Development of a Method for the Fast Detection of Extended-Spectrum β-Lactamase- and Plasmid-Mediated AmpC β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae from Dogs and Cats in the USA
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
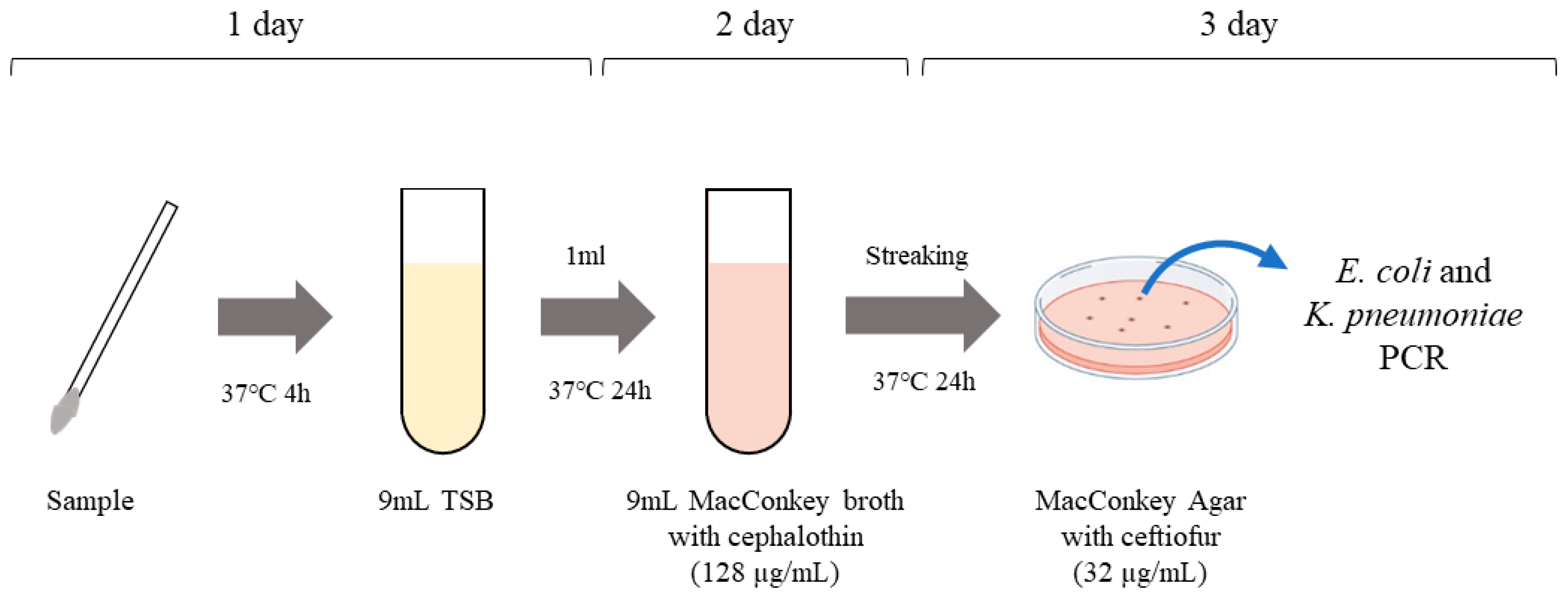
2.1. Study Design
2.2. Sampling
2.3. Isolation of ESBL- and pAmpC β-Lactamase-Producing E. coli and K. pneumoniae
2.4. Antimicrobial Susceptibility Testing
3. Results and Discussion
3.1. Isolation of ESBL and pAmpC-producing Escherichia coli and Klebsiella pneumoniae
3.2. Characterization of ESBL and pAmpC-Producing Escherichia coli and Klebsiella pneumoniae
3.3. Antimicrobial Resistance Phenotypes
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Z.; Wang, Y.; Chen, Y.; Huang, M.; Wang, Y.; Shen, Z.; Xia, Z.; Li, G. Antimicrobial resistance of bacterial pathogens isolated from canine urinary tract infections. Vet. Microbiol. 2020, 241, 108540. [Google Scholar] [CrossRef]
- Darwich, L.; Seminati, C.; Burballa, A.; Nieto, A.; Durán, I.; Tarradas, N.; Molina-López, R.A. Antimicrobial susceptibility of bacterial isolates from urinary tract infections in companion animals in Spain. Vet. Rec. 2021, 188, e60. [Google Scholar] [CrossRef]
- Smoglica, C.; Evangelisti, G.; Fani, C.; Marsilio, F.; Trotta, M.; Messina, F.; Di Francesco, C.E. Antimicrobial Resistance Profile of Bacterial Isolates from Urinary Tract Infections in Companion Animals in Central Italy. Antibiotics 2022, 11, 1363. [Google Scholar] [CrossRef]
- Toombs-Ruane, L.J.; Marshall, J.C.; Benschop, J.; Drinković, D.; Midwinter, A.C.; Biggs, P.J.; Grange, Z.; Baker, M.G.; Douwes, J.; Roberts, M.G.; et al. Extended spectrum β-lactamase-and AmpC β-lactamase-producing Enterobacterales associated with urinary tract infections in the New Zealand community: A case-control study. Int. J. Infect. Dis. 2022, 128, 325–334. [Google Scholar] [CrossRef]
- Mahrouki, S.; Hammami, S.; Mansouri, R.; Abbassi, M.S. Overview of ESBLproducing Escherichia coli of Animal Origin in Tunisia: In the Way of the Global Spread of CTX-M β-Lactamases. Archi. Cli. Micro. 2015, 6, 4. [Google Scholar]
- Marques, C.; Belas, A.; Aboim, C.; Cavaco-Silva, P.; Trigueiro, G.; Gama, L.T.; Pomba, C. Evidence of Sharing of Klebsiella pneumoniae Strains between Healthy Companion Animals and Cohabiting Humans. J. Clin. Microbiol. 2019, 57, e01537-618. [Google Scholar] [CrossRef]
- Prescott, J.F.; Hanna, W.J.; Reid-Smith, R.; Drost, K. Antimicrobial drug use and resistance in dogs. Can. Vet. J. 2002, 43, 107–116. [Google Scholar]
- Pallett, A.; Hand, K. Complicated urinary tract infections: Practical solutions for the treatment of multiresistant Gram-negative bacteria. J. Antimicrob. Chemother. 2010, 65, iii25–iii33. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lee, M.K.; Kim, T.H. Management of Extended-Spectrum Beta-Lactamase-Positive Gram-Negative Bacterial Urologic Infections Urogenit. Tract. Infect. 2015, 10, 84–91. [Google Scholar]
- Lee, S.; Han, S.W.; Kim, K.W.; Song, D.Y.; Kwon, K.T. Third-generation cephalosporin resistance of community-onset Escherichia coli and Klebsiella pneumoniae bacteremia in a secondary hospital. Korean J. Intern. Med. 2014, 29, 49–56. [Google Scholar] [CrossRef]
- Zogg, A.L.; Simmen, S.; Zurfluh, K.; Stephan, R.; Schmitt, S.N.; Nüesch-Inderbinen, M. High Prevalence of Extended-Spectrum β-Lactamase Producing Enterobacteriaceae Among Clinical Isolates From Cats and Dogs Admitted to a Veterinary Hospital in Switzerland. Front. Vet. Sci. 2018, 5, 62. [Google Scholar] [CrossRef]
- Coque, T.M.; Novais, A.; Carattoli, A.; Poirel, L.; Pitout, J.; Peixe, L.; Baquero, F.; Cantón, R.; Nordmann, P. Dissemination of clonally related Escherichia coli strains expressing extended-spectrum beta-lactamase CTX-M-15. Emerg. Infect. Dis. 2008, 14, 195–200. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine—5th Revision; World Health Organization: Geneva, Switzerland, 2016; Available online: http://apps.who.int/iris/bitstream/10665/255027/1/9789241512220-eng.pdf?ua=1 (accessed on 25 January 2023).
- Kaur, J.; Chopra, S.; Sheevani; Mahajan, G. Modified Double Disc Synergy Test to Detect ESBL Production in Urinary Isolates of Escherichia coli and Klebsiella pneumoniae. J. Clin. Diagn. Res. 2013, 7, 229–233. [Google Scholar]
- Drieux, L.; Brossier, F.; Sougakoff, W.; Jarlier, V. Phenotypic detection of extended-spectrum β-lactamase production in Enterobacteriaceae: Review and bench guide. Clin. Microbiol. Infect. 2008, 1, 90–103. [Google Scholar] [CrossRef]
- Naseer, F.; Iqbal, R.; Ikram, N.; Shoaib, M.; Javaid Asad, M.; Mehmood, R.T.; Niazi, A.; Asghar, A.; Ishfaq, B. Phenotypic cofirmatory disc diffusion test (PCDDT), double disc synergy test (DDST), E-test OS diagnostic tool for detection of extended spectrum beta lactamase (ESΒL) producing Uropathogens. J. Appl. Biotechnol. Bioeng. 2017, 3, 344–349. [Google Scholar] [CrossRef][Green Version]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef]
- Tayebeh, F.; Amani, J.; Nazarian, S.; Moradyar, M.; Mirhosseini, S. Molecular Diagnosis of Clinically Isolated Klebsiella pneumoniae Strains by PCR-ELISA. J. App. Biotech. Rep. 2016, 3, 501–505. [Google Scholar]
- Candrian, U.; Furrer, B.; Hofelein, C.; Meyer, R.; Jermini, M.; Lüthy, J. Detection of Escherichia coli and identification of enterotoxigenic strains by primer-directed enzymatic amplification of specific DNA sequences. Int. J. Food Microbiol. 1991, 12, 339–351. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 5th ed.; CLSI standard VET01; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Pimenta, A.C.; Martins, J.M.; Fernandes, R.; Moreira, I.S. Ligand-induced structural changes in TEM-1 probed by molecular dynamics and relative binding free energy calculations. J. Chem. Inf. Model. 2013, 53, 2648–2658. [Google Scholar] [CrossRef]
- Rodríguez-González, M.J.; Jiménez-Pearson, M.A.; Duarte, F.; Poklepovich, T.; Campos, J.; Araya-Sánchez, L.N.; Chirino-Trejo, M.; Barquero-Calvo, E. Multidrug-Resistant CTX-M and CMY-2 Producing Escherichia coli Isolated from Healthy Household Dogs from the Great Metropolitan Area, Costa Rica. Microb. Drug Resist. 2020, 26, 1421–1428. [Google Scholar] [CrossRef]
- Liu, J.H.; Wei, S.Y.; Ma, J.Y.; Zeng, Z.L.; Lü, D.H.; Yang, G.X.; Chen, Z.L. Detection and characterisation of CTX-M and CMY-2 β-lactamases among Escherichia coli isolates from farm animals in Guangdong Province of China. Int. J. Antimicrob. Agents 2007, 29, 576–581. [Google Scholar] [CrossRef]
- Briñas, L.; Moreno, M.A.; Zarazaga, M.; Porrero, C.; Sáenz, Y.; García, M.; Dominguez, L.; Torres, C. Detection of CMY-2, CTX-M-14, and SHV-12 β-Lactamases in Escherichia coli Fecal-Sample Isolates from Healthy Chickens. Antimicrob. Agents Chemother. 2003, 47, 2056–2058. [Google Scholar] [CrossRef]
- Shin, S.W.; Jung, M.; Won, H.G.; Belaynehe, K.M.; Yoon, I.J.; Yoo, H.S. Characteristics of Transmissible CTX-M- and CMY-Type β-Lactamase Producing Escherichia coli Isolates Collected from Pig and Chicken Farms in South Korea. J. Microbiol. Biotechnol. 2017, 27, 1716–1723. [Google Scholar] [CrossRef]
- Hong, J.S.; Song, W.; Park, H.M.; Oh, J.Y.; Chae, J.C.; Shin, S.; Jeong, S.H. Clonal Spread of Extended-Spectrum Cephalosporin-Resistant Enterobacteriaceae Between Companion Animals and Humans in South Korea. Front. Microbiol. 2019, 10, 1371. [Google Scholar] [CrossRef]
- Palmeira, J.D.; Cunha, M.V.; Carvalho, J.; Ferreira, H.; Fonseca, C.; Torres, R.T. Emergence and Spread of Cephalosporinases in Wildlife: A Review. Animals 2021, 11, 1765. [Google Scholar] [CrossRef]
- Chiaverini, A.; Cornacchia, A.; Centorotola, G.; Tieri, E.E.; Sulli, N.; Del Matto, I.; Iannitto, G.; Petrone, D.; Petrini, A.; Pomilio, F. Phenotypic and Genetic Characterization of Klebsiella pneumoniae Isolates from Wild Animals in Central Italy. Animals 2022, 12, 1347. [Google Scholar] [CrossRef]
- Rana, C.; Rajput, S.; Behera, M.; Gautam, D.; Vikas, V.; Vats, A.; Roshan, M.; Ghorai, S.M.; De, S. Global epidemiology of CTX-M-type β-lactam resistance in human and animal. Comp. Immunol. Microbiol. Infect. Dis. 2022, 86, 101815. [Google Scholar] [CrossRef]
- Ewers, C.; Bethe, A.; Semmler, T.; Guenther, S.; Wieler, L.H. Extended-spectrum β-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: A global perspective. Clin. Microbiol. Infect. 2012, 18, 646–655. [Google Scholar] [CrossRef]
- Smet, A.; Martle, A.; Persoons, D.; Dewulf, J.; Heyndrickx, M.; Catry, B.; Herman, L.; Haesebrouck, F.; Butaye, P. Diversity of extended-spectrum β-lactamases and class C β-lactamases among cloacal Escherichia coli isolates in Belgian broiler farms. Antimicrob. Agents Chemother. 2008, 52, 1238–1243. [Google Scholar] [CrossRef]
- Widodo, A.; Effendi, M.H.; Khairullah, A.R. Extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli from livestock. Sys. Rev. Pharm. 2020, 11, 382–392. [Google Scholar]
- Darwich, L.; Vidal, A.; Seminati, C.; Albamonte, A.; Casado, A.; López, F.; Molina-López, R.A.; Migura-Garcia, L. High prevalence and diversity of extended-spectrum β-lactamase and emergence of OXA-48 producing Enterobacterales in wildlife in Catalonia. PLoS ONE 2019, 14, e0210686. [Google Scholar] [CrossRef]
- Livermore, D.M.; Canton, R.; Gniadkowski, M.; Nordmann, P.; Rossolini, G.M.; Arlet, G.; Ayala, J.; Coque, T.M.; Kern-Zdanowicz, I.; Luzzaro, F.; et al. CTX-M: Changing the face of ESBLs in Europe. J. Antimicrob. Chemother. 2007, 59, 165–174. [Google Scholar] [CrossRef]
- Garcês, A.; Lopes, R.; Silva, A.; Sampaio, F.; Duque, D.; Brilhante-Simões, P. Bacterial Isolates from Urinary Tract Infection in Dogs and Cats in Portugal, and Their Antibiotic Susceptibility Pattern: A Retrospective Study of 5 Years (2017–2021). Antibiotics 2022, 11, 1520. [Google Scholar] [CrossRef]
- Naas, T.; Zerbib, M.; Girlich, D.; Nordmann, P. Integration of a transposon Tn1-encoded inhibitor-resistant β-lactamase gene, blaTEM-67 from Proteus mirabilis, into the Escherichia coli chromosome. Antimicrob. Agents Chemother. 2003, 47, 19–26. [Google Scholar] [CrossRef]
- Pomba, C.; da Fonseca, J.D.; Baptista, B.C.; Correia, J.D.; Mart´ınez-Mart´ınez, L. Detection of the pandemic O25-ST131 human virulent Escherichia coli CTX-M-15-producing clone harboring the qnrB2 and aac(6’)-Ib-cr genes in a Dog. Antimicrob. Agents Chemother. 2009, 53, 327–328. [Google Scholar] [CrossRef]
- Costa, D.; Poeta, P.; Sàenz, Y.; Vinué, L.; Rojo-Bezares, B.; Jouini, A.; Zarazaga, M.; Rodrigues, J.; Torres, C. Detection of Escherichia coli harbouring extended-spectrum beta-lactamases of the CTX-M, TEM and SHV classes in faecal samples of wild animals in Portugal. J. Antimicrob. Chemother. 2006, 58, 1311–1312. [Google Scholar] [CrossRef]
- Marchese, A.; Arlet, G.; Schito, G.C.; Lagrange, P.H.; Philippon, A. Characterization of FOX-3, an AmpC-type plasmid-mediated β-lactamase from an Italian isolate of Klebsiella oxytoca. Antimicrob. Agents Chemother. 1998, 42, 464–467. [Google Scholar] [CrossRef]
- Nakayama, T.; Kumeda, Y.; Kawahara, R.; Yamaguchi, T.; Yamamoto, Y. Carriage of colistin-resistant, extended-spectrum β-lactamase-producing Escherichia coli harboring the mcr-1 resistance gene after short-term international travel to Vietnam. Infect. Drug Resist. 2018, 11, 391–395. [Google Scholar] [CrossRef]
- Bartoloni, A.; Pallecchi, L.; Riccobono, E.; Mantella, A.; Magnelli, D.; Di Maggio, T.; Villagran, A.L.; Lara, Y.; Saavedra, C.; Strohmeyer, M.; et al. Relentless increase of resistance to fluoroquinolones and expanded spectrum cephalosporins in Escherichia coli: 20 years of surveillance in resource-limited settings from Latin America. Clin. Microbiol. Infect. 2013, 9, 356–361. [Google Scholar] [CrossRef]


| No. | DDST a Test | Bacteria | Animal | Origin | ESBL and pAmpC β-Lactamase Genes | Resistance Pattern of Cephalosporins b |
|---|---|---|---|---|---|---|
| 1 | + | K. pneumoniae | Canine | Wound | TEM, OXA-1, CTX-M-1, CIT | CEP, FOX, CPD |
| 2 | + | K. pneumoniae | Canine | Wound | TEM, OXA-1, CTX-M-1, CIT | CEP, FOX, CPD |
| 3 | + | K. pneumoniae | Canine | Wound | TEM, OXA-1, CTX-M-1, CIT | CEP, FOX, CPD |
| 4 | + | K. pneumoniae | Canine | Urine | TEM, OXA-1, CTX-M-1 | CEP, FOX, CPD |
| 5 | + | K. pneumoniae | Canine | Urine | TEM, OXA-1, CTX-M-1 | CEP, FOX, CPD |
| 6 | + | K. pneumoniae | Canine | Abscess | TEM, OXA-1, CTX-M-1 | CEP, FOX, CPD |
| 7 | + | K. pneumoniae | Canine | Wound | TEM, OXA-1, CTX-M-1 | CEP, CPD |
| 8 | + | K. pneumoniae | Canine | Urine | TEM, OXA-1, CTX-M-1 | CEP, CPD |
| 9 | + | K. pneumoniae | Canine | Urine | TEM, OXA-1, CTX-M-1 | CEP, CPD |
| 10 | + | K. pneumoniae | Canine | Urine | TEM, OXA-1, CTX-M-1 | CEP, CPD |
| 11 | + | K. pneumoniae | Canine | Ear | TEM, OXA-1 | CEP, CPD |
| 12 | + | K. pneumoniae | Canine | Drain | TEM, OXA-1 | CEP, CPD |
| 13 | + | K. pneumoniae | Canine | Wound | TEM, OXA-1 | CEP, CPD |
| 14 | + | K. pneumoniae | Canine | Ear | TEM, OXA-1 | CEP, CPD |
| 15 | + | K. pneumoniae | Feline | Abscess | TEM, OXA-1 | CEP, CPD |
| 16 | + | K. pneumoniae | Canine | Skin swab | CIT | CEP, FOX, CPD |
| 17 | + | K. pneumoniae | Canine | Wound | CTX-M-1 | CEP, CPD |
| 18 | + | K. pneumoniae | Canine | Bronchial aspirate | TEM | CEP, CPD |
| 19 | + | K. pneumoniae | Canine | Biopsy lung | TEM | CEP, FOX |
| 20 | + | K. pneumoniae | Canine | Nasopharyngeal swab | TEM | CEP, FOX |
| 21 | + | K. pneumoniae | Canine | Wound | TEM | CEP, CPD |
| 22 | + | K. pneumoniae | Equine | Catheter | TEM | CEP |
| 23 | + | K. pneumoniae | Equine | wound | TEM | CEP |
| 24 | - | K. pneumoniae | Canine | Wound | - | - |
| 25 | - | K. pneumoniae | Canine | Urine | - | - |
| 26 | - | K. pneumoniae | Equine | Gastric aspirate | - | - |
| 27 | - | K. pneumoniae | Equine | Tracheal aspirate | - | - |
| 28 | - | K. pneumoniae | Canine | Urine | - | - |
| 29 | - | K. pneumoniae | Canine | Wound | - | - |
| 30 | - | K. pneumoniae | Canine | Prostatic fluid | - | - |
| 31 | - | K. pneumoniae | Canine | Systemic Infection | - | - |
| 32 | - | K. pneumoniae | Canine | Urine | - | - |
| 33 | - | K. pneumoniae | Canine | Ear | - | - |
| 34 | - | K. pneumoniae | Canine | Urine | - | - |
| 35 | - | K. pneumoniae | Canine | Ear | - | - |
| 36 | - | K. pneumoniae | Canine | Wound | - | - |
| 37 | - | K. pneumoniae | Canine | Urine | - | - |
| 38 | - | K. pneumoniae | Canine | Urine | - | - |
| 39 | - | K. pneumoniae | Canine | Urine | - | - |
| 40 | - | K. pneumoniae | Canine | Skin swab | - | - |
| 41 | - | K. pneumoniae | Canine | Ascitic fluid | - | - |
| 42 | - | K. pneumoniae | Canine | Urine | - | - |
| 43 | - | K. pneumoniae | Canine | Urine | - | - |
| 44 | - | K. pneumoniae | Canine | Urine | - | - |
| 45 | - | K. pneumoniae | Canine | Urine | - | - |
| 46 | - | K. pneumoniae | Canine | Urine | - | - |
| 47 | + | E. coli | Canine | Urine | TEM, OXA-1, CTX-M-1 | CEP, CPD |
| 48 | + | E. coli | Canine | Urine | TEM, CIT | CEP, FOX, CPD |
| 49 | + | E. coli | Canine | Urine | OXA-1, CTX-M-1 | CEP, CPD |
| 50 | + | E. coli | Canine | Urine | CIT | CEP, FOX, CPD |
| 51 | + | E. coli | Canine | Urine | CIT | CEP, FOX, CPD |
| 52 | + | E. coli | Canine | Urine | CIT | CEP, FOX, CPD |
| 53 | + | E. coli | Canine | Urine | CIT | CEP, FOX, CPD |
| 54 | + | E. coli | Canine | Urine | CIT | CEP, CPD |
| 55 | + | E. coli | Canine | Urine | CIT | CEP, CPD |
| 56 | + | E. coli | Canine | Urine | CTX-M-1 | CEP, CPD |
| 57 | + | E. coli | Canine | Urine | TEM | CEP |
| 58 | + | E. coli | Canine | Urine | TEM | CEP |
| 59 | + | E. coli | Canine | Urine | TEM | CEP |
| 60 | - | E. coli | Canine | Urine | - | - |
| 61 | - | E. coli | Canine | Urine | - | - |
| 62 | - | E. coli | Canine | Urine | - | - |
| 63 | - | E. coli | Canine | Urine | - | - |
| 64 | - | E. coli | Canine | Urine | - | - |
| 65 | - | E. coli | Canine | Urine | - | - |
| 66 | - | E. coli | Canine | Urine | - | - |
| 67 | - | E. coli | Canine | Urine | - | - |
| 68 | - | E. coli | Canine | Urine | - | - |
| 69 | - | E. coli | Canine | Urine | - | - |
| 70 | - | E. coli | Canine | Urine | - | - |
| 71 | - | E. coli | Canine | Urine | - | - |
| 72 | - | E. coli | Canine | Urine | - | - |
| Bacteria | No. of Grown Bacteria (%) | ||||||
|---|---|---|---|---|---|---|---|
| First-Generation Cephalosporin | Third-Generation Cephalosporin | ||||||
| Cephalothin (μg/mL) | Ceftiofur (μg/mL) | Ceftriaxone (μg/mL) | |||||
| 128 | 16 | 32 | 64 | 16 | 32 | 64 | |
| ESBL- and pAmpC β-lactamase-producing E. coli (n = 13) | 13 (100.0) | 13 (100.0) | 13 (100.0) | 7 (53.8) | 10 (76.9) | 7 (53.8) | 5 (38.5) |
| Non ESBL- and pAmpC β-lactamase-producing E. coli (n = 13) | 10 (76.9) | 5 (38.5) | 0 (0.0) | 0 (0.0) | 3 (23.1) | 1 (7.7) | 1 (7.7) |
| ESBL- and pAmpC β-lactamase-producing K. pneumoniae (n = 23) | 23 (100.0) | 23 (100.0) | 22 (95.7) | 16 (69.6) | 20 (87.0) | 18 (78.3) | 14 (60.9) |
| Non ESBL- and pAmpC β-lactamase-producing K. pneumoniae (n = 23) | 17 (73.9) | 13 (61.5) | 0 (0.0) | 0 (0.0) | 8 (34.8) | 5 (21.7) | 4 (17.4) |
| Total Number of Samples | No. of Samples That Grew or Did Not Grow in MacConkey Broth Containing Cephalothin (%) | No. of Samples That Grew or Did Not Grow on MacConkey Agar Containing Ceftiofur (%) | ||
|---|---|---|---|---|
| 109 | Grew | 99 (90.8) | Grew | 71 (65.1) |
| Did not grow | 28 (25.7) | |||
| Did not grow | 10 (9.2) | Grew | 0 (0.0) | |
| Did not grow | 10 (9.2) | |||
| No. of Samples | Bacteria | No. of Bacteria Isolates | No. of Bacteria Isolates Detected β-Lactamase Gene(s) |
|---|---|---|---|
| Grew on MacConkey agar containing ceftiofur | |||
| 71 | E. coli | 58 | 58 |
| K. pneumoniae | 20 | 19 | |
| Did not grow on MacConkey agar containing ceftiofur | |||
| 38 | E. coli | 32 | 0 |
| K. pneumoniae | 10 | 0 | |
| Genotype | No. of ESBL and pAmpC β-Lactamase Genes | |
|---|---|---|
| E. coli | K. pneumoniae | |
| CIT | 43 | 7 |
| TEM | 25 | 4 |
| CTX-M-1 | 24 | 0 |
| CTX-M-9 | 4 | 3 |
| OXA-1 | 7 | 0 |
| CTX-M-2 | 4 | 2 |
| EBC | 4 | 1 |
| ACC | 1 | 3 |
| FOX | 1 | 3 |
| DHA | 1 | 0 |
| Total | 114 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, K.-W. Development of a Method for the Fast Detection of Extended-Spectrum β-Lactamase- and Plasmid-Mediated AmpC β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae from Dogs and Cats in the USA. Animals 2023, 13, 649. https://doi.org/10.3390/ani13040649
Seo K-W. Development of a Method for the Fast Detection of Extended-Spectrum β-Lactamase- and Plasmid-Mediated AmpC β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae from Dogs and Cats in the USA. Animals. 2023; 13(4):649. https://doi.org/10.3390/ani13040649
Chicago/Turabian StyleSeo, Kwang-Won. 2023. "Development of a Method for the Fast Detection of Extended-Spectrum β-Lactamase- and Plasmid-Mediated AmpC β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae from Dogs and Cats in the USA" Animals 13, no. 4: 649. https://doi.org/10.3390/ani13040649
APA StyleSeo, K.-W. (2023). Development of a Method for the Fast Detection of Extended-Spectrum β-Lactamase- and Plasmid-Mediated AmpC β-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae from Dogs and Cats in the USA. Animals, 13(4), 649. https://doi.org/10.3390/ani13040649





