Evaluation of the Thermal Response of the Horns in Dairy Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. General Study Set-Up
2.3. Focal Animals
2.3.1. Farm A
2.3.2. Farm B
2.3.3. Farm C
2.4. Horn Temperature in Relation to Behaviour and Environmental Conditions
2.4.1. Behavioural Observations
2.4.2. Infrared Temperature Measurements
2.5. Horn Temperature in Relation to Rumination
2.6. Data Processing
HLI and THI Calculation
2.7. Statistical Analysis
2.7.1. Inter- and Intra-Observer Reliability
2.7.2. Horn Temperature in Relation to Behaviour and Environmental Conditions
2.7.3. Horn Temperature in Relation to Rumination
3. Results
3.1. Inter- and Intra-Observer Reliability
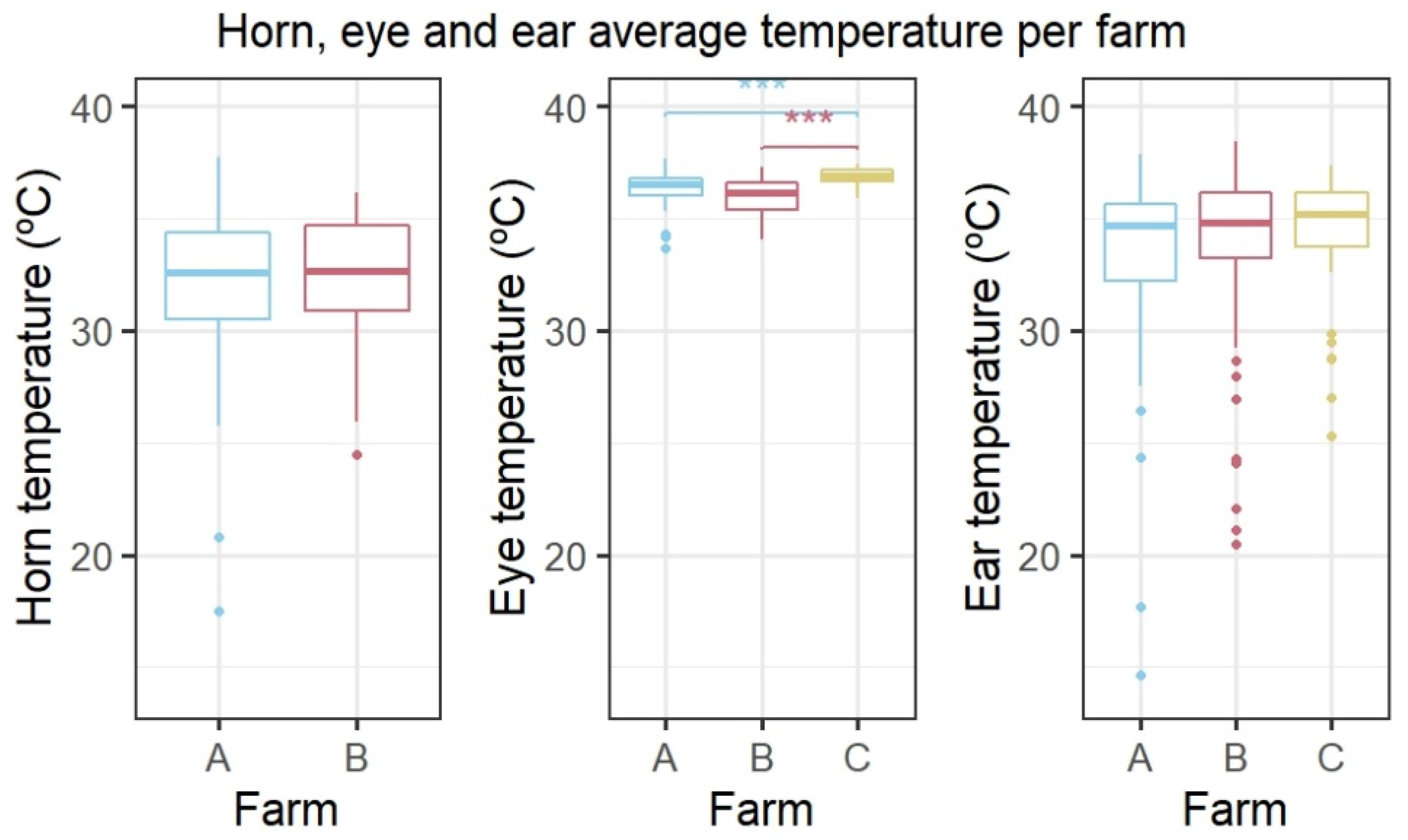
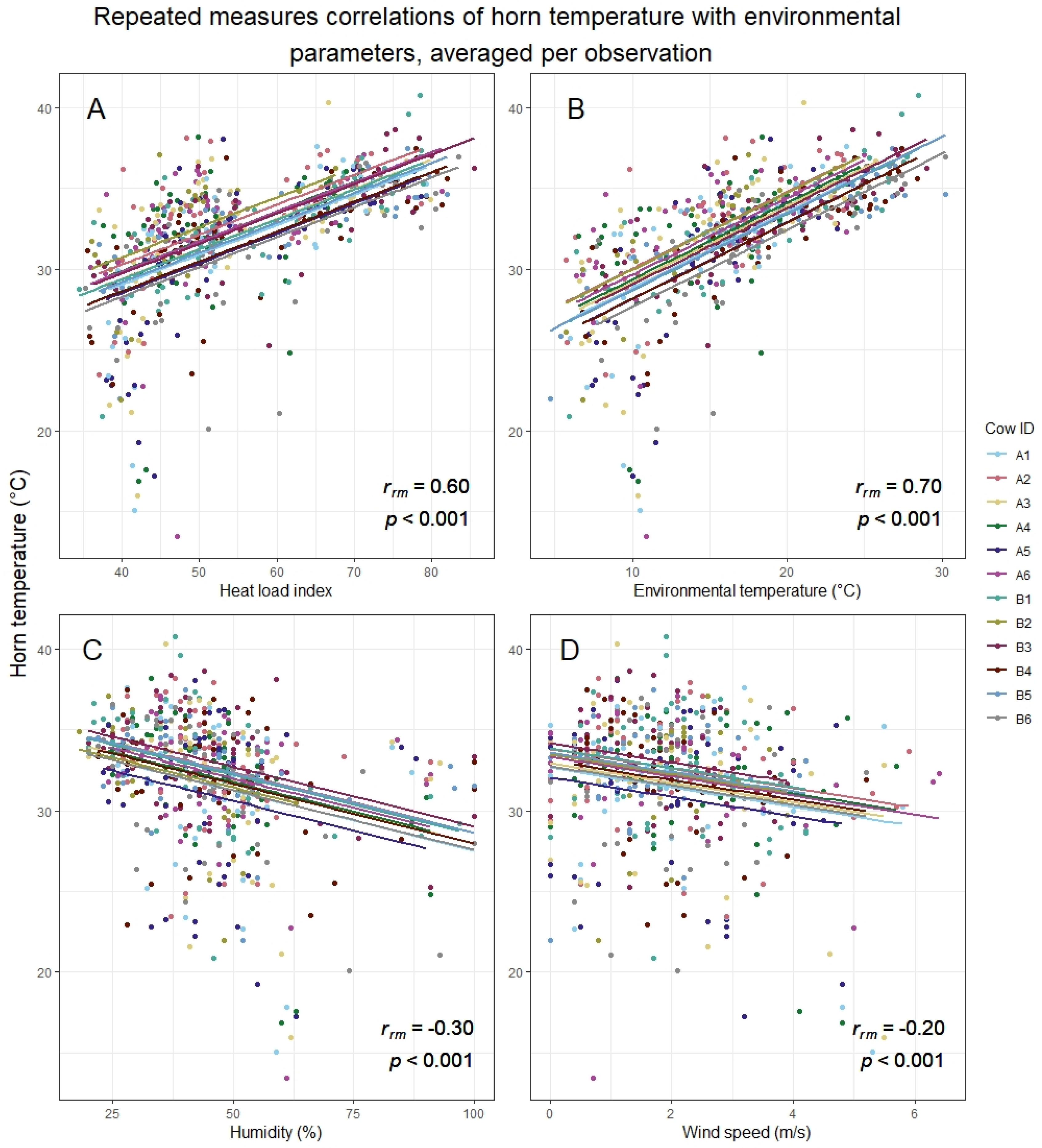
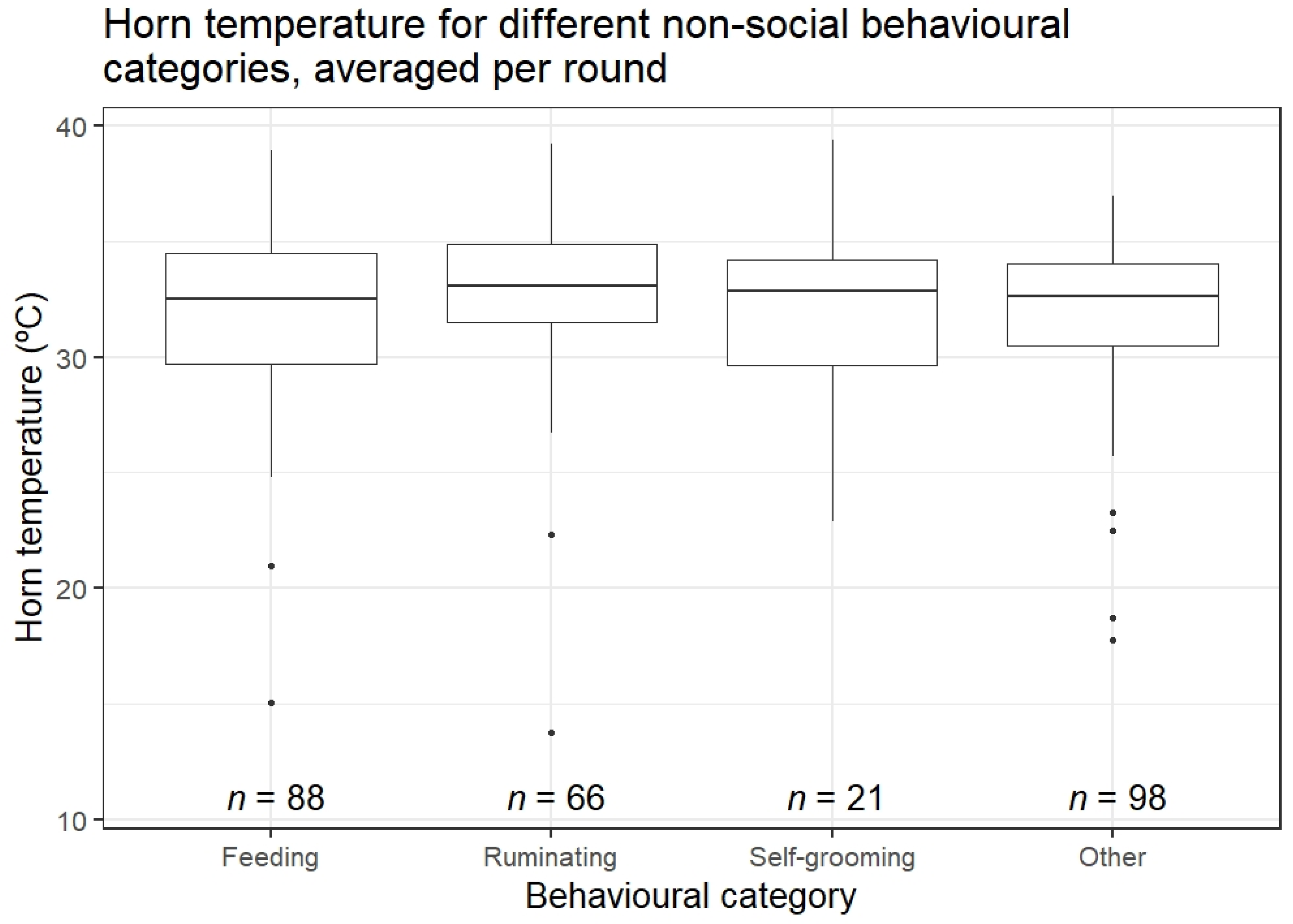
3.2. Horn Temperature in Relation to Behaviour and Environmental Conditions
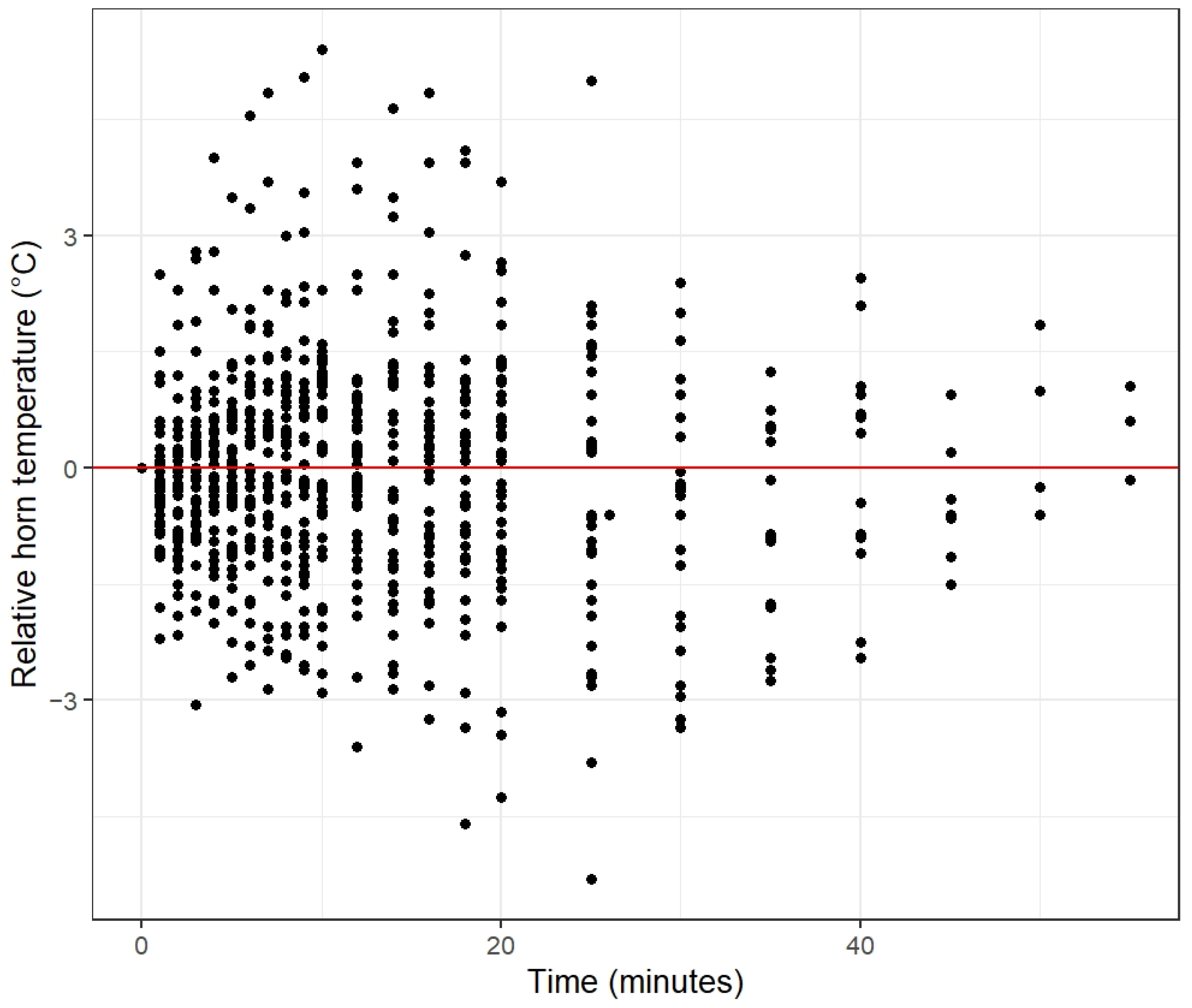
3.3. Horn Temperature in Relation to Rumination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cozzi, G.; Gottardo, F.; Brscic, M.; Contiero, B.; Irrgang, N.; Knierim, U.; Pentelescu, O.; Windig, J.J.; Mirabito, L.; Eveillard, F.K. Dehorning of Cattle in the EU Member States: A Quantitative Survey of the Current Practices. Livest. Sci. 2015, 179, 4–11. [Google Scholar]
- Nordquist, R.E.; Van der Staay, F.J.; Van Eerdenburg, F.J.C.M.; Velkers, F.C.; Fijn, L.; Arndt, S.S. Mutilating Procedures, Management Practices, and Housing Conditions That May Affect the Welfare of Farm Animals: Implications for Welfare Research. Animals 2017, 7, 12. [Google Scholar] [PubMed]
- Stafford, K.J.; Mellor, D.J. Dehorning and Disbudding Distress and Its Alleviation in Calves. Vet. J. 2005, 169, 337–349. [Google Scholar] [CrossRef]
- Kling-Eveillard, F.; Knierim, U.; Irrgang, N.; Gottardo, F.; Ricci, R.; Dockès, A.C. Attitudes of Farmers towards Cattle Dehorning. Livest. Sci. 2015, 179, 12–21. [Google Scholar] [CrossRef]
- Knierim, U.; Irrgang, N.; Roth, B.A. To Be or Not to Be Horned—Consequences in Cattle. Livest. Sci. 2015, 179, 29–37. [Google Scholar] [CrossRef]
- Stookey, J.M.; Goonewardene, L.A. A Comparison of Production Traits and Welfare Implications between Horned and Polled Beef Bulls. Can. J. Anim. Sci. 1996, 76, 1–5. [Google Scholar] [CrossRef]
- Stafford, K.J.; Mellor, D.J. Addressing the Pain Associated with Disbudding and Dehorning in Cattle. Appl. Anim. Behav. Sci. 2011, 135, 226–231. [Google Scholar] [CrossRef]
- Stock, M.L.; Baldridge, S.L.; Griffin, D.; Coetzee, J.F. Bovine Dehorning: Assessing Pain and Providing Analgesic Management. Vet. Clin. Food Anim. Pract. 2013, 29, 103–133. [Google Scholar] [CrossRef]
- Sylvester, S.P.; Stafford, K.J.; Mellor, D.J.; Bruce, R.A.; Ward, R.N. Acute Cortisol Responses of Calves to Four Methods of Dehorning by Amputation. Aust. Vet. J. 1998, 76, 123–126. [Google Scholar] [CrossRef]
- Sylvester, S.P.; Stafford, K.J.; Mellor, D.J.; Bruce, R.A.; Ward, R.N. Behavioural Responses of Calves to Amputation Dehorning with and without Local Anaesthesia. Aust. Vet. J. 2004, 82, 697–700. [Google Scholar] [CrossRef]
- Winder, C.B.; Miltenburg, C.L.; Sargeant, J.M.; LeBlanc, S.J.; Haley, D.B.; Lissemore, K.D.; Godkin, M.A.; Duffield, T.F. Effects of Local Anesthetic or Systemic Analgesia on Pain Associated with Cautery Disbudding in Calves: A Systematic Review and Meta-Analysis. J. Dairy Sci. 2018, 101, 5411–5427. [Google Scholar] [CrossRef] [PubMed]
- Neave, H.W.; Daros, R.R.; Costa, J.H.C.; von Keyserlingk, M.A.G.; Weary, D.M. Pain and Pessimism: Dairy Calves Exhibit Negative Judgement Bias Following Hot-Iron Disbudding. PLoS ONE 2013, 8, e80556. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.S.; Goerlich, V.C.; van der Staay, F.J. A Dynamic Concept of Animal Welfare: The Role of Appetitive and Adverse Internal and External Factors and the Animal’s Ability to Adapt to Them. Front. Anim. Sci. 2022, 3, 908513. [Google Scholar] [CrossRef]
- Bro-Jørgensen, J. The Intensity of Sexual Selection Predicts Weapon Size in Male Bovids. Evol. Int. J. Org. Evol. 2007, 61, 1316–1326. [Google Scholar] [CrossRef]
- Preston, B.T.; Stevenson, I.R.; Pemberton, J.M.; Coltman, D.W.; Wilson, K. Overt and Covert Competition in a Promiscuous Mammal: The Importance of Weaponry and Testes Size to Male Reproductive Success. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.R.; Kruuk, L.E.B. Function of Weaponry in Females: The Use of Horns in Intrasexual Competition for Resources in Female Soay Sheep. Biol. Lett. 2007, 3, 651–654. [Google Scholar] [CrossRef]
- Stankowich, T.; Caro, T. Evolution of Weaponry in Female Bovids. Proc. R. Soc. B Biol. Sci. 2009, 276, 4329–4334. [Google Scholar] [CrossRef]
- DeVries, T.J.; Vankova, M.; Veira, D.M.; Von Keyserlingk, M.A.G. Usage of Mechanical Brushes by Lactating Dairy Cows. J. Dairy Sci. 2007, 90, 2241–2245. [Google Scholar] [CrossRef]
- Simonsen, H.B. Grooming Behaviour of Domestic Cattle. Nord. Vet. Med. 1979, 31, 1–5. [Google Scholar]
- Budras, K.-D.; Habel, R.E.; Habel, R.E.; Wunsche, A.; Buda, S. Bovine Anatomy: An Illustrated Text; Schlütersche: Hannover, Germany, 2003; ISBN 3899930002. [Google Scholar]
- König, H.E.; Bragulla, H.; Hans-Georg, H.-G. Veterinary Anatomy of Domestic Mammals: Textbook and Colour Atlas; Schattauer Verlag: Stuttgart, Germany, 2007; ISBN 3794524853. [Google Scholar]
- Edmondson, M.A. Local and Regional Anesthesia in Cattle. Vet. Clin. North Am. Food Anim. Pract. 2008, 24, 211–226. [Google Scholar] [CrossRef]
- Romanovsky, A.A. The Thermoregulation System and How It Works. Handb. Clin. Neurol. 2018, 156, 3–43. [Google Scholar]
- Taylor, C.R. The Vascularity and Possible Thermoregulatory Function of the Horns in Goats. Physiol. Zool. 1966, 39, 127–139. [Google Scholar] [CrossRef]
- Johnson, K.G.; Callahan, S.M.; Strack, R. Temperature and Humidity of Expired Air of Sheep. Aust. J. Biol. Sci. 1988, 41, 309–314. [Google Scholar] [CrossRef]
- Langman, V.A.; Maloiy, G.M.O.; Schmidt-Nielsen, K.; Schroter, R.C. Nasal Heat Exchange in the Giraffe and Other Large Mammals. Respir. Physiol. 1979, 37, 325–333. [Google Scholar] [CrossRef]
- Irrgang, N. Horns in Cattle-Implications of Keeping Horned Cattle or Not; Universität Kassel: Kassel, Germany, 2013. [Google Scholar]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Picard, K.; Thomas, D.W.; Festa-Bianchet, M.; Belleville, F.; Laneville, A. Differences in the Thermal Conductance of Tropical and Temperate Bovid Horns. Ecoscience 1999, 6, 148–158. [Google Scholar] [CrossRef]
- Parés Casanova, P.M.; Kucherova, I. Possible Tendency of Longer Horns towards Shorter Ears in Goats. Adv. Agric. Biol. 2014, 1, 17–19. [Google Scholar] [CrossRef]
- Tattersall, G.J.; Arnaout, B.; Symonds, M.R.E. The Evolution of the Avian Bill as a Thermoregulatory Organ. Biol. Rev. 2017, 92, 1630–1656. [Google Scholar] [CrossRef]
- Spengler, A.; Hurni, B.; Streiff, R.; Bigler, M.; Haeni, R.; Ivemeyer, S.; Knösel, M.; Letsch, A.; Loeffler, T.; Lutke Schipholt, H. Die Bedeutung der Hörner Für Die Kuh; Forschungsinstitut für biologischen Landbau (FiBL), Schweiz, Verein für biologisch-dynamische Landwirtschaft: Demeter, Bioland, 2015; ISBN 3037362723. [Google Scholar]
- Ohl, F.; van der Staay, F.J. Animal Welfare: At the Interface between Science and Society. Vet. J. 2012, 192, 13–19. [Google Scholar] [CrossRef]
- Bang, N.N.; Gaughan, J.B.; Hayes, B.J.; Lyons, R.E.; McNeill, D.M. Application of Infrared Thermal Technology to Assess the Level of Heat Stress and Milk Yield Reduction of Cows in Tropical Smallholder Dairy Farms. J. Dairy Sci. 2022, 105, 8454–8469. [Google Scholar] [CrossRef]
- Byrne, D.T.; Berry, D.P.; Esmonde, H.; McHugh, N. Temporal, Spatial, Inter-, and Intra-Cow Repeatability of Thermal Imaging1. J. Anim. Sci. 2017, 95, 970–979. [Google Scholar] [CrossRef]
- Rao, T.K.S.; Chauhan, I.S.; Kumar, P.; Gamit, K.C. Elements of Behaviour in Cattle-An Overview. Vet. Res. Int. 2015, 3, 71–80. [Google Scholar]
- Cuthbertson, H.; Tarr, G.; Loudon, K.; Lomax, S.; White, P.; McGreevy, P.; Polkinghorne, R.; González, L.A. Using Infrared Thermography on Farm of Origin to Predict Meat Quality and Physiological Response in Cattle (Bos Taurus) Exposed to Transport and Marketing. Meat Sci. 2020, 169, 108173. [Google Scholar] [CrossRef]
- Macmillan, K.; Colazo, M.G.; Cook, N.J. Evaluation of Infrared Thermography Compared to Rectal Temperature to Identify Illness in Early Postpartum Dairy Cows. Res. Vet. Sci. 2019, 125, 315–322. [Google Scholar] [CrossRef]
- Martello, L.S.; da Luz e Silva, S.; da Costa Gomes, R.; da Silva Corte, R.R.P.; Leme, P.R. Infrared Thermography as a Tool to Evaluate Body Surface Temperature and Its Relationship with Feed Efficiency in Bos Indicus Cattle in Tropical Conditions. Int. J. Biometeorol. 2016, 60, 173–181. [Google Scholar] [CrossRef]
- Talukder, S.; Kerrisk, K.L.; Ingenhoff, L.; Thomson, P.C.; Garcia, S.C.; Celi, P. Infrared Technology for Estrus Detection and as a Predictor of Time of Ovulation in Dairy Cows in a Pasture-Based System. Theriogenology 2014, 81, 925–935. [Google Scholar] [CrossRef]
- Uddin, J.; McNeill, D.M.; Lisle, A.T.; Phillips, C.J.C. A Sampling Strategy for the Determination of Infrared Temperature of Relevant External Body Surfaces of Dairy Cows. Int. J. Biometeorol. 2020, 64, 1583–1592. [Google Scholar] [CrossRef]
- Franchi, G.A.; Jensen, M.B.; Herskin, M.S.; McNeill, D.M.; Phillips, C.J.C. Assessing Response to Dry-off in Dairy Cows Kept Outdoors Using Spontaneous Behaviours and Infrared Thermography—A Pilot Study. Trop. Anim. Health Prod. 2021, 53, 46. [Google Scholar] [CrossRef]
- Uddin, J.; Phillips, C.J.C.; Goma, A.A.; McNeill, D.M. Relationships between Infrared Temperature and Laterality. Appl. Anim. Behav. Sci. 2019, 220, 104855. [Google Scholar] [CrossRef]
- Cuthbertson, H.; Tarr, G.; González, L.A. Methodology for Data Processing and Analysis Techniques of Infrared Video Thermography Used to Measure Cattle Temperature in Real Time. Comput. Electron. Agric. 2019, 167, 105019. [Google Scholar] [CrossRef]
- Bikker, J.P.; van Laar, H.; Rump, P.; Doorenbos, J.; van Meurs, K.; Griffioen, G.M.; Dijkstra, J. Technical Note: Evaluation of an Ear-Attached Movement Sensor to Record Cow Feeding Behavior and Activity. J. Dairy Sci. 2014, 97, 2974–2979. [Google Scholar] [CrossRef] [PubMed]
- Lecorps, B.; Kappel, S.; Weary, D.M.; von Keyserlingk, M.A.G. Social Proximity in Dairy Calves Is Affected by Differences in Pessimism. PLoS ONE 2019, 14, e0223746. [Google Scholar] [CrossRef]
- Fogsgaard, K.K.; Røntved, C.M.; Sørensen, P.; Herskin, M.S. Sickness Behavior in Dairy Cows during Escherichia Coli Mastitis. J. Dairy Sci. 2012, 95, 630–638. [Google Scholar] [CrossRef]
- Meen, G.H.; Schellekens, M.A.; Slegers, M.H.M.; Leenders, N.L.G.; van Erp-van der Kooij, E.; Noldus, L.P.J.J. Sound Analysis in Dairy Cattle Vocalisation as a Potential Welfare Monitor. Comput. Electron. Agric. 2015, 118, 111–115. [Google Scholar] [CrossRef]
- Johnsen, J.F.; Ellingsen, K.; Grøndahl, A.M.; Bøe, K.E.; Lidfors, L.; Mejdell, C.M. The Effect of Physical Contact between Dairy Cows and Calves during Separation on Their Post-Separation Behavioural Response. Appl. Anim. Behav. Sci. 2015, 166, 11–19. [Google Scholar] [CrossRef]
- Lürzel, S.; Bückendorf, L.; Waiblinger, S.; Rault, J.-L. Salivary Oxytocin in Pigs, Cattle, and Goats during Positive Human-Animal Interactions. Psychoneuroendocrinology 2020, 115, 104636. [Google Scholar] [CrossRef]
- Rousing, T.; Wemelsfelder, F. Qualitative Assessment of Social Behaviour of Dairy Cows Housed in Loose Housing Systems. Appl. Anim. Behav. Sci. 2006, 101, 40–53. [Google Scholar] [CrossRef]
- Seifi, H.A.; LeBlanc, S.J.; Leslie, K.E.; Duffield, T.F. Metabolic Predictors of Post-Partum Disease and Culling Risk in Dairy Cattle. Vet. J. 2011, 188, 216–220. [Google Scholar] [CrossRef]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–266. [Google Scholar] [CrossRef]
- Hoffmann, G.; Herbut, P.; Pinto, S.; Heinicke, J.; Kuhla, B.; Amon, T. Animal-Related, Non-Invasive Indicators for Determining Heat Stress in Dairy Cows. Biosyst. Eng. 2020, 199, 83–96. [Google Scholar] [CrossRef]
- Montanholi, Y.R.; Odongo, N.E.; Swanson, K.C.; Schenkel, F.S.; McBride, B.W.; Miller, S.P. Application of Infrared Thermography as an Indicator of Heat and Methane Production and Its Use in the Study of Skin Temperature in Response to Physiological Events in Dairy Cattle (Bos Taurus). J. Therm. Biol. 2008, 33, 468–475. [Google Scholar] [CrossRef]
- Sathiyabarathi, M.; Jeyakumar, S.; Manimaran, A.; Jayaprakash, G.; Pushpadass, H.A.; Sivaram, M.; Ramesha, K.P.; Das, D.N.; Kataktalware, M.A.; Prakash, M.A. Infrared Thermography: A Potential Noninvasive Tool to Monitor Udder Health Status in Dairy Cows. Vet. world 2016, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-Invasive Measurement of Stress in Dairy Cows Using Infrared Thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Xudong, Z.; Xi, K.; Ningning, F.; Gang, L. Automatic Recognition of Dairy Cow Mastitis from Thermal Images by a Deep Learning Detector. Comput. Electron. Agric. 2020, 178, 105754. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- RStudio Team Rstudio. Integrated Development for R; BPC: Boston, MA, USA, 2020. [Google Scholar]
- Gaughan, J.B.; Mader, T.L.; Holt, S.M.; Lisle, A. A New Heat Load Index for Feedlot Cattle. J. Anim. Sci. 2008, 86, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Petrov, R.; Lott, S.; Binns, P.; Cork, R. Measuring the Microclimate of Eastern Australian Feedlots; Project No. FLOT. 317; Meat & Livestock Australia Limited: Sidney, Australia, 2003; ISBN 9781741915723. [Google Scholar]
- KNMI Daggegevens van Het Weer in Nederland. Available online: https://www.knmi.nl/nederland-nu/klimatologie/daggegevens (accessed on 23 September 2021).
- Bouraoui, R.; Lahmar, M.; Majdoub, A.; Belyea, R. The Relationship of Temperature-Humidity Index with Milk Production of Dairy Cows in a Mediterranean Climate. Anim. Res. 2002, 51, 479–491. [Google Scholar] [CrossRef]
- Gantner, V.; Mijić, P.; Kuterovac, K.; Solić, D.; Gantner, R. Temperature-Humidity Index Values and Their Significance on the Daily Production of Dairy Cattle. Mljekarstvo Časopis Za Unaprjeđenje Proizv. I Prerade Mlijeka 2011, 61, 56–63. [Google Scholar]
- Kibler, H.H. Environmental Physiology and Shelter Engineering with Special Reference to Domestic Animals. In LXVII, Thermal Effects of Various Temperature-Humidity Combinations on Holstein Cattle as Measured by Eight Physiological Responses; University of Missouri, College of Agriculture, Agricultural Experiment Station: Columbia, MO, USA, 1964; Volume 862. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. Dplyr: A Grammar of Data Manipulation, 2021. Available online: https://dplyr.tidyverse.org/ (accessed on 1 July 2021).
- Millard, S.P. EnvStats: An R Package for Environmental Statistics; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-8455-4. [Google Scholar]
- Bakdash, J.Z.; Marusich, L.R. Repeated Measures Correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef]
- Ogle, D.H.; Doll, J.C.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis. R Package Version 0.9.3.9000. 2022. Available online: https://Github.Com/FishR-Core-Team/FSA (accessed on 1 July 2021).
- Auguie, B. GridExtra: Miscellaneous Functions for “Grid” Graphics. R Package Version 2.3. 2017. Available online: https://cran.r-project.org/web/packages/gridExtra/index.html (accessed on 1 July 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using {lme4}. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Højsgaard, S.; Halekoh, U. DoBy: Groupwise Statistics, LSmeans, Linear Contrasts, Utilities; R Package Version 4.5-15. 2021. Available online: https://cran.r-project.org/web/packages/doBy/index.html (accessed on 1 July 2021).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer International Publishing: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. Ggpubr: “ggplot2” Based Publication Ready Plots. R Package Version 0.4. 0 438. 2020. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 1 July 2021).
- Grosjean, P.; Ibanez, F. Pastecs: Package for Analysis of Space-Time Ecological Series. R Package Version 1. p. 21. 2018. Available online: https://cran.r-project.org/web/packages/pastecs/pastecs.pdf (accessed on 1 July 2021).
- Wickham, H.; Bryan, J. Readxl: Read Excel Files; R Package Version 1.3.1. 2019. Available online: https://cran.r-project.org/web/packages/readxl/index.html (accessed on 1 July 2021).
- Cicchetti, D. V Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 159–174. [Google Scholar] [CrossRef]
- Mader, T.L.; Davis, M.S.; Brown-Brandl, T. Environmental Factors Influencing Heat Stress in Feedlot Cattle. J. Anim. Sci. 2006, 84, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Salles, M.S.V.; da Silva, S.C.; Salles, F.A.; Roma, L.C.; El Faro, L.; Bustos Mac Lean, P.A.; Lins de Oliveira, C.E.; Martello, L.S. Mapping the Body Surface Temperature of Cattle by Infrared Thermography. J. Therm. Biol. 2016, 62, 63–69. [Google Scholar] [CrossRef]
- George, W.D.; Godfrey, R.W.; Ketring, R.C.; Vinson, M.C.; Willard, S.T. Relationship among Eye and Muzzle Temperatures Measured Using Digital Infrared Thermal Imaging and Vaginal and Rectal Temperatures in Hair Sheep and Cattle. J. Anim. Sci. 2014, 92, 4949–4955. [Google Scholar] [CrossRef]
- Baars, T.; Jahreis, G.; Lorkowski, S.; Rohrer, C.; Vervoort, J.; Hettinga, K. Short Communication: Changes under Low Ambient Temperatures in the Milk Lipodome and Metabolome of Mid-Lactation Cows after Dehorning as a Calf. J. Dairy Sci. 2019, 102, 2698–2702. [Google Scholar] [CrossRef] [PubMed]
- Mendl, M.; Burman, O.H.P.; Parker, R.M.A.; Paul, E.S. Cognitive Bias as an Indicator of Animal Emotion and Welfare: Emerging Evidence and Underlying Mechanisms. Appl. Anim. Behav. Sci. 2009, 118, 161–181. [Google Scholar] [CrossRef]
- CBS; PBL; RIVM. WUR Temperature Trends: The Netherlands and Worldwide, 1906–2015. Available online: https://www.environmentaldata.nl (accessed on 16 November 2022).
- Herbut, P.; Angrecka, S.; Walczak, J. Environmental Parameters to Assessing of Heat Stress in Dairy Cattle—A Review. Int. J. Biometeorol. 2018, 62, 2089–2097. [Google Scholar] [CrossRef]
- Schafberg, R.; Swalve, H.H. The History of Breeding for Polled Cattle. Livest. Sci. 2015, 179, 54–70. [Google Scholar] [CrossRef]
- Wythes, J.R.; Horder, J.C.; Lapworth, J.W.; Cheffins, R.C. Effect of Tipped Horns on Cattle Bruising. Vet. Rec. 1979, 104, 390–392. [Google Scholar] [CrossRef]
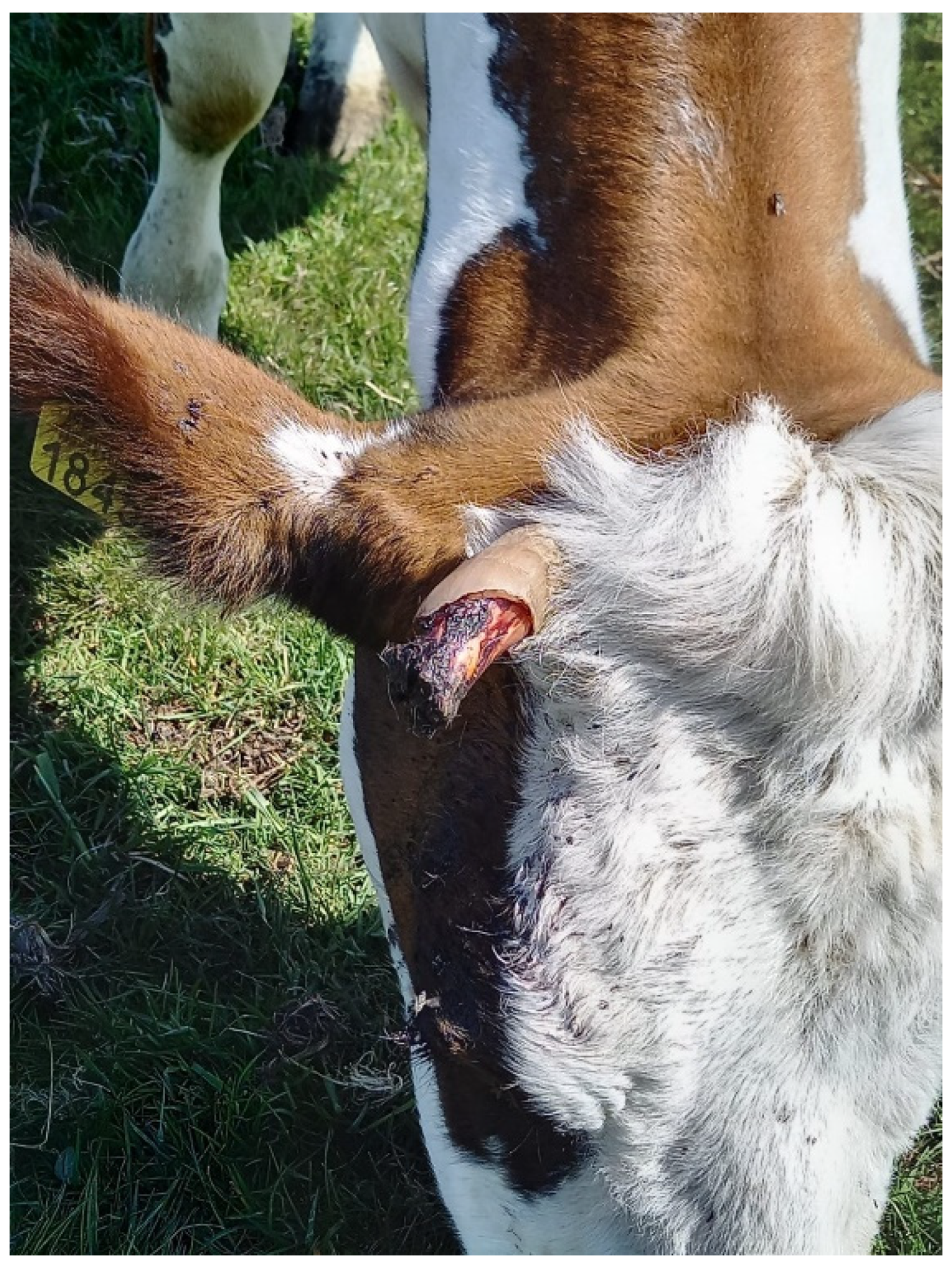
| Days of Observation | Rounds | Observations | Minutes Observed |
|---|---|---|---|
| 25 | 100 (50 each farm) Max 5 rounds per observation day | 2 (farms) × 50 (rounds) × 6 (cows) = 600 | 50 (rounds) × 5 (minutes observed per cow per round) = 250 min per cow = 4 h 10 m |
| Category | Behaviour | Description |
|---|---|---|
| Non-social | Feeding | Cow is feeding on grass or other fodder; bouts of ≤10 s are ignored and disruptions of ≤10 s are allowed. |
| Self-grooming | Rubbing parts of the body or head against other body parts, or licking body parts [47]. | |
| Ruminating | Regurgitation, chewing, and swallowing of previously eaten food; bouts of ≤10 s are ignored and disruptions ≤10 s are allowed [47]. | |
| Other | Other non-social behaviours, e.g., drinking, being alert, being milked. |
| Parameter | Environmental Temperature (°C) | Humidity (%) | Wind Speed (m/s) | HLI | Horn Temperature (°C) | Eye Temperature (°C) | Ear Temperature (°C) |
|---|---|---|---|---|---|---|---|
| Mean | 16.1 | 49 | 1.7 | 55.1 | 32 | 36.4 | 33.7 |
| Min | 4.7 | 18 | 0 | 34 | 12 | 26 | 9 |
| Max | 30.2 | 100 | 6.4 | 89 | 54 | 45 | 55 |
| Df | AIC | p-Value | |
|---|---|---|---|
| <none> | 1949.7 | ||
| Time | 15 | 1931.7 | 0.68 |
| Farm | 1 | 1948.5 | 0.38 |
| Windspeed | 3 | 1954.5 | 0.01 |
| THI | 4 | 1978.5 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algra, M.; de Keijzer, L.; Arndt, S.S.; van Eerdenburg, F.J.C.M.; Goerlich, V.C. Evaluation of the Thermal Response of the Horns in Dairy Cattle. Animals 2023, 13, 500. https://doi.org/10.3390/ani13030500
Algra M, de Keijzer L, Arndt SS, van Eerdenburg FJCM, Goerlich VC. Evaluation of the Thermal Response of the Horns in Dairy Cattle. Animals. 2023; 13(3):500. https://doi.org/10.3390/ani13030500
Chicago/Turabian StyleAlgra, Marijke, Lara de Keijzer, Saskia S. Arndt, Frank J. C. M. van Eerdenburg, and Vivian C. Goerlich. 2023. "Evaluation of the Thermal Response of the Horns in Dairy Cattle" Animals 13, no. 3: 500. https://doi.org/10.3390/ani13030500
APA StyleAlgra, M., de Keijzer, L., Arndt, S. S., van Eerdenburg, F. J. C. M., & Goerlich, V. C. (2023). Evaluation of the Thermal Response of the Horns in Dairy Cattle. Animals, 13(3), 500. https://doi.org/10.3390/ani13030500






