g) | Geographical Area | |||
|---|---|---|---|---|
| South | North | |||
| Age | ||||
| ≤3 Years (n = 12) | 4–7 Years (n = 9) | ≤3 Years (n = 12) | 4–7 Years (n = 12) | |
| Essential | ||||
| Cu | 3656 ± 470 a | 2655 ± 419 | 2127 ± 292 a | 2810 ± 393 |
| Fe | 274,410 ± 31,770 | 396,322 ± 67,369 | 219,244 ± 23,922 | 334,995 ± 52,108 |
| Mn | 1484 ± 240 | 1097 ± 268 | 924 ± 160 | 1359 ± 282 |
| Zn | 68,499 ± 13,812 | 48,165 ± 6450 | 48,301 ± 8898 | 69,526 ± 13,521 |
| Se | 1599 ± 142 | 1362 ± 191 | 1315 ± 143 | 1398 ± 109 |
| Toxic | ||||
| Al | 339 ± 84 | 207 ± 25 | 376 ± 63 | 466 ± 124 |
| As | 21.6 ± 4.3 | 21.8 ± 8.4 | 19.8 ± 4.6 | 23.7 ± 6.5 |
| Cd | 10.7 ± 1.5 b | 31.9 ± 7.6 b,d | 11.4 ± 2.6 | 13.0 ± 2.6 d |
| Pb | 14.8 ± 4.7 c | 27.9 ± 4.8 c,e | 7.82 ± 0.73 | 16.5 ± 10.6 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forte, G.; Ariu, F.; Bocca, B.; Solinas, G.; Leoni, G.G.; Podda, A.; Madeddu, R.; Bogliolo, L. Heavy Metal(loid) Accumulation in the Ovarian Tissue of Free-Ranging Queens and Bitches Inhabiting Highly Polluted Urban Environments. Animals 2023, 13, 650. https://doi.org/10.3390/ani13040650
Forte G, Ariu F, Bocca B, Solinas G, Leoni GG, Podda A, Madeddu R, Bogliolo L. Heavy Metal(loid) Accumulation in the Ovarian Tissue of Free-Ranging Queens and Bitches Inhabiting Highly Polluted Urban Environments. Animals. 2023; 13(4):650. https://doi.org/10.3390/ani13040650
Chicago/Turabian StyleForte, Giovanni, Federica Ariu, Beatrice Bocca, Giuliana Solinas, Giovanni Giuseppe Leoni, Andrea Podda, Roberto Madeddu, and Luisa Bogliolo. 2023. "Heavy Metal(loid) Accumulation in the Ovarian Tissue of Free-Ranging Queens and Bitches Inhabiting Highly Polluted Urban Environments" Animals 13, no. 4: 650. https://doi.org/10.3390/ani13040650
APA StyleForte, G., Ariu, F., Bocca, B., Solinas, G., Leoni, G. G., Podda, A., Madeddu, R., & Bogliolo, L. (2023). Heavy Metal(loid) Accumulation in the Ovarian Tissue of Free-Ranging Queens and Bitches Inhabiting Highly Polluted Urban Environments. Animals, 13(4), 650. https://doi.org/10.3390/ani13040650










