Radiographic Left Atrial Size Measurement of Dogs in Different Mitral Valve Disease Stages with Four Different Methods
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchanan, J.W.; Bücheler, J. Vertebral scale system to measure canine heart size in radiographs. J. Am. Vet. Med. Assoc. 1995, 206, 194–199. [Google Scholar]
- Mostafa, A.A.; Berry, C.R. Radiographic assessment of the cardiac silhouette in clinically normal large- and small-breed dogs. Am. J. Vet. Res. 2017, 78, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Costanza, D.; Greco, A.; Piantedosi, D.; Bruzzese, D.; Pasolini, M.P.; Coluccia, P.; Castiello, E.; Baptista, C.S.; Meomartino, L. The heart to single vertebra ratio: A new objective method for radiographic assessment of cardiac silhouette size in dogs. Vet. Radiol. Ultrasound. 2023, 64, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Marbella Fernández, D.; García, V.; Santana, A.J.; Montoya-Alonso, J.A. The thoracic inlet heart size, a new approach to radiographic cardiac measurement. Animals 2023, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Lord, P.F.; Hansson, K.; Carnabuci, C.; Kvart, C.; Häggström, J. Radiographic heart size and its rate of increase as tests for onset of congestive heart failure in Cavalier King Charles Spaniels with mitral valve regurgitation. J. Vet. Intern. Med. 2011, 25, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, E.L.; Visser, L.C.; Phillips, K.L.; Johnson, L.R. Diagnostic value of vertebral left atrial size as determined from thoracic radiographs for assessment of left atrial size in dogs with myxomatous mitral valve disease. J. Am. Vet. Med. Assoc. 2018, 253, 1038–1045. [Google Scholar] [CrossRef]
- Mikawa, S.; Nagakawa, M.; Ogi, H.; Akabane, R.; Koyama, Y.; Sakatani, A.; Ogawa, M.; Miyakawa, H.; Shigemoto, J.; Tokuriki, T.; et al. Use of vertebral left atrial size for staging of dogs with myxomatous valve disease. J. Vet. Cardiol. 2020, 30, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yun, T.; Koo, Y.; Chae, Y.; Ku, D.; Chang, D.; Kang, B.T.; Yang, M.P.; Kim, H. Change of vertebral left atrial size in dogs with preclinical myxomatous mitral valve disease prior to the onset of congestive heart failure. J. Vet. Cardiol. 2022, 42, 23–33. [Google Scholar] [CrossRef]
- Borgarelli, M.; Buchanan, J.W. Historical review, epidemiology and natural history of degenerative mitral valve disease. J. Vet. Cardiol. 2012, 14, 93–101. [Google Scholar] [CrossRef]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef]
- Szatmári, V.; Hofman, Z.M.M.; van Bijsterveldt, N.J.; Tellegen, A.R.; Vilaplana Grosso, F.R. A novel standardized method for aiding to determine left atrial enlargement on lateral thoracic radiographs in dogs. Animals 2023, 13, 2178. [Google Scholar] [CrossRef]
- Sánchez Salguero, X.; Prandi, D.; Llabrés-Díaz, F.; Manzanilla, E.G.; Badiella, L.; Bussadori, C. Heart to spine measurements to detect left atrial enlargement in dogs with mitral valve insufficiency. Ir. Vet. J. 2019, 72, 14. [Google Scholar] [CrossRef]
- Sánchez Salguero, X.; Prandi, D.; Llabrés-Díaz, F.; Manzanilla, E.G.; Bussadori, C. A radiographic measurement of left atrial size in dogs. Ir. Vet. J. 2018, 71, 25. [Google Scholar] [CrossRef] [PubMed]
- Stepien, R.L.; Rak, M.B.; Blume, L.M. Use of radiographic measurements to diagnose stage B2 preclinical myxomatous mitral valve disease in dogs. J. Am. Vet. Med. Assoc. 2020, 256, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.; Gavaghan, B.J.; Meyers, F.E. Radiographic quantification of left atrial size in dogs with myxomatous mitral valve disease. J. Vet. Intern. Med. 2021, 35, 747–754. [Google Scholar] [CrossRef]
- An, S.; Hwang, G.; Noh, S.A.; Yoon, Y.; Lee, H.C.; Hwang, T.S. A Retrospective Study of Radiographic Measurements of Small Breed Dogs with Myxomatous Mitral Valve Degeneration: A New Modified Vertebral Left Atrial Size. J. Vet. Clin. 2023, 40, 31–37. [Google Scholar] [CrossRef]
- Bagardi, M.; Manfredi, M.; Zani, D.D.; Brambilla, P.G.; Locatelli, C. Interobserver variability of radiographic methods for the evaluation of left atrial size in dogs. Vet. Radiol. Ultrasound. 2021, 62, 161–174. [Google Scholar] [CrossRef]
- Vezzosi, T.; Puccinelli, C.; Citi, S.; Tognetti, R. Two radiographic methods for assessing left atrial enlargement and cardiac remodeling in dogs with myxomatous mitral valve disease. J. Vet. Cardiol. 2021, 34, 55–63. [Google Scholar] [CrossRef]
- Levicar, C.; Grandos-Soler, J.L.; Freise, F.; Raue, J.F.; Nolte, I.; Bach, J.-P. Comparison of different radiographic scores with associated echocardiographic measurements and prediction of heart enlargement in dogs with and without myxomatous mitral valve disease. J. Vet. Cardiol. 2022, 44, 1–12. [Google Scholar] [CrossRef]
- Ross, E.S.; Visser, L.C.; Sbardellati, N.; Potter, B.M.; Ohlendorf, A.; Scansen, B.A. Utility of vertebral left atrial size and vertebral heart size to aid detection of congestive heart failure in dogs with respiratory signs. J. Vet. Intern. Med. 2023, 37, 2021–2029. [Google Scholar] [CrossRef]
- Vereb, M.; Atkins, C.E.; Adin, D.; Blondel, T.; Coffman, M.; Lee, S.; Guillot, E.; Ward, J.L. Efficacy of a mitral regurgitation severity index to predict long-term outcome in dogs with myxomatous mitral valve disease. J. Vet. Intern. Med. 2023. [Google Scholar] [CrossRef]
- Wiegel, P.S.; Mach, R.; Nolte, I.; Freise, F.; Levicar, C.; Merhof, K.; Bach, J.P. Breed-specific values for vertebral heart score (VHS), vertebral left atrial size (VLAS), and radiographic left atrial dimension (RLAD) in pugs without cardiac disease, and their relationship to Brachycephalic Obstructive Airway Syndrome (BOAS). PLoS ONE 2022, 17, e0274085. [Google Scholar] [CrossRef] [PubMed]
- Wesselowski, S.; Gordon, S.G.; Meddaugh, N.; Saunders, A.B.; Häggström, J.; Cusack, K.; Janacek, B.W.; Matthews, D.J. Prediction of clinically important acquired cardiac disease without an echocardiogram in large breed dogs using a combination of clinical, radiographic and electrocardiographic variables. J. Vet. Cardiol. 2022, 40, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Bagardi, M.; Locatelli, C.; Manfredi, M.; Bassi, J.; Spediacci, C.; Ghilardi, S.; Zani, D.D.; Brambilla, P.G. Breed-specific vertebral heart score, vertebral left atrial size, and radiographic left atrial dimension in Cavalier King Charles Spaniels: Reference interval study. Vet. Radiol. Ultrasound. 2021, 63, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Baisan, R.A.; Vulpe, V. Vertebral heart size and vertebral left atrial size reference ranges in healthy Maltese dogs. Vet. Radiol. Ultrasound. 2022, 63, 18–22. [Google Scholar] [CrossRef]
- Puccinelli, C.; Citi, S.; Vezzosi, T.; Garibaldi, S.; Tognetti, R. A radiographic study of breed-specific vertebral heart score and vertebral left atrial size in Chihuahuas. Vet. Radiol. Ultrasound. 2021, 62, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.E.; Fink, E.A. Tracheal diameter: Analysis of radiographic measurements in brachycephalic and nonbrachycephalic dogs. J. Am. Anim. Hosp. Assoc. 1982, 18, 570–576. [Google Scholar]
- Vezzosi, T.; Puccinelli, C.; Tognetti, R.; Pelligra, T.; Citi, S. Radiographic vertebral left atrial size: A reference interval study in healthy adult dogs. Vet. Radiol. Ultrasound. 2020, 61, 507–511. [Google Scholar] [CrossRef]
- Jepsen-Grant, K.; Pollard, R.E.; Johnson, L.R. Vertebral heart scores in eight dog breeds. Vet. Radiol. Ultrasound. 2013, 54, 3–8. [Google Scholar] [CrossRef]
- Duler, L.; Visser, L.C.; Jackson, K.N.; Phillips, K.L.; Pollard, R.E.; Wanamaker, M.W. Evaluation of radiographic predictors of left heart enlargement in dogs with known or suspected cardiovascular disease. Vet. Radiol. Ultrasound. 2021, 62, 271–281. [Google Scholar] [CrossRef]
- Levicar, C.; Nolte, L.; Granados-Soler, J.L.; Freise, F.; Raue, J.F.; Bach, J.-P. Methods of heart and left atrial size in dogs with and without myxomatous mitral valve disease: Intra- and Interobserver agreement and practicability of different methods. Animals 2022, 12, 2531. [Google Scholar] [CrossRef] [PubMed]
- Poad, M.H.; Manzi, T.J.; Oyama, M.A.; Gelzer, A.R. Utility of radiographic measurements to predict echocardiographic left heart enlargement in dogs with preclinical myxomatous mitral valve disease. J. Vet. Intern. Med. 2020, 34, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Wesselowski, S.; Gordon, S.G.; Fries, R.; Saunders, A.B.; Sykes, K.T.; Vitt, J.; Boutet, B.; Häggström, J.; Kadotani, S.; Stack, J.; et al. Use of physical examination, electrocardiography, radiography, and biomarkers to predict echocardiographic stage B2 myxomatous mitral valve disease in preclinical Cavalier King Charles Spaniels. J. Vet. Cardiol. 2023, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Woolley, R.; Smith, P.; Munro, E.; Smith, S.; Swift, S.; Devine, C.; Corcoran, B.; French, A. Effects of treatment type of vertebral heart size in dogs with myxomatous mitral valve disease. Intern. J. Appl. Res. Vet. Med. 2007, 5, 43–48. [Google Scholar]
- Rishniw, M.; Caivano, D.; Dickson, D.; Vatne, L.; Harris, J.; Matos, J.N. Two-dimensional echocardiographic left- atrial-to-aortic 498 ratio in healthy adult dogs: A reexamination of reference intervals. J. Vet. Cardiol. 2019, 26, 29–38. [Google Scholar] [CrossRef]
- Rishniw, M.; Brown, D. The ACVIM consensus statement definition of left ventricular enlargement in myxomatous mitral valve disease does not always represent left ventricular enlargement. J. Vet. Cardiol. 2022, 42, 92–102. [Google Scholar] [CrossRef]
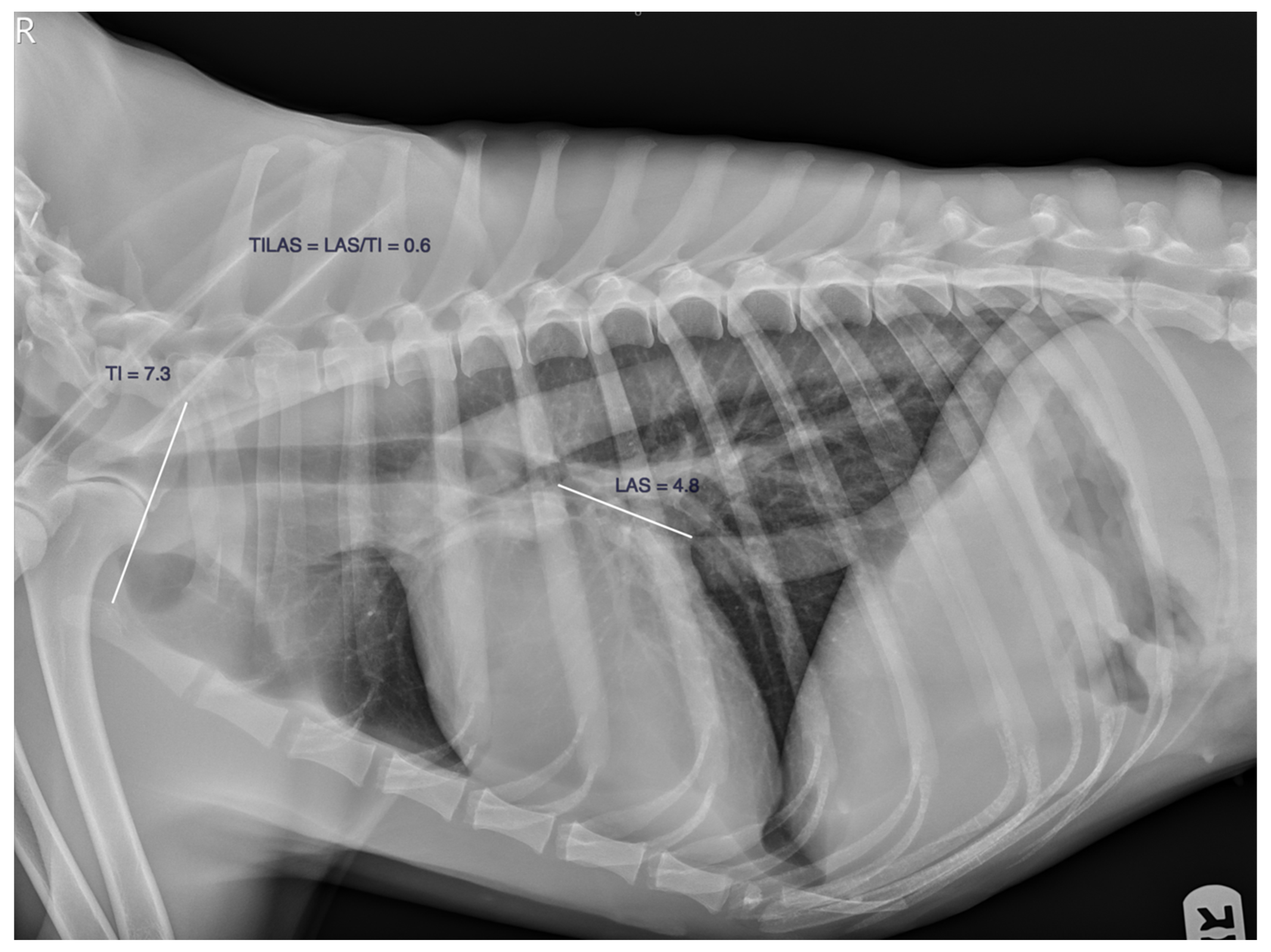
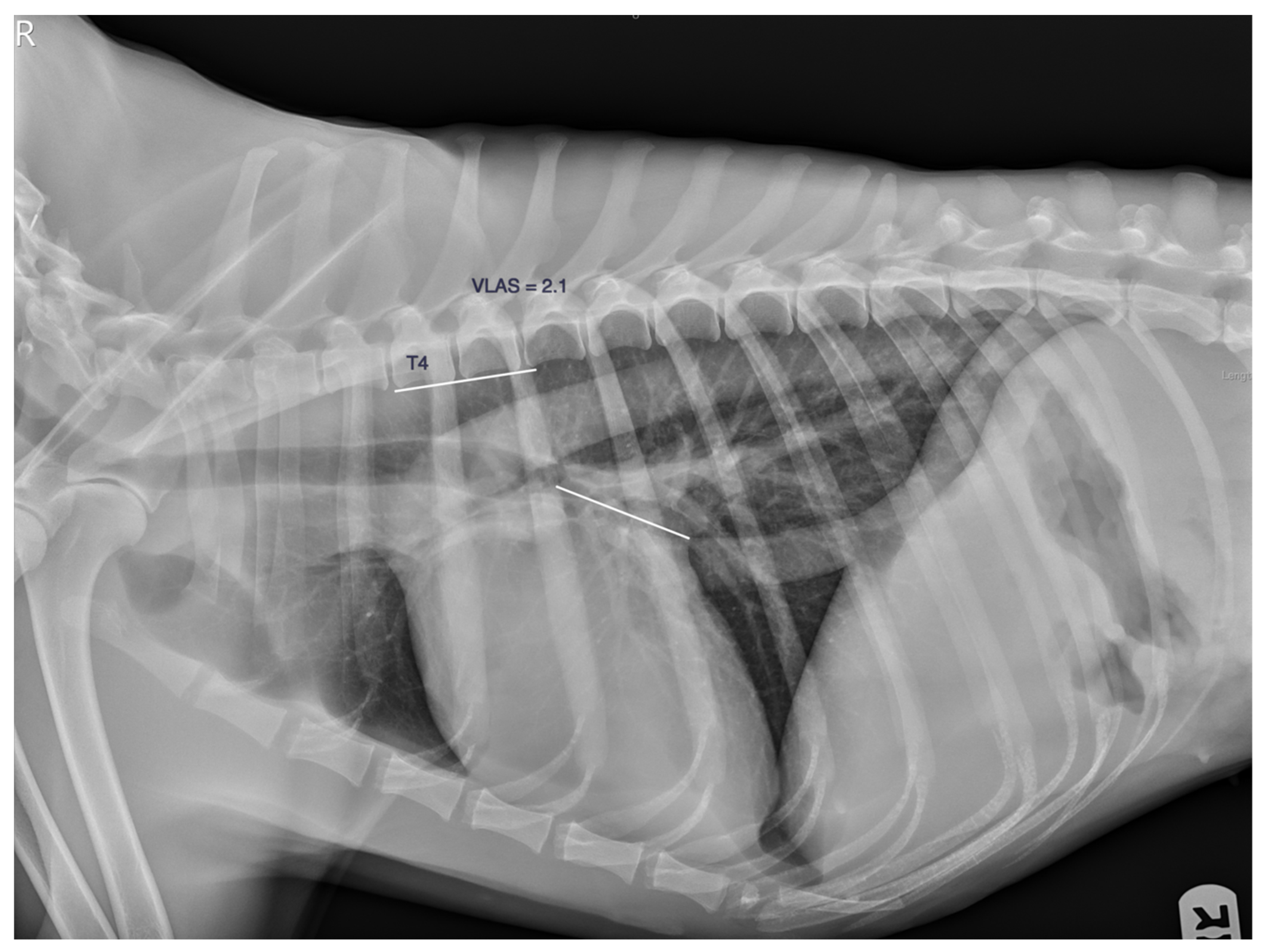
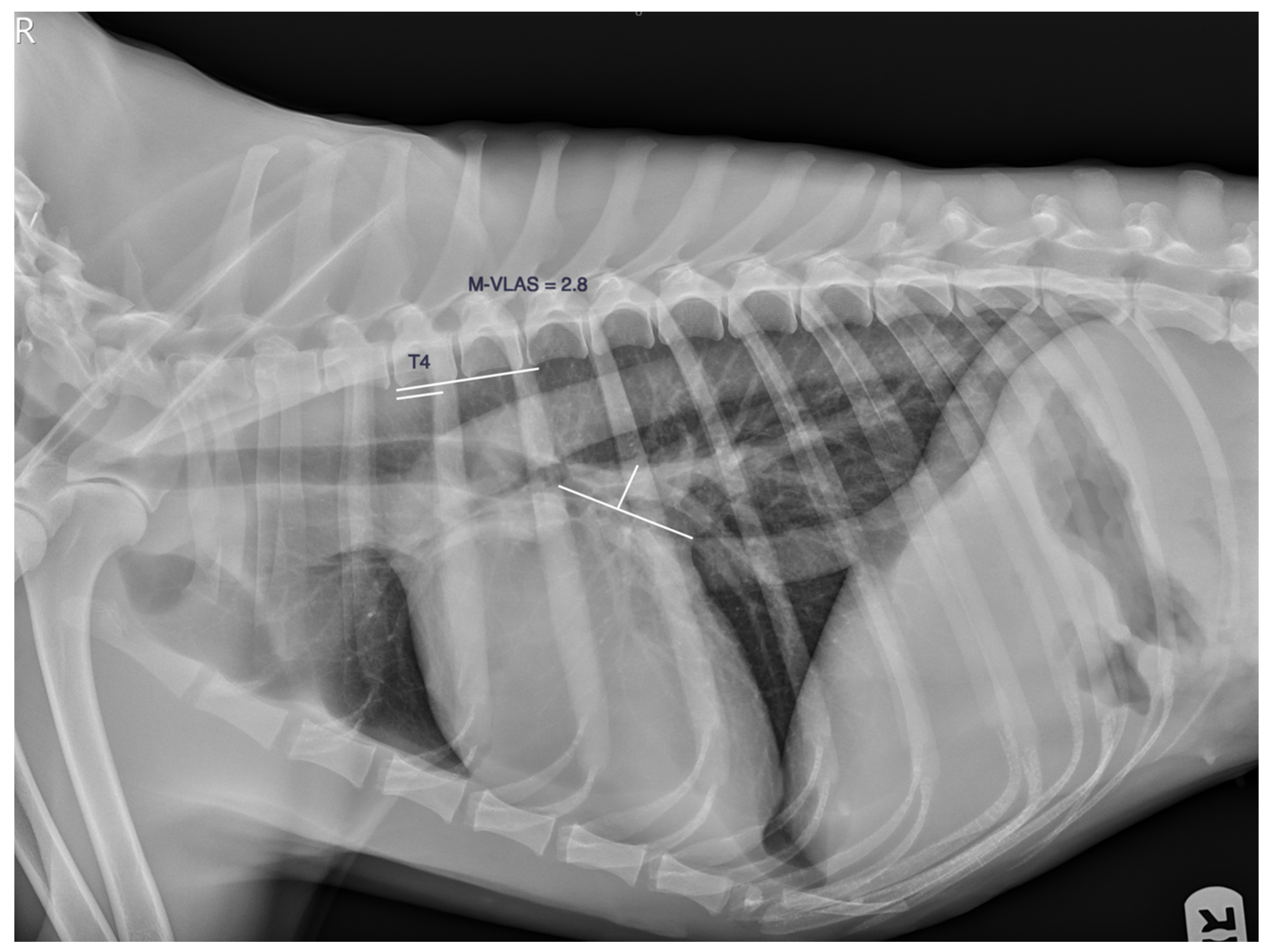
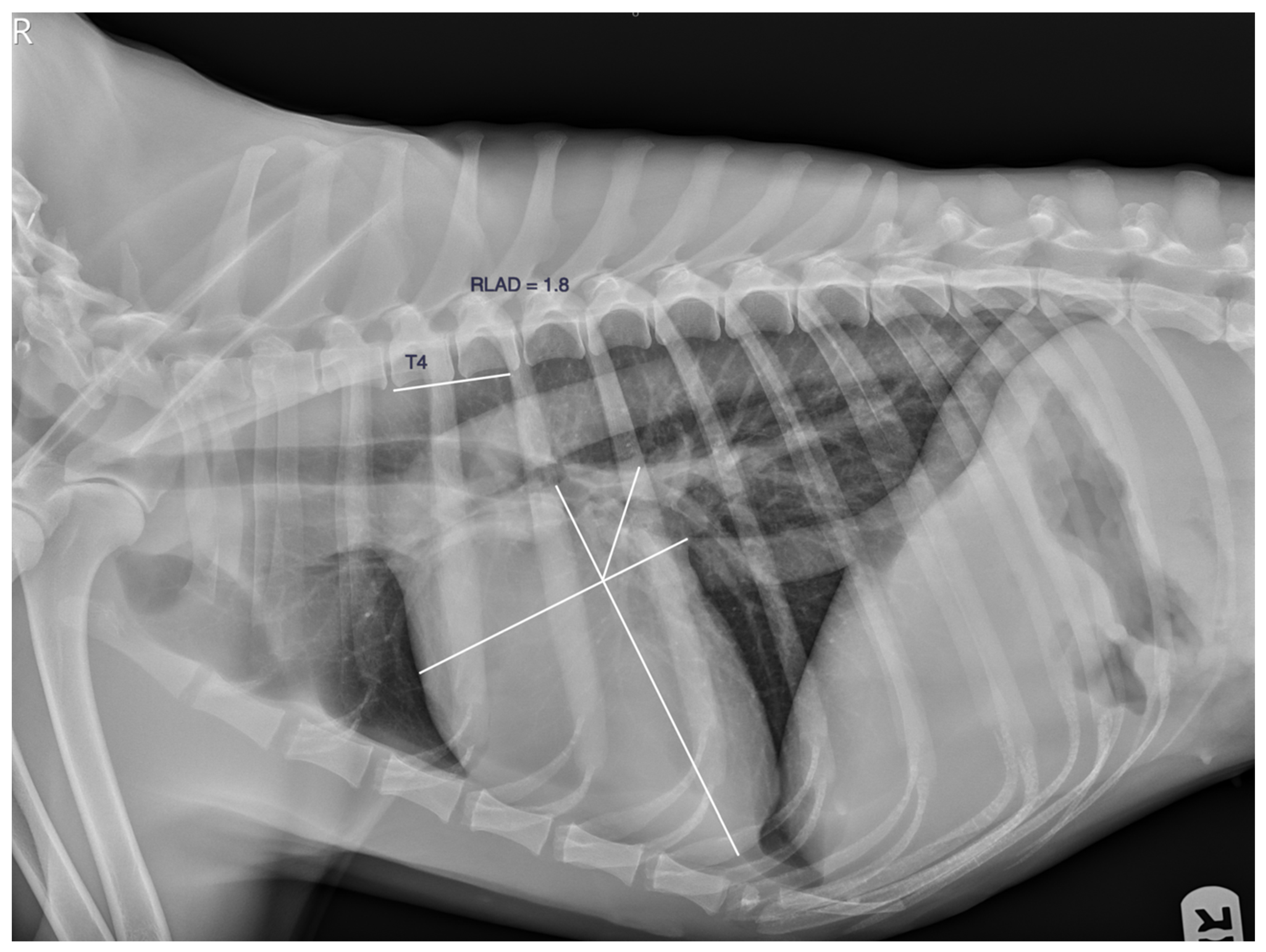
| n | Age (y) | Weight (Kg) | VLAS (v) | M-VLAS (v) | RLAD (v) | TILAS | |
|---|---|---|---|---|---|---|---|
| General | 135 | 7.5 (1–16) | 14.02 (1.8–44.4) | 1.86 ± 0.28 | 2.62 ± 0.47 | 1.82 ± 0.40 | 0.52 ± 0.15 |
| Male | 72 | 7.8 (1–16) | 13.41 (1.8–44.4) | 1.86 ± 0.26 | 2.63 ± 0.45 | 1.81 ± 0.37 | 0.52 ± 0.10 |
| Female | 63 | 7.2 (1–14.3) | 14.56 (2.1–34.6) | 1.86 ± 0.30 | 2.61 ± 0.49 | 1.83 ± 0.44 | 0.53 ± 0.19 |
| <10 kg | 68 | 8.4 (1–15.3) | 5.2 (1.8–9.9) | * 1.84 ± 0.28 | * 2.57 ± 0.42 | * 1.71 ± 0.34 | * 0.53 ± 0.09 |
| ≥10–<20 kg | 26 | 2.8 (1–16) | 13.75 (10.2–18.2) | * 1.77 ± 0.26 | 2.54 ± 0.36 | * 1.80 ± 0.41 | * 0.47 ± 0.09 |
| ≥20–<30 kg | 25 | 6.5 (1–14.3) | 24.35 (20.1–29.8) | 1.87 ± 0.27 | * 2.65 ± 0.44 | * 1.91 ± 0.40 | 0.54 ± 0.28 |
| ≥30 kg | 16 | 6.1 (2–11.4) | 34.15 (30–44.4) | * 2.10 ± 0.36 | * 2.88 ± 0.73 | * 2.19 ± 0.46 | 0.54 ± 0.13 |
| Control (36) | Stage B1 (35) | Stage B2 (17) | Stage C (40) | |
|---|---|---|---|---|
| Age (years) | 8.3 (1.1–15.4) | 12.1 (5.5–15.9) | 12.7 (7.4–19) | 11.4 (7.9–17.7) |
| Weight (kg) | 7.60 (2.1–18.2) | 7.78 (2.6–24.1) | 7.38 (2–19.1) | 6.3 (2.7–25) |
| VLAS (v) | * 1.79 ± 0.29 (1.69–1.89) | 2.00 ± 0.32 (1.89–2.11) | 2.57 ± 0.35 (2.39–2.75) | 2.88 ± 0.55 (2.60–3.06) |
| M–VLAS (v) | * 2.47 ± 0.44 (2.32–2.62) | 2.78 ± 0.52 (2.60–2.96) | 3.54 ± 0.80 (3.13–3.95) | 4.26 ± 0.90 (3.97–4.55) |
| RLAD (v) | * 1.63 ± 0.35 (1.51–1.75) | 1.89 ± 0.39 (1.76–2.02) | 2.48 ± 0.43 (2.26–2.70) | 3.00 ± 0.49 (2.84–3.16) |
| TILAS | * 0.51 ± 0.08 (0.48–0.54) | 0.57 ± 0.14 (0.52–0.62) | 0.75 ± 0.13 (0.69–0.81) | 0.84 ± 0.18 (0.78–0.90) |
| MVD Dogs (C-B2/B1) | Cutoff | Se (%) | Sp (%) | FP (%) | FN (%) | YI (Se + Sp) − 1 | AUC |
|---|---|---|---|---|---|---|---|
| TILAS | 0.8 | 77 | 83 | 17 | 23 | 0.60 | 0.91 |
| VLAS (v) | 2.5 | 69 | 97 | 3 | 31 | 0.66 | 0.93 |
| M-VLAS (v) | 3.6 | 71 | 97 | 3 | 29 | 0.68 | 0.90 |
| RLAD (v) | 2.3 | 81 | 91 | 9 | 19 | 0.72 | 0.94 |
| Control Dogs and MVD Dogs | Cutoff | Se (%) | Sp (%) | FP (%) | FN (%) | YI (Se + Sp) − 1 | AUC |
|---|---|---|---|---|---|---|---|
| TILAS | 0.8 | 79 | 92 | 8 | 21 | 0.70 | 0.94 |
| VLAS (v) | 2.4 | 79 | 92 | 8 | 21 | 0.70 | 0.95 |
| M-VLAS (v) | 3.3 | 82 | 90 | 10 | 18 | 0.73 | 0.93 |
| RLAD (v) | 2.3 | 82 | 94 | 6 | 18 | 0.77 | 0.97 |
| Radiographic LAS Method | Study | n | MVD Stage | Cutoff | Sensitivity (%) | Specificity (%) | AUC |
|---|---|---|---|---|---|---|---|
| VLAS | Malcolm, 2018 [6] | 103 | 26 B2 22 C–D | ≥2.5 (LAE) | 67 | 84 | 0.84 |
| Poad, 2019 [32] | 70 | 34 B2 | >2.3 (B2) | 71.8 | 74.4 | 0.767 | |
| Duler, 2020 [30] | 183 | 32 B2 33 C–D | >2.3 (LAE) | 90.3 | 73.6 | 0.89 | |
| >2.5 (B2) | 69 | 85.7 | 0.84 | ||||
| Lam, 2021 [15] | 64 | 21 B2 21 C | ≥2.4 (LAE) | 80.5 | 96.6 | 0.95 | |
| Bagardi, 2021 [17] | 74 | 16 B2 24 C 3 D | ≥2.43 (LAE) | 66 | 88 | 0.82 | |
| Stepien, 2020 [14] | 56 | 30 B2 | ≥2.5 (B2) | 70 | 84 | 0.79 | |
| MIkawa, 2020 [7] | 97 | 19 B2 14 C–D | ≥2.5 (LHE) | 86 | 84 | 0.87 | |
| Vezzosi, 2021 [18] | 111 | 32 B2 32 C–D | ≥2.4 (B2) | 66 | 100 | 0.90 | |
| ≥2.2 (LAE) | 90 | 80 | 0.93 | ||||
| Levicar 2022 [19] | 200 | 50 B2 50 C–D | >2.3 (B2) | 72 | 78 | 0.81 | |
| * Wesselowski, 2022 [23] | 455 | 39 B1–B2 20 C–D | ≥2.2 (LAE, LVE or both) | 64.4 | 75.2 | 0.722 | |
| Lee, 2022 [8] | 41 | 17 CHF | >2.45 (CHF) | 93.3 | 47.6 | 0.71 | |
| ** Wesselowski, 2023 [33] | 226 | 45 B2 | >2.3 (LHE) | 51.1 | 92.3 | 0.783 | |
| Ross, 2023 [20] | 114 | 57 CHF | >2.3 (CHF) | 93 | 82.5 | 0.92 | |
| Vereb 2023 [21] | 869 | 234 B2 | >2.5 (CHF) | 62 | 80 | 0.76 | |
| Present study | 136 | 25 B2 40 C | ≥2.5 (LHE) | 69 | 97 | 0.93 | |
| RLAD | Salguero, 2017 [13] | 77 | 15B2 28 C | ≥1.8 (LAE) | 93.5 | 96.8 | 0.9691 |
| Levicar 2022 [19] | 200 | 50 B2 50 C–D | ≥2.0 (B2) | 75 | 83 | 0.85 | |
| Bagardi, 2021 [17] | 74 | 16 B2 24 C 3 D | ≥1.9 (LAE) | 71 | 82 | 0.81 | |
| Lam, 2021 [15] | 64 | 21 B2 21 C | ≥1.7 (LAE) | 100 | 72.4 | 0.93 | |
| Vezzosi, 2021 [18] | 111 | 32 B2 32 C–D | ≥1.8 (B2) | 100 | 93 | 0.99 | |
| ≥1.8 (LAE) | 90 | 95 | 0.98 | ||||
| Present study | 136 | 25 B2 40 C | ≥2.3 (LHE) | 81 | 91 | 0.94 | |
| M-VLAS | Lam, 2021 [15] | 64 | 21 B2 21 C | ≥3.4 | 92.7 | 93.1 | 0.97 |
| Vereb, 2023 [21] | 869 | 234 B2 | >3.5 (CHF) | 62 | 80 | 0.75 | |
| Present study | 136 | 25 B2 40 C | ≥3.6 (LHE) | 71 | 97 | 0.90 | |
| TILAS | Present study | 136 | 25 B2 40 C | ≥0.8 (LHE) | 77 | 83 | 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marbella Fernández, D.; Montoya-Alonso, J.A. Radiographic Left Atrial Size Measurement of Dogs in Different Mitral Valve Disease Stages with Four Different Methods. Animals 2023, 13, 3835. https://doi.org/10.3390/ani13243835
Marbella Fernández D, Montoya-Alonso JA. Radiographic Left Atrial Size Measurement of Dogs in Different Mitral Valve Disease Stages with Four Different Methods. Animals. 2023; 13(24):3835. https://doi.org/10.3390/ani13243835
Chicago/Turabian StyleMarbella Fernández, David, and Jose Alberto Montoya-Alonso. 2023. "Radiographic Left Atrial Size Measurement of Dogs in Different Mitral Valve Disease Stages with Four Different Methods" Animals 13, no. 24: 3835. https://doi.org/10.3390/ani13243835
APA StyleMarbella Fernández, D., & Montoya-Alonso, J. A. (2023). Radiographic Left Atrial Size Measurement of Dogs in Different Mitral Valve Disease Stages with Four Different Methods. Animals, 13(24), 3835. https://doi.org/10.3390/ani13243835







