Seasonal Activity of Adult Ticks Ixodes persulcatus (Acari, Ixodidae) in the North-West of the Distribution Area
Abstract
:Simple Summary
Abstract
1. Introduction
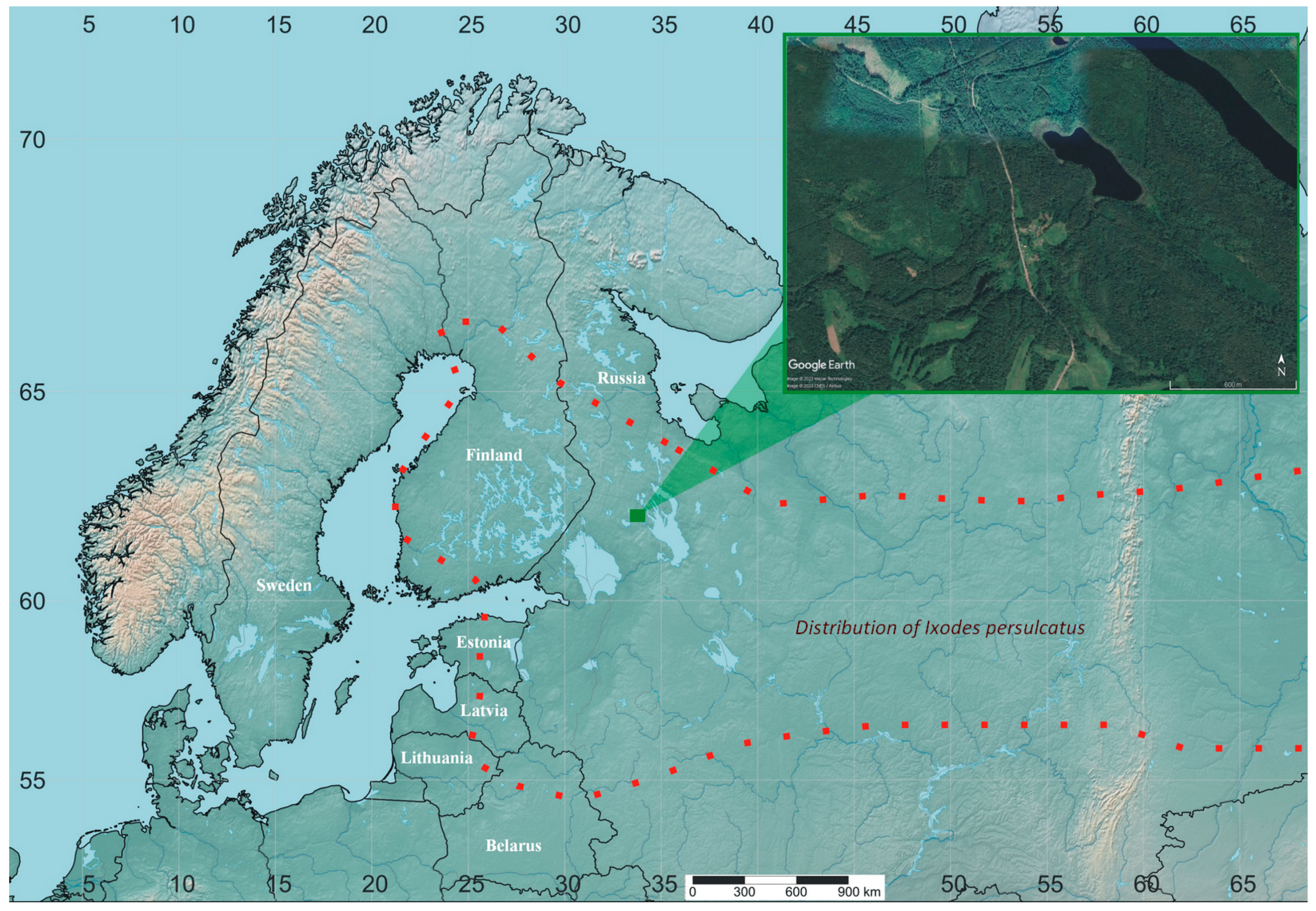
2. Materials and Methods
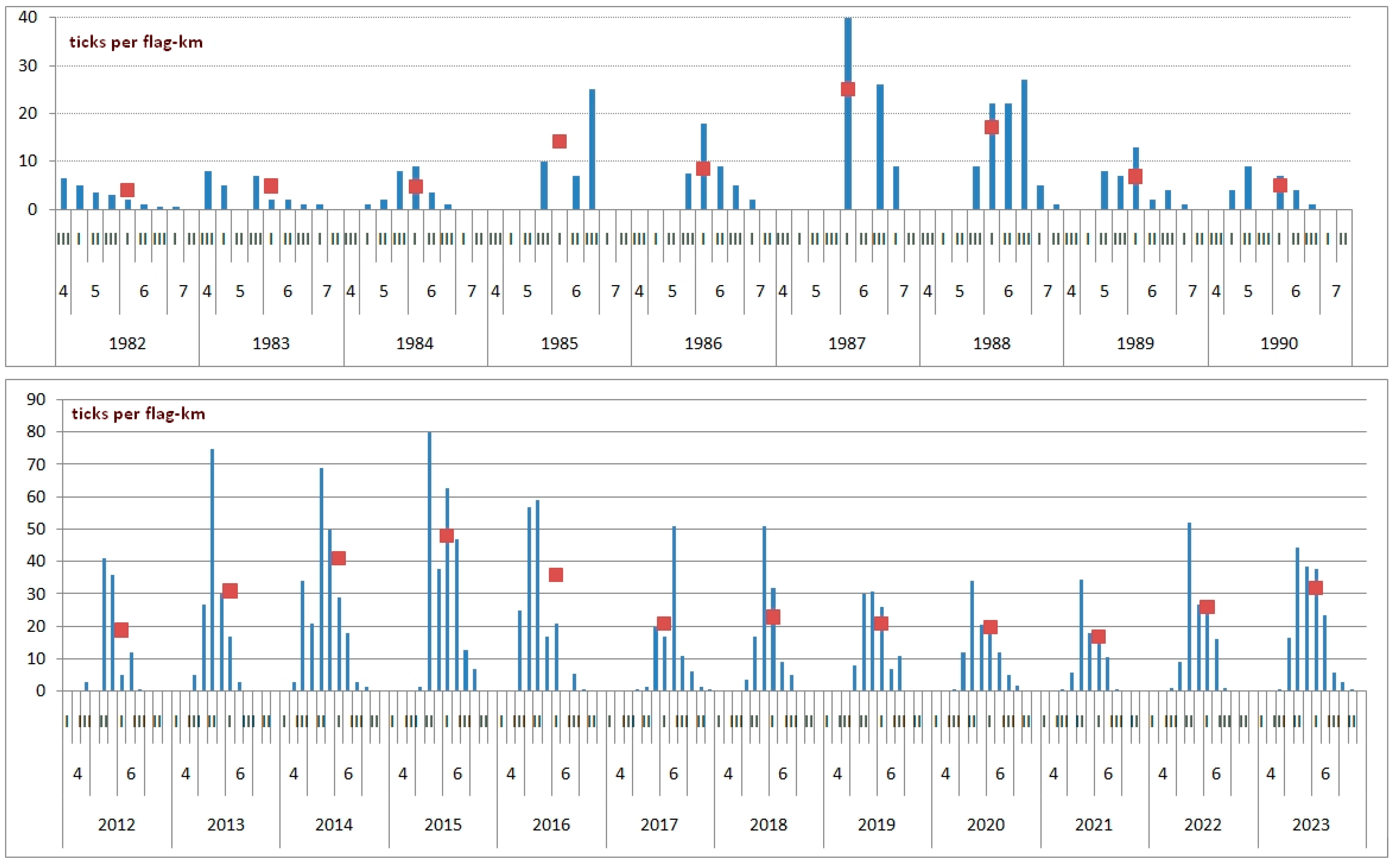
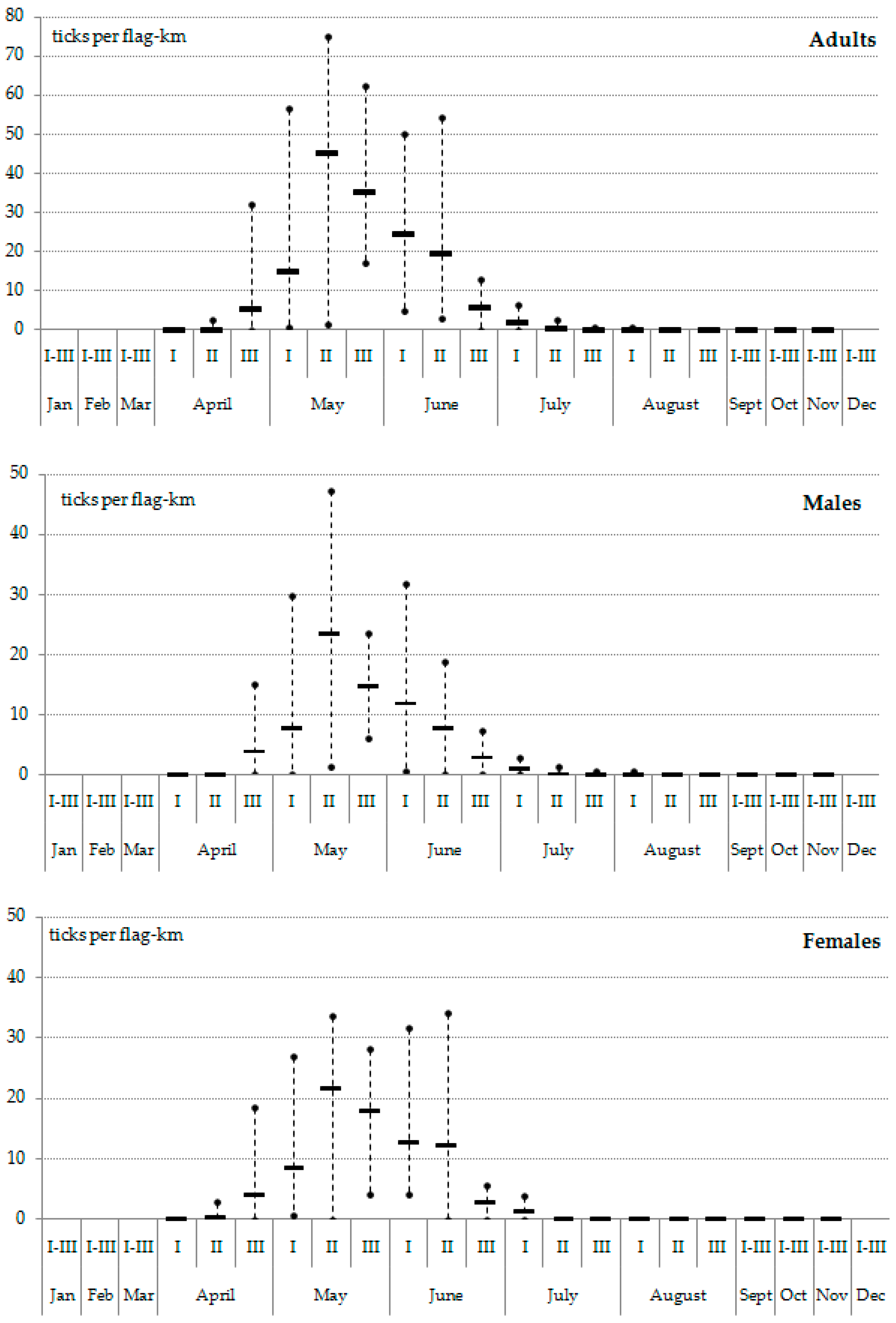
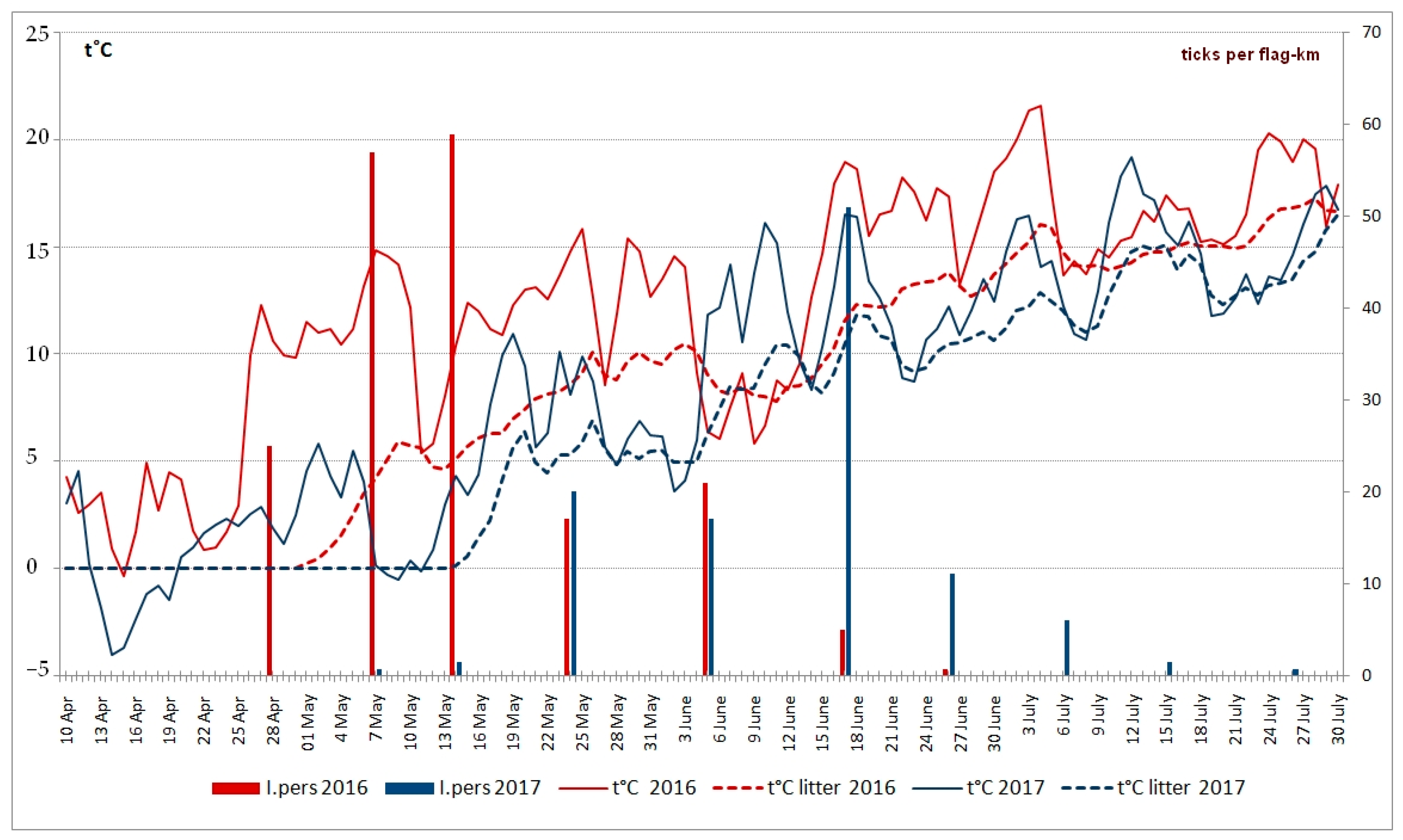
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balashov, Y.S. Parasite-Host Relationships of Arthropods with Terrestrial Vertebrates; Nauka: Leningrad, Russia, 1982. [Google Scholar]
- Filippova, N.A. Ixodid ticks of the subfamily Ixodinae. In Fauna of the USSR: Arachnoides; Nauka: Leningrad, Russia, 1977; Volume 4. [Google Scholar]
- Filippova, N.A. History of the species range of ixodid ticks, vectors of pathogens with natural nidality (Acari, Ixodidae), as a prerequisite of their intraspecific biodiversity. Entmol. Rev. 2017, 97, 255–275. [Google Scholar] [CrossRef]
- Korenberg, E.I.; Sirotkin, M.B.; Kovalevskii, Y.V. Adaptive Features of the Biology of Closely Related Species of Ixodid Ticks That Determine Their Distribution (Illustrated on the Example of the Taiga Tick Ixodes persulcatus Sch. 1930 and the Castor Bean Tick lxodes ricinus L. 1758). Biol. Bull. Rev. 2021, 11, 602–615. [Google Scholar] [CrossRef]
- Wang, S.S.; Liu, J.Y.; Wang, B.Y.; Wang, W.J.; Cui, X.M.; Jiang, J.F.; Sun, Y.; Guo, W.B.; Pan, Y.S.; Zhou, Y.H.; et al. Geographical Distribution of Ixodes persulcatus and Associated Pathogens: Analysis of Integrated Data from a China Field Survey and Global Published Data. One Health 2023, 16, 100508. [Google Scholar] [CrossRef]
- Babenko, L.V. On geographic variation in the seasonal dynamics of activity of Ixodes ricinus and I. persulcatus ticks and on factors responsible for long-term fluctuations of their abundance. Med. Parazitol. Parazit. Bolezn. 1958, 27, 639–653. [Google Scholar]
- Korenberg, E.I.; Lebedeva, N.N.; Zhukov, V.I. Geographic variation and types of seasonal activity of adult Ixodes persulcatus P. Sch. Ticks. Bull. Mosc. Soc. Nat. Biol. Ser. 1974, 79, 34–43. [Google Scholar]
- Babenko, L.V.; Repkina, L.V. Seasonal activity and duration of active life. In Taiga Tick Ixodes Persulcatus Schulze (Acarina, Ixodidae): Morphology, Systematics, Ecology, and Medical Significance; Filippova, N., Ed.; Nauka: Leningrad, Russia, 1985; pp. 218–238. [Google Scholar]
- Korenberg, E.I. Seasonal population dynamics of Ixodes ticks and tick-borne encephalitis virus. Exp. Appl. Acarol. 2000, 24, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Kheysin, E.M. Behaviour of adult Ixodes persulcatus P. Sch. depending on environmental temperature and humidity. Zool. Zhurnal 1953, 32, 77–87. [Google Scholar]
- Kheisin, E.M.; Pavlovskaya, O.; Malakhova, R.P.; Rybak, V.F. Duration of the developmental cycle of Ixodes persulcatus under natural conditions of the Karelo-Finnish SSR. Proc. Karelo-Finn. Univ. 1955, 6, 102–123. [Google Scholar]
- Kheisin, E.M. On the northern boundary of the range of ticks in the Karelian Finnish SSR. Zool. Zhurnal 1950, 29, 572–574. [Google Scholar] [PubMed]
- Lutta, A.S.; Shul’man, R.E. On the western boundary of Ixodes persulcatus distribution in the territory of Karelian-Finnish ASSR. Zool. Zhurnal 1954, 33, 1231–1235. [Google Scholar]
- Bugmyrin, S.V.; Bespyatova, L.A.; Korotkov, Y.S. Long-term dynamics of Ixodes persulcatus (Acari: Ixodidae) abundance in the north–west of its range (Karelia, Russia). Exp. Appl. Acarol. 2019, 77, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.T.; Värv, K.; Fröjdman, I.; Jääskeläinen, A.; Rundgren, K.; Versteirt, V.; Estrada-Peña, A.; Medlock, J.M.; Golovljova, I. First evidence of established populations of the taiga tick Ixodes persulcatus (Acari: Ixodidae) in Sweden. Parasites Vectors 2016, 9, 377. [Google Scholar] [CrossRef] [PubMed]
- Laaksonen, M.; Sajanti, E.; Sormunen, J.J.; Penttinen, R.; Hänninen, J.; Ruohomäki, K.; Sääksjärvi, I.; Vesterinen, E.J.; Vuorinen, I.; Hytönen, J.; et al. Crowdsourcing-based nationwide tick collection reveals the distribution of Ixodes ricinus and I. persulcatus and associated pathogens in Finland. Emerg. Microbes Infect. 2017, 6, e31. [Google Scholar] [CrossRef] [PubMed]
- Tokarevich, N.K.; Tronin, A.A.; Blinova, O.V.; Buzinov, R.V.; Boltenkov, V.P.; Yurasova, E.D.; Nurse, J. The impact of climate change on the expansion of Ixodes persulcatus habitat and the incidence of tick-borne encephalitis in the north of European Russia. Glob. Health Action 2011, 4, 8448. [Google Scholar] [CrossRef] [PubMed]
- Bugmyrin, S.V.; Romanova, L.Y.; Belova, O.A.; Kholodilov, I.S.; Bespyatova, L.A.; Chernokhaeva, L.L.; Gmyl, L.V.; Klimentov, A.S.; Ivannikova, A.Y.; Polienko, A.E.; et al. Pathogens in Ixodes persulcatus and Ixodes ricinus ticks (Acari, Ixodidae) in Karelia (Russia). Ticks Tick Borne Dis. 2022, 13, 102045. [Google Scholar] [CrossRef]
- Bugmyrin, S.V.; Poutonen, T.B.; Pakhomova, T.N.; Bespyatova, L.A.; Chevskaya, V.E.; Kocherova, N.A. Ticks and tick-borne infections in Karelia: Analysis of ticks brought by citizens to be tested at the center for hygiene and epidemiology in the Republic of Karelia (Petrozavodsk). Parazitologia 2023, 57, 3–19. [Google Scholar]
- Shorthouse, D.P. SimpleMappr, an Online Tool to Produce Publication-Quality Point Maps. 2010. Available online: https://www.simplemappr.net (accessed on 19 November 2023).
- Pakanen, V.M.; Sormunen, J.J.; Sippola, E.; Blomqvist, D.; Kallio, E.R. Questing abundance of adult taiga ticks Ixodes persulcatus and their Borrelia prevalence at the north-western part of their distribution. Parasites Vectors 2020, 13, 384. [Google Scholar] [CrossRef]
- Romanenko, V.; Leonovich, S. Long-term monitoring and population dynamics of ixodid ticks in Tomsk city (Western Siberia). Exp. Appl. Acarol. 2015, 66, 103–118. [Google Scholar] [CrossRef]
- Zemskaya, A.A. Seasonal activity of adult ticks Ixodes persulcatus P. Sch in the eastern part of the Russian plain. Folia Parasitol. 1984, 31, 269–276. [Google Scholar]
- Belozerov, V.N. Ecological rhythms in ixodid ticks and their regulation. Parazitol. Sb. 1981, 30, 22–46. [Google Scholar]
- Gray, J.S.; Kahl, O.; Lane, R.S.; Levin, M.L.; Tsao, J.I. Diapause in ticks of the medically important Ixodes ricinus species complex. Ticks Tick Borne Dis. 2016, 7, 992–1003. [Google Scholar] [CrossRef]
- Bugmyrin, S.V.; Gorbach, V.V. Mark-release-recapture of ticks: A case study of estimating the abundance of Ixodes persulcatus (Acari, Ixodidae). Med. Vet. Entomol. 2022, 36, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Skorokhodova, S.B. Nature calendar (1966–2005) of «Kivach» Reserve. Proc. State Nat. Reserve 2006, 3, 48–79. [Google Scholar]
- Ovaskainen, O.; Skorokhodova, S.; Yakovleva, M.; Sukhov, A.; Kutenkov, A.; Kutenkova, N.; Shcherbakov, A.; Meyke, E.; Delgado, M.M. Community-level phenological response to climate change. Proc. Natl. Acad. Sci. USA 2013, 110, 13434–13439. [Google Scholar] [CrossRef] [PubMed]
- Skorohodova, S.B.; Scsherbakov, A.N. Trends of phenological phenomena beginning in Kivach Reserve during the 1966–2005 years. Proc. State Nat. Reserve 2011, 5, 207–221. [Google Scholar]
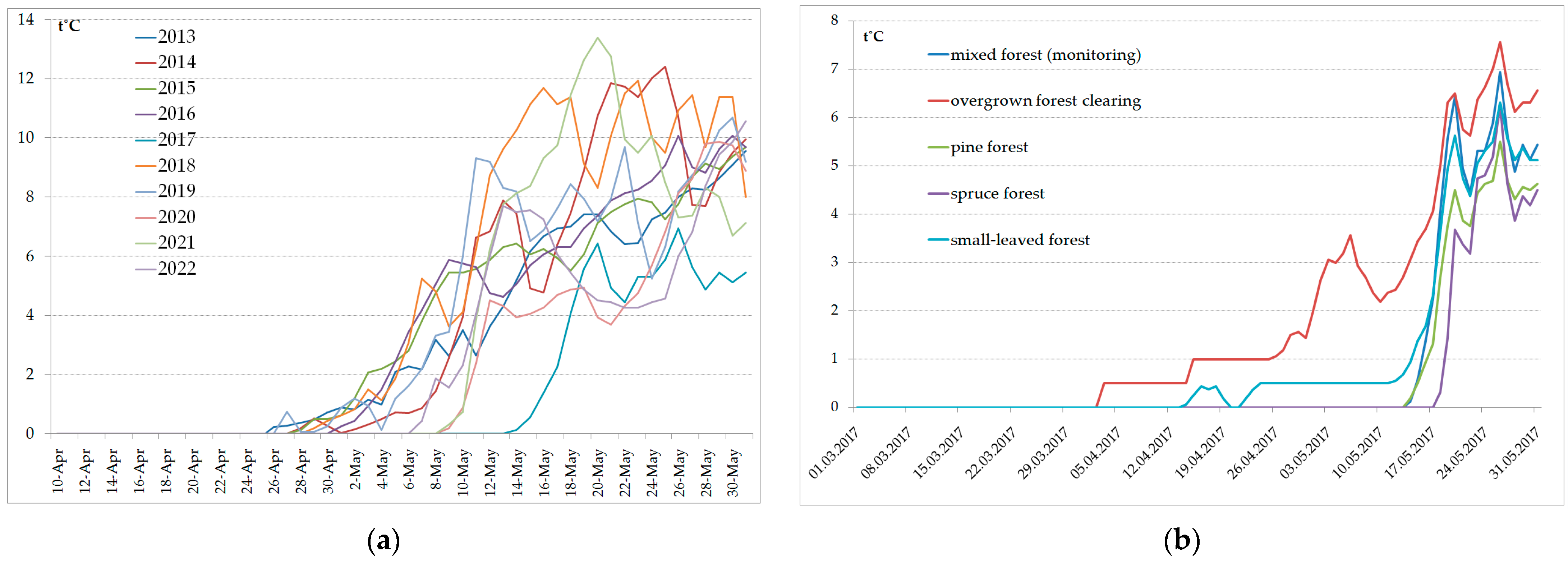
| Year | Beginning of Activity | Peak of Activity | ||||
|---|---|---|---|---|---|---|
| Ten-Day Period/Month | Temperature, °C | Ten-Day Period/Month | Temperature, °C | |||
| Litter | Air | Litter | Air | |||
| 2013 | III/04 | 2 | 6 | II/05 | 6 | 13 |
| 2014 | II/04 | 0 | 5 | II/05 | 7 | 12 |
| 2015 | I/05 | 3 | 8 | II/05 | 6 | 9 |
| 2016 | III/04 | 0 | 6 | II/05 | 6 | 10 |
| 2017 | I/05 | 0 | 3 | II/06 | 10 | 13 |
| 2018 | I/05 | 3 | 8 | III/05 | 11 | 14 |
| 2019 | I/05 | 2 | 7 * | III/05 | 8 | 10 |
| 2020 | III/04 | 0 | 2 * | II/05 | 4 | 5 * |
| 2021 | III/04 | 0 | 3 * | II/05 | 7 | 15 * |
| 2022 | III/04 | 0 | 3 * | II/05 | 6 | 8 * |
| 2023 | III/04 | 2 | 7 * | II/05 | 10 | 12 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bugmyrin, S.V.; Bespyatova, L.A. Seasonal Activity of Adult Ticks Ixodes persulcatus (Acari, Ixodidae) in the North-West of the Distribution Area. Animals 2023, 13, 3834. https://doi.org/10.3390/ani13243834
Bugmyrin SV, Bespyatova LA. Seasonal Activity of Adult Ticks Ixodes persulcatus (Acari, Ixodidae) in the North-West of the Distribution Area. Animals. 2023; 13(24):3834. https://doi.org/10.3390/ani13243834
Chicago/Turabian StyleBugmyrin, Sergey V., and Lyubov A. Bespyatova. 2023. "Seasonal Activity of Adult Ticks Ixodes persulcatus (Acari, Ixodidae) in the North-West of the Distribution Area" Animals 13, no. 24: 3834. https://doi.org/10.3390/ani13243834
APA StyleBugmyrin, S. V., & Bespyatova, L. A. (2023). Seasonal Activity of Adult Ticks Ixodes persulcatus (Acari, Ixodidae) in the North-West of the Distribution Area. Animals, 13(24), 3834. https://doi.org/10.3390/ani13243834






