Responses of Micropterus salmoides under Ammonia Stress and the Effects of a Potential Ammonia Antidote
Abstract
Simple Summary
Abstract
1. Introduction
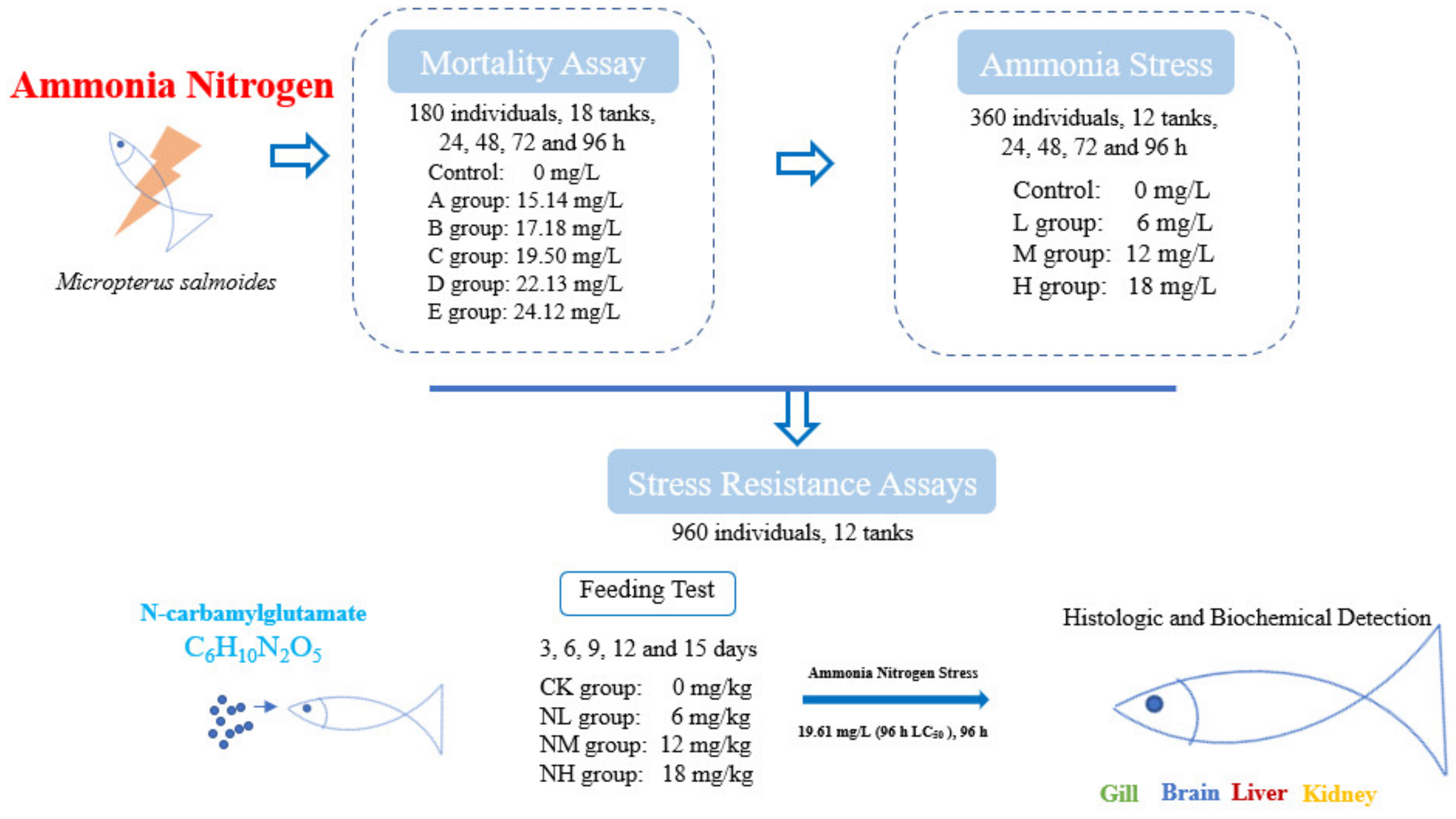
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Fish and Culture Conditions
2.3. Cumulative Mortality Assay
2.4. Ammonia Stress Assays
2.5. Stress Resistance Assays
2.5.1. Effect of NCG on M. salmoides
2.5.2. Effect of NCG on Ammonia Nitrogen Resistance
2.6. Index Detection
2.6.1. Sample Collecting
2.6.2. Histological Detection
2.6.3. Biochemical Detection
2.7. Statistical Analysis
3. Results
3.1. Analysis of Mortality
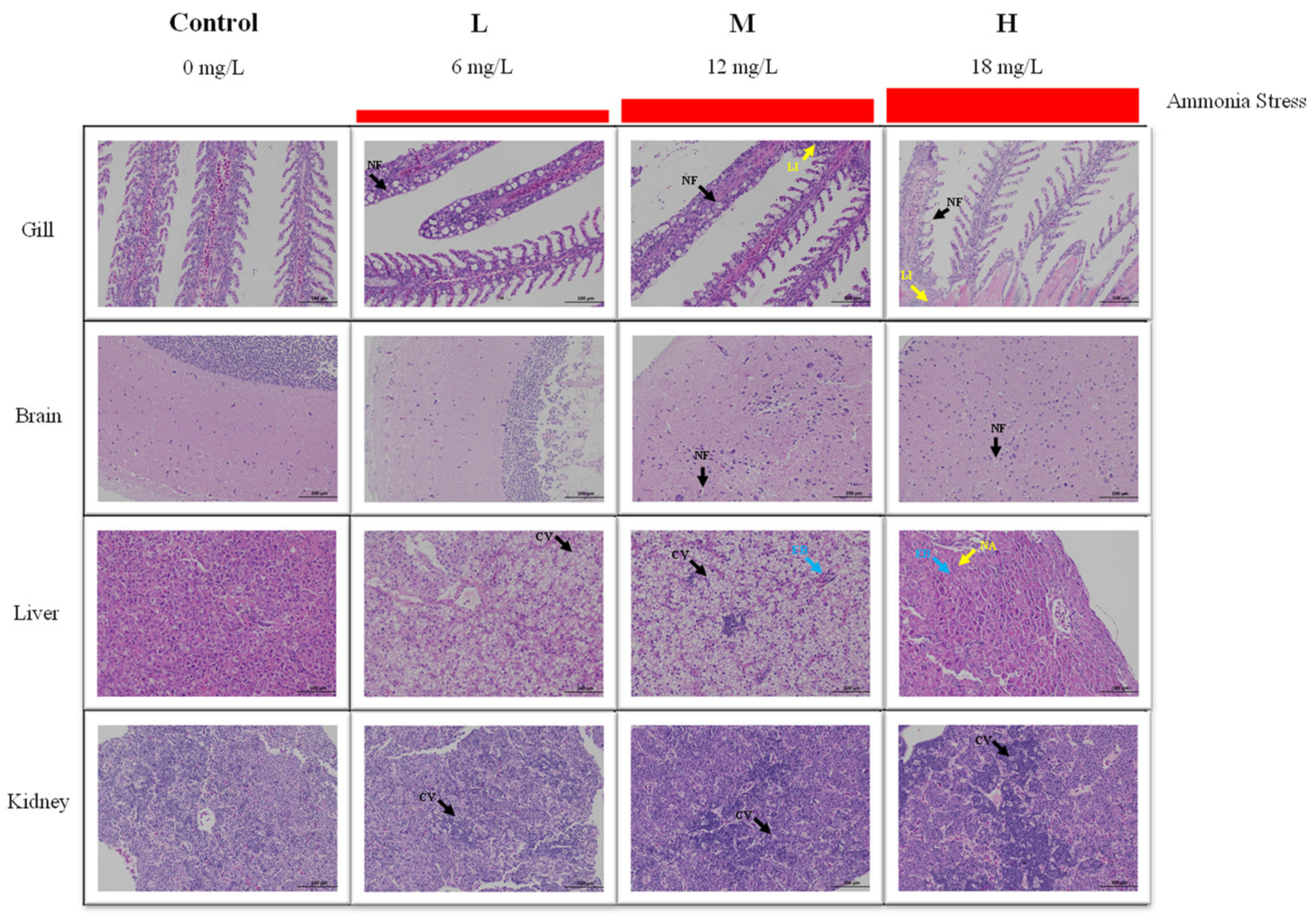
3.2. Histological Analysis under Ammonia Stress
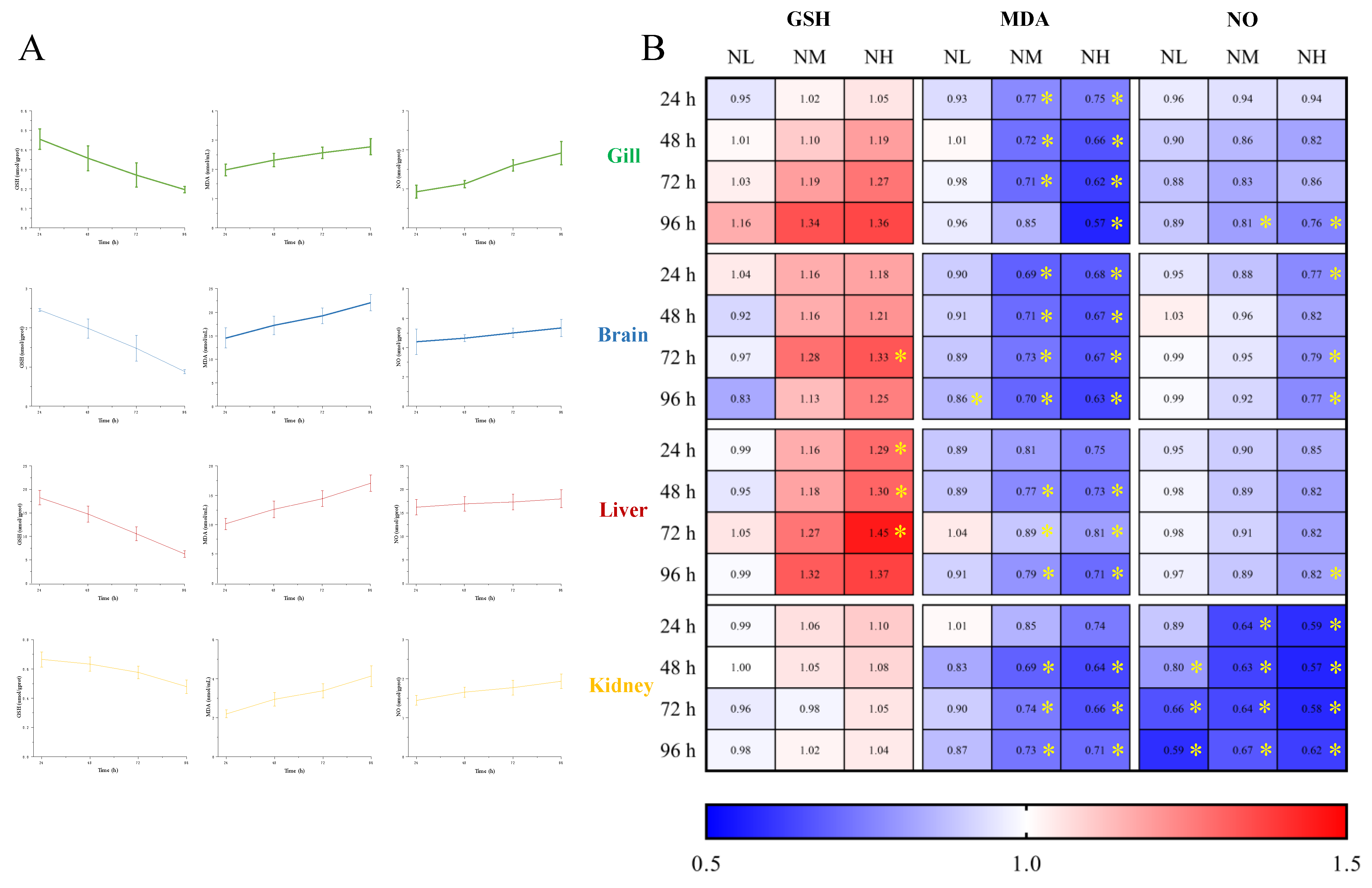
3.3. Biochemical Analysis under Ammonia Stress
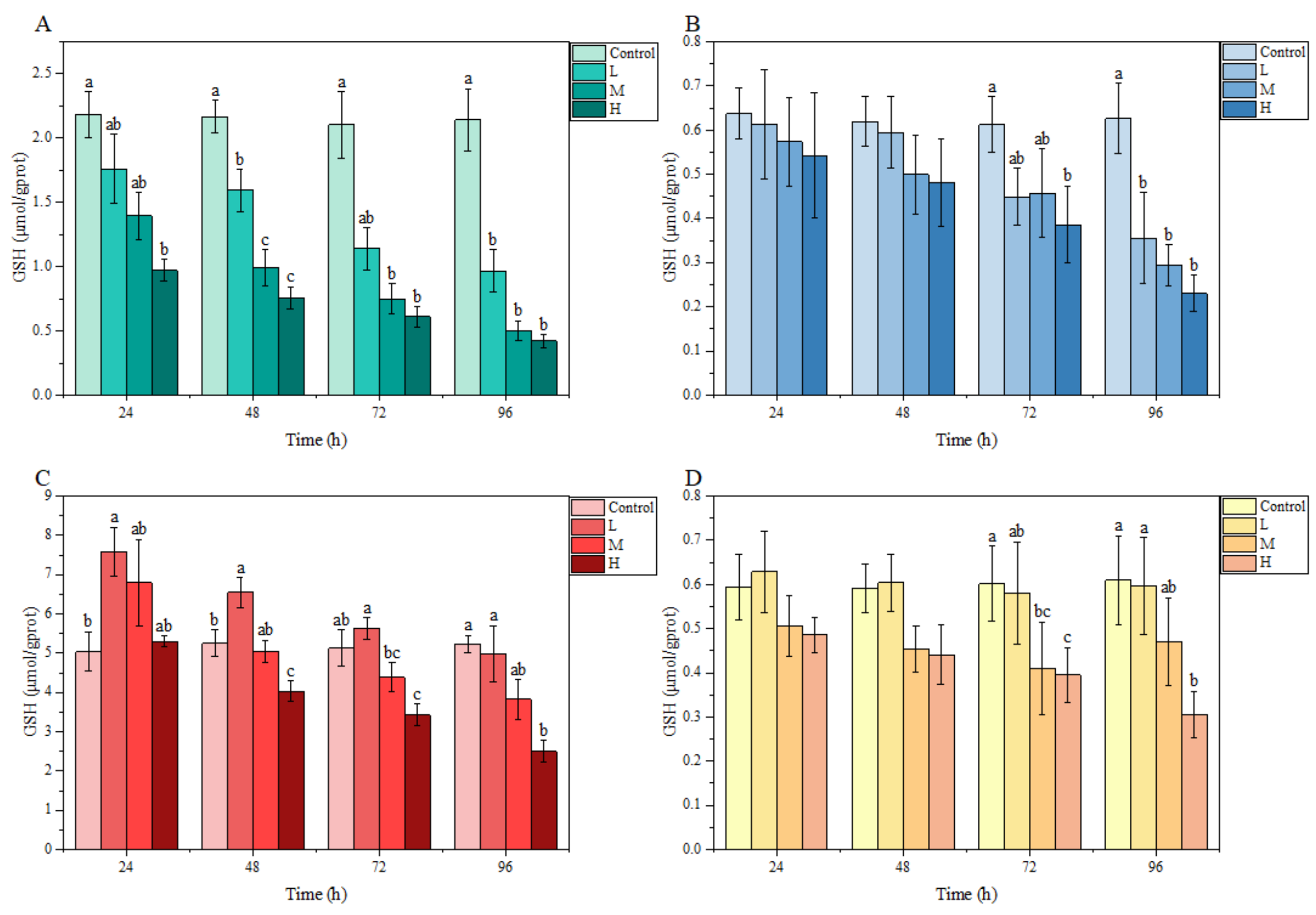
3.3.1. GSH Content
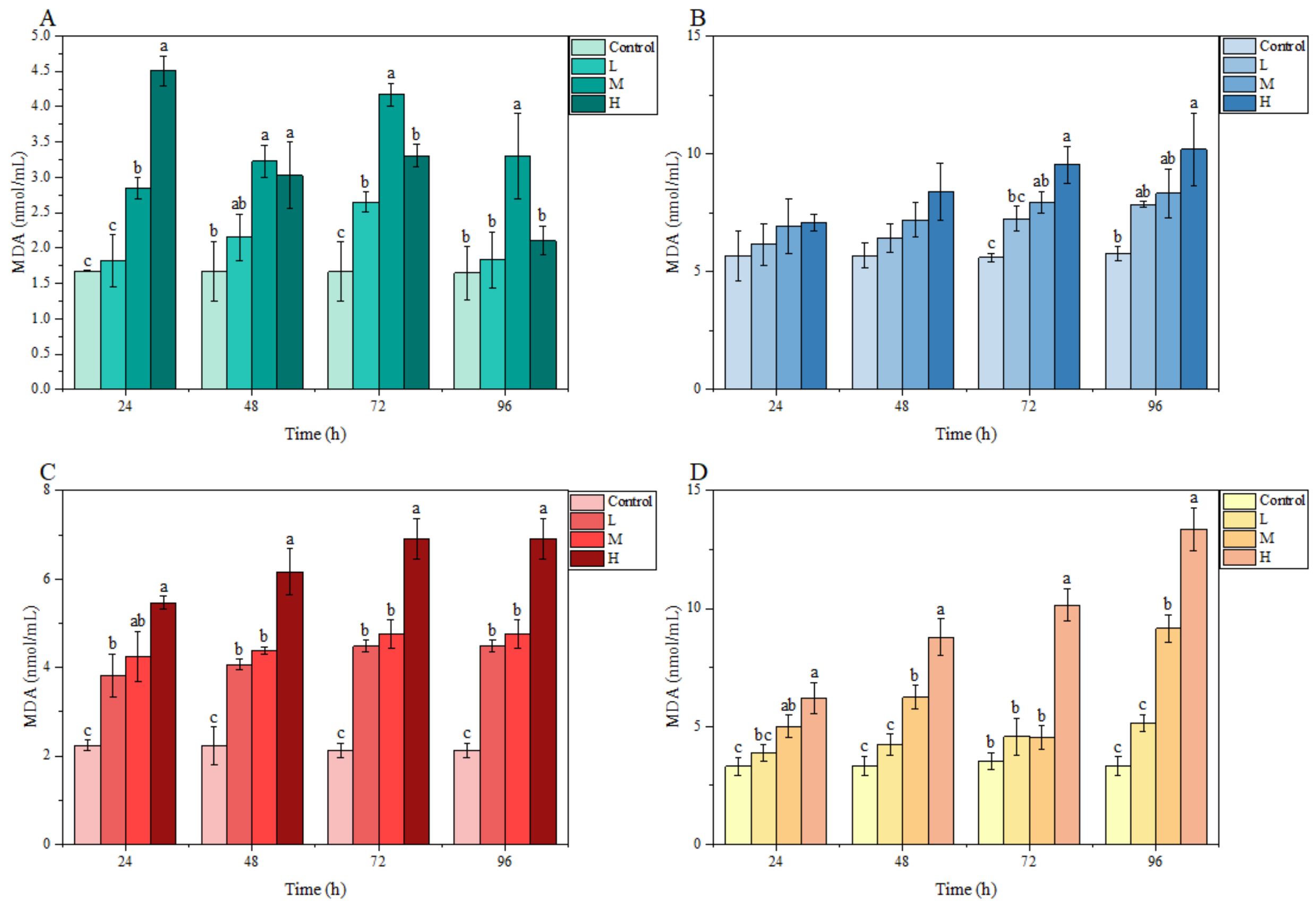
3.3.2. MDA Content
3.3.3. NO Content
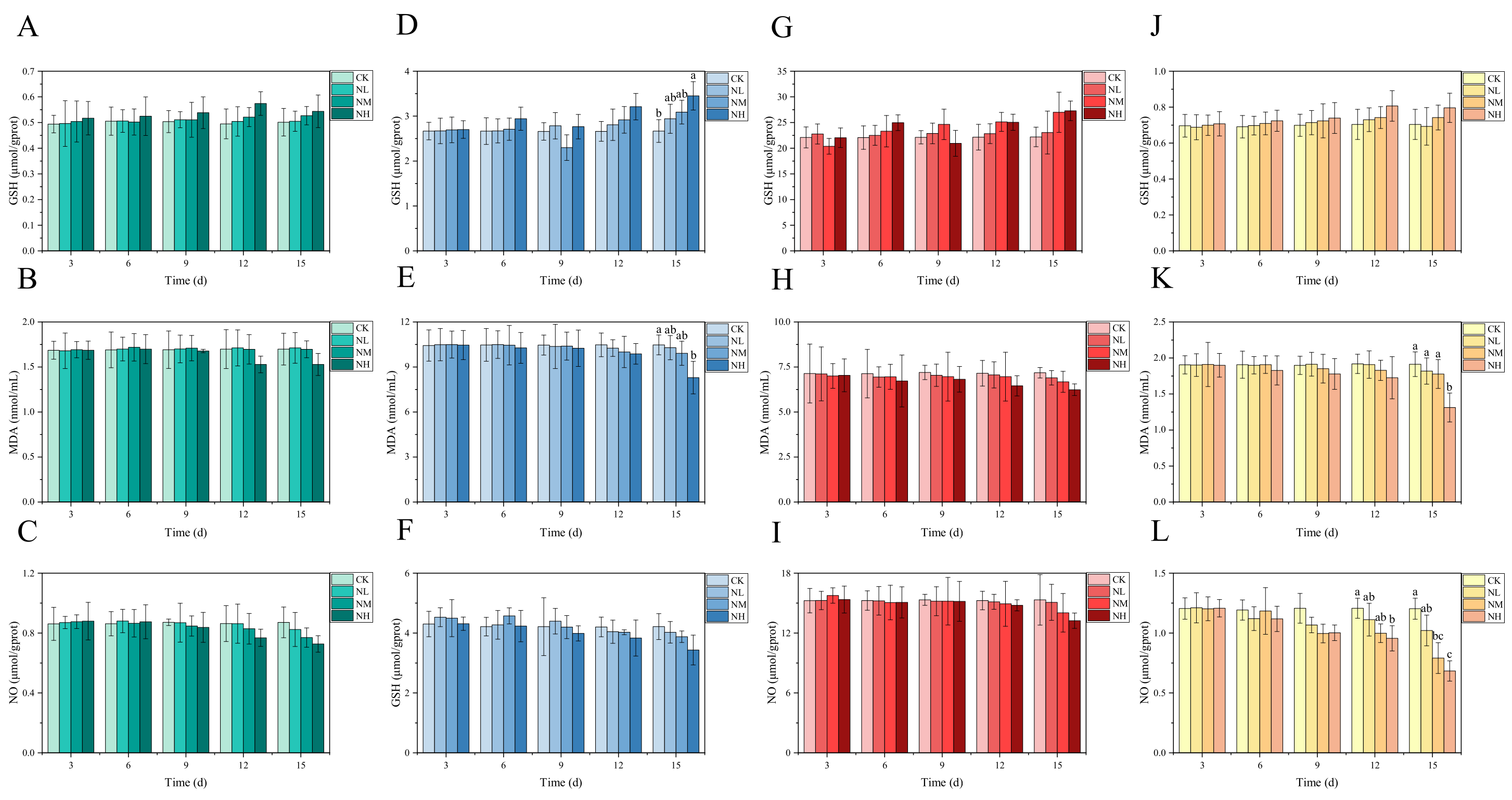
3.4. Analysis of NCG Impact in Feeding Test
3.5. Analysis of NCG Impact on Ammonia Nitrogen Resistance
3.5.1. Effects of NCG on Tissue Structure under Ammonia Stress
3.5.2. Effects of NCG on Biochemical Indexes under Ammonia Stress
4. Discussion
4.1. Effects of Ammonia Stress on Mortality and Histology
4.2. Effect of NCG on Stress Tolerance on M. salmoides
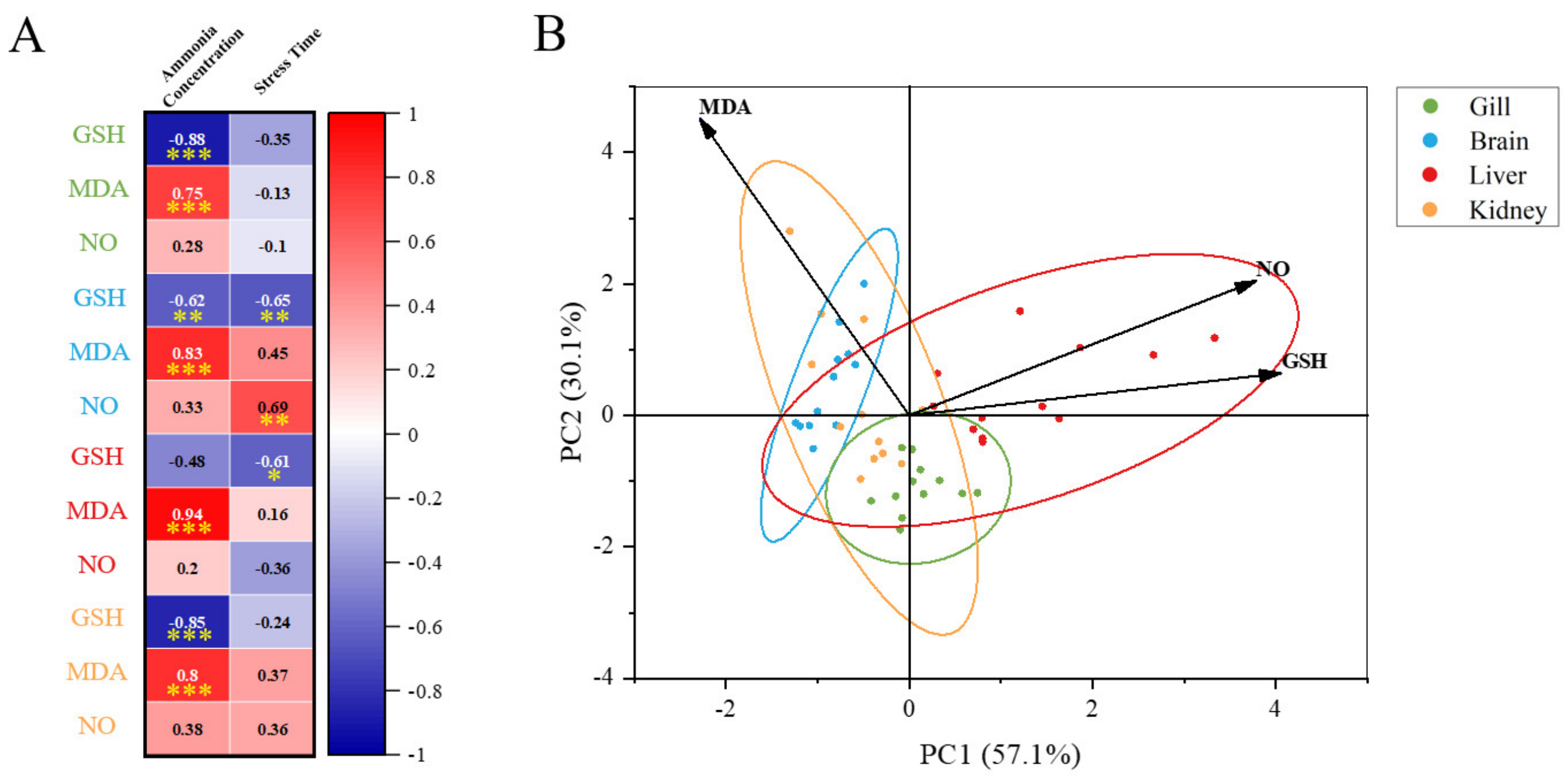
4.3. Tissue Specific Responses to Ammonia Stress and NCG Addition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, J.; Lutz-Carrillo, D.J.; Quan, Y.; Liang, S. Taxonomic status and genetic diversity of cultured largemouth bass Micropterus salmoides in China. Aquaculture 2008, 278, 27–30. [Google Scholar] [CrossRef]
- Xu, Z.; Cao, J.; Qin, X.; Qiu, W.; Mei, J.; Xie, J. Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and tissue structure in fish exposed to ammonia nitrogen: A review. Animals 2021, 11, 3304. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chen, S.; Ouyang, K.; Kuang, Y.; Yang, H.; Wang, Y.; Tang, R.; Zhang, X.; Li, D.; Li, L. Evaluation of ammonia nitrogen exposure in immune defenses present on spleen and head-kidney of Wuchang bream (Megalobrama amblycephala). Int. J. Mol. Sci. 2022, 23, 3129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, J.; Qi, Q.; Xu, M.; Xu, R. Effects of ammonia exposure on anxiety behavior, oxidative stress and inflammation in guppy (Poecilia reticulate). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 265, 109539. [Google Scholar] [CrossRef]
- Liu, M.; Guo, H.; Zhu, K.; Liu, B.; Liu, B.; Guo, L.; Zhang, N.; Yang, J.; Jiang, S.; Zhang, D. Effects of acute ammonia exposure and recovery on the antioxidant response and expression of genes in the Nrf2-keap1 signaling pathway in the juvenile golden pompano (Trachinotus ovatus). Aquat Toxicol. 2021, 240, 105969. [Google Scholar] [CrossRef]
- Lu, J.; Yao, T.; Shi, S.; Ye, L. Effects of acute ammonia nitrogen exposure on metabolic and immunological responses in the Hong Kong oyster Crassostrea hongkongensis. Ecotoxicol. Environ. Saf. 2022, 237, 113518. [Google Scholar] [CrossRef]
- Zhuo, H.; Liu, J. Nuclear Factor Interleukin 3 (NFIL3) Participates in regulation of the NF-ΚB-mediated inflammation and antioxidant system in Litopenaeus vannamei under ammonia-n stress. Fish Shellfish Immunol. 2022, 131, 1192–1205. [Google Scholar] [CrossRef]
- Kuang, Y.; Guo, H.; Ouyang, K.; Wang, X.; Li, D.; Li, L. Nano-TiO2 aggravates immunotoxic effects of chronic ammonia stress in zebrafish (Danio rerio) intestine. Comp Biochem Physiol C Toxicol Pharmacol 2023, 266, 109548. [Google Scholar] [CrossRef]
- Esam, F.; Khalafalla, M.M.; Gewaily, M.S.; Abdo, S.; Hassan, A.M.; Dawood, M.A.O. Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in Nile tilapia. Ecotoxicol. Environ. Saf. 2022, 231, 113187. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Salah, A.S.; Elsheshtawy, A.; Rizk, M.; Abualreesh, M.H.; Abdel-Daim, M.M.; Salem, S.M.R.; Askary, A.E.; Assar, D.H. Exploring the multimodal role of Yucca schidigera extract in protection against Chronic ammonia exposure targeting: Growth, metabolic, stress and inflammatory responses in Nile tilapia (Oreochromis niloticus L.). Animals 2021, 11, 2072. [Google Scholar] [CrossRef]
- Kaleo, I.V.; Gao, Q.; Liu, B.; Sun, C.; Zhou, Q.; Zhang, H.; Shan, F.; Xiong, Z.; Bo, L.; Song, C. Effects of Moringa oleifera leaf extract on growth performance, physiological and immune response, and related immune gene expression of Macrobrachium rosenbergii with vibrio anguillarum and ammonia stress. Fish Shellfish Immunol. 2019, 89, 603–613. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Kalhor, N.; Yousefi, M.; Adineh, H.; Moghadam, M.S.; Sheikhzadeh, N.; Moonmanee, T.; Hoseinifar, S.H.; Van Doan, H. Effects of dietary Plantago ovata seed extract administration on growth performance and immune function of common carp (Cyprinus carpio) fingerling exposed to ammonia toxicity. Vet. Res. Commun. 2022, 18. [Google Scholar] [CrossRef]
- Liu, M.; Gao, Q.; Sun, C.; Liu, B.; Liu, X.; Zhou, Q.; Zheng, X.; Xu, P.; Liu, B. Effects of dietary tea tree oil on the growth, physiological and non-specific immunity response in the giant freshwater prawn (Macrobrachium rosenbergii) under high ammonia stress. Fish Shellfish Immunol. 2022, 120, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Tadese, D.A.; Wangari, M.R.; Zhou, Q.; Zheng, X.; Liu, B.; Tamiru, M.; Dagne, A.; Janssens, G.P.J.; Zhao, Y. Amelioration of ammonia-induced intestinal oxidative stress by dietary Clostridium butyricum in giant freshwater prawn (Macrobrachium rosenbergii). Fish Shellfish Immunol. 2022, 131, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Mokrani, A.; Ji, K.; Ge, X.; Ren, M.; Pan, L.; Sun, A. Effects of dietary arginine on intestinal antioxidant status and immunity involved in Nrf2 and NF-ΚB signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala. Fish Shellfish Immunol. 2018, 82, 243–249. [Google Scholar] [CrossRef]
- Mommsen, T.P. Paradigms of growth in fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 207–219. [Google Scholar] [CrossRef]
- Xiao, L.; Cao, W.; Liu, G.; Fang, T.; Wu, X.; Jia, G.; Chen, X.; Zhao, H.; Wang, J.; Wu, C.; et al. Arginine, n-carbamylglutamate, and glutamine exert protective effects against oxidative stress in rat intestine. Anim. Nutr. 2016, 2, 242–248. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, Y.; Wang, M.; Loor, J.J.; Wang, H. N-carbamylglutamate and l-arginine supplementation improve hepatic antioxidant status in intrauterine growth-retarded suckling lambs. RSC Adv. 2020, 10, 11173–11181. [Google Scholar] [CrossRef]
- Huang, H.Y.; Chen, P.; Liang, X.F.; Wu, X.F.; Wang, C.P.; Gu, X.; Xue, M. Dietary n-carbamylglutamate (NCG) alleviates liver metabolic disease and hepatocyte apoptosis by suppressing ERK1/2-MTOR-S6K1 signal pathway via promoting endogenous arginine synthesis in Japanese seabass (Lateolabrax japonicus). Fish Shellfish Immunol. 2019, 90, 338–348. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Y.; Dong, X.; Tan, B.; Liu, H.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Yang, Y. Ammonia toxicity induces oxidative stress, inflammatory response and apoptosis in hybrid grouper (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatu). Front. Mar. Sci. 2021, 8, 66743. [Google Scholar] [CrossRef]
- Hao, M.; Zuo, Q.; Zhang, W.; Feng, Y.; Wang, L.; Yu, L.; Zhang, X.; Li, J.; Huang, Z. Toxicological assessment of ammonia exposure on Carassius auratus red var. living in landscape waters. Bull. Environ. Contam Toxicol. 2019, 103, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Benli, A.C.K.; Köksal, G.; Ozkul, A. Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): Effects on gill, liver and kidney histology. Chemosphere 2008, 72, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Cong, M.; Wu, H.; Cao, T.; Ji, C.; Lv, J. Effects of ammonia nitrogen on gill mitochondria in clam Ruditapes philippinarum. Environ. Toxicol. Pharmacol. 2019, 65, 46–52. [Google Scholar] [CrossRef]
- Rodrigues, R.V.; Schwarz, M.H.; Delbos, B.C.; Carvalho, E.L.; Romano, L.A.; Sampaio, L.A. Acute exposure of juvenile cobia Rachycentron canadum to nitrate induces gill, esophageal and brain damage. Aquaculture 2011, 322–323, 223–226. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhang, M.; Jiang, H.; Wang, R.; Qian, Y.; Li, M. Ammonia stress disrupts intestinal microbial community and amino acid metabolism of juvenile yellow catfish (Pelteobagrus fulvidraco). Ecotoxicol. Environ. Saf. 2021, 227, 112932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xia, S.; Zhu, J.; Miao, L.; Ren, M.; Lin, Y.; Ge, X.; Sun, S. Growth performance, physiological response and histology changes of juvenile blunt snout bream, Megalobrama amblycephala exposed to chronic ammonia. Aquaculture 2019, 506, 424–436. [Google Scholar] [CrossRef]
- Cheng, C.; Yang, F.; Ling, R.; Liao, S.; Miao, Y.; Ye, C.; Wang, A. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef]
- Gao, X.; Fei, F.; Huang, B.; Meng, X.; Zhang, T.; Zhao, K.; Chen, H.; Xing, R.; Liu, B. Alterations in hematological and biochemical parameters, oxidative stress, and immune response in Takifugu rubripes under acute ammonia exposure. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 243, 108978. [Google Scholar] [CrossRef]
- Seo, J.S.; Haque, M.N.; Nam, S.E.; Kim, B.M.; Rhee, J.S. Inorganic nitrogen compounds reduce immunity and induce oxidative stress in red seabream. Fish Shellfish Immunol. 2020, 104, 237–244. [Google Scholar] [CrossRef]
- Xiao, W.; Loscalzo, J. Metabolic responses to reductive stress. Antioxid Redox Signal 2020, 32, 1330–1347. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar]
- Ghasemi, M. Nitric Oxide: Antidepressant mechanisms and inflammation. Adv. Pharmacol. 2019, 86, 121–152. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Wang, L.; Qiu, X.-M.; Hao, Q.; Li, D.-J. Anti-inflammatory effects of a Chinese herbal medicine in atherosclerosis via estrogen receptor β mediating nitric oxide production and NF-ΚB suppression in endothelial cells. Cell Death Dis. 2013, 4, e551. [Google Scholar] [CrossRef] [PubMed]
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H.M.N. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders—A review. Recent Pat Inflamm Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, S.; Lin, X.; Zeng, W.; Mi, Y.; Zhang, C. Effect of dietary n-carbamylglutamate on development of ovarian follicles via enhanced angiogenesis in the chicken. Poult Sci. 2020, 99, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, J.; Fang, X.; Jiang, X.; Sun, Y.; Zhang, Y. Effects of dietary n-carbamylglutamate on growth performance, apparent digestibility, nitrogen metabolism and plasma metabolites of fattening Holstein bulls. Animals 2021, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Peng, A.; Yu, Y.; Guo, S.; Wang, M.; Coleman, D.N.; Loor, J.J.; Wang, H. N-carbamylglutamate and l-arginine promote intestinal absorption of amino acids by regulating the mTOR signaling pathway and amino acid and peptide transporters in suckling lambs with intrauterine growth restriction. J. Nutr. 2019, 149, 923–932. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, X.; Liang, X.; Wu, X.; Gu, X.; Han, J.; Xue, M. N-carbamoylglutamate improves lipid metabolism, inflammation, and apoptosis responses in visceral adipocytes of Japanese seabass (Lateolabrax japonicus), in Vivo and in Vitro. Anim. Nutr. 2021, 7, 707–715. [Google Scholar] [CrossRef]
- Pritchard, J.B. The gill and homeostasis: Transport under stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1269–R1271. [Google Scholar] [CrossRef]
- Zhong, Y.; Duan, Z.; Su, M.; Lin, Y.; Zhang, J. Inflammatory responses associated with hyposaline stress in gill epithelial cells of the spotted scat Scatophagus argus. Fish Shellfish Immunol. 2021, 114, 142–151. [Google Scholar] [CrossRef] [PubMed]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P.E. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef] [PubMed]
- Mooney, T.J.; Pease, C.; Trenfield, M.; van Dam, R.; Harford, A.J. Modeling the pH-ammonia toxicity relationship for Hydra viridissima in soft waters with low ionic concentrations. Environ. Toxicol. Chem. 2018, 37, 1189–1196. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, S.; Ge, X.; Xia, S.; Zhu, J.; Miao, L.; Lin, Y.; Liang, H.; Pan, W.; Su, Y.; et al. Acute effects of ammonia exposure on the plasma and haematological parameters and histological structure of the juvenile blunt snout bream, Megalobrama amblycephala, and post-exposure recovery. Aquac. Res. 2018, 49, 1008–1019. [Google Scholar] [CrossRef]
- Van der Linden, A.; Verhoye, M.; Nilsson, G.E. Does anoxia induce cell swelling in carp brains? In vivo MRI measurements in crucian carp and common carp. J. Neurophysiol. 2001, 85, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xu, J.; Xu, X.; Wang, Q.; Kong, Q.; Xu, F.; Du, Y. Organ-specific responses to total ammonia nitrogen stress on juvenile grass carp (Ctenopharyngodon idellus). Environ. Sci. Pollut. Res. Int. 2019, 26, 10826–10834. [Google Scholar] [CrossRef]


| Group | Ammonia Concentration (mg/L) | Cumulative Number of Deaths (Individual) | |||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | ||
| Control | 0 | 0 | 0 | 0 | 0 |
| A | 15.14 | 0 | 0 | 0 | 1 |
| B | 17.18 | 0 | 1 | 7 | 10 |
| C | 19.50 | 2 | 8 | 11 | 16 |
| D | 22.13 | 8 | 12 | 16 | 20 |
| E | 25.12 | 11 | 14 | 21 | 29 |
| LC50 (mg/L) | 25.78 | 24.40 | 21.90 | 19.61 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Guo, X.; Tu, J.; Shi, X.; Gan, L.; Zhang, M.; Jiang, H.; Zhang, X.; Shao, J. Responses of Micropterus salmoides under Ammonia Stress and the Effects of a Potential Ammonia Antidote. Animals 2023, 13, 397. https://doi.org/10.3390/ani13030397
Wang Z, Guo X, Tu J, Shi X, Gan L, Zhang M, Jiang H, Zhang X, Shao J. Responses of Micropterus salmoides under Ammonia Stress and the Effects of a Potential Ammonia Antidote. Animals. 2023; 13(3):397. https://doi.org/10.3390/ani13030397
Chicago/Turabian StyleWang, Zhenlu, Xingchen Guo, Jiao Tu, Xuan Shi, Lei Gan, Muzi Zhang, Haibo Jiang, Xiaoxue Zhang, and Jian Shao. 2023. "Responses of Micropterus salmoides under Ammonia Stress and the Effects of a Potential Ammonia Antidote" Animals 13, no. 3: 397. https://doi.org/10.3390/ani13030397
APA StyleWang, Z., Guo, X., Tu, J., Shi, X., Gan, L., Zhang, M., Jiang, H., Zhang, X., & Shao, J. (2023). Responses of Micropterus salmoides under Ammonia Stress and the Effects of a Potential Ammonia Antidote. Animals, 13(3), 397. https://doi.org/10.3390/ani13030397






