Phage-Based Biosanitation Strategies for Minimizing Persistent Salmonella and Campylobacter Bacteria in Poultry
Abstract
Simple Summary
Abstract
1. Introduction
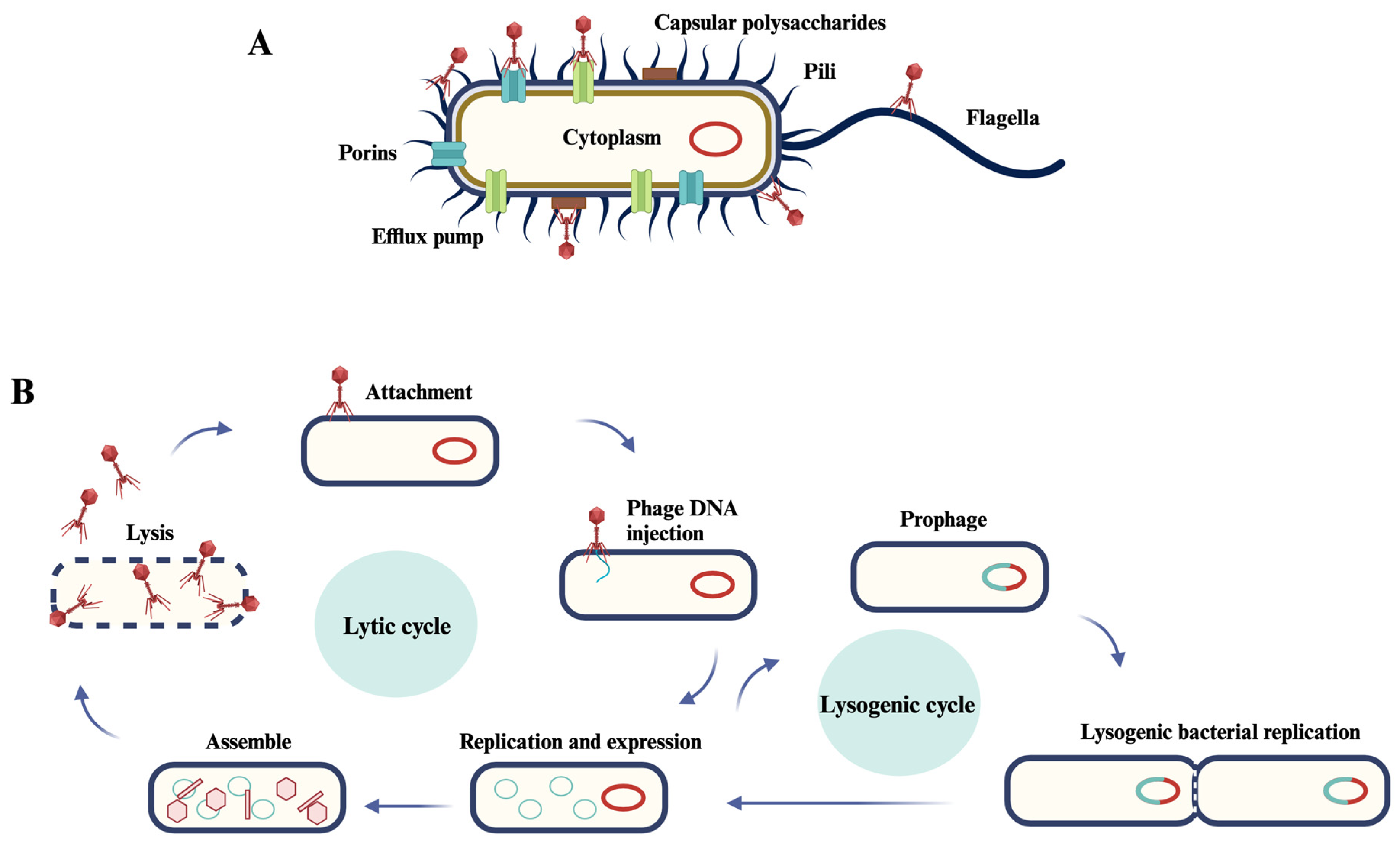
2. General Considerations about Phages
3. Current Utilities and Legislations in the Use of Phage in Poultry
4. Current Characteristics of Cleaning and Disinfection Used in Poultry Production and the Main Challenges: Biofilms
5. Biofilms and Bacteriophages
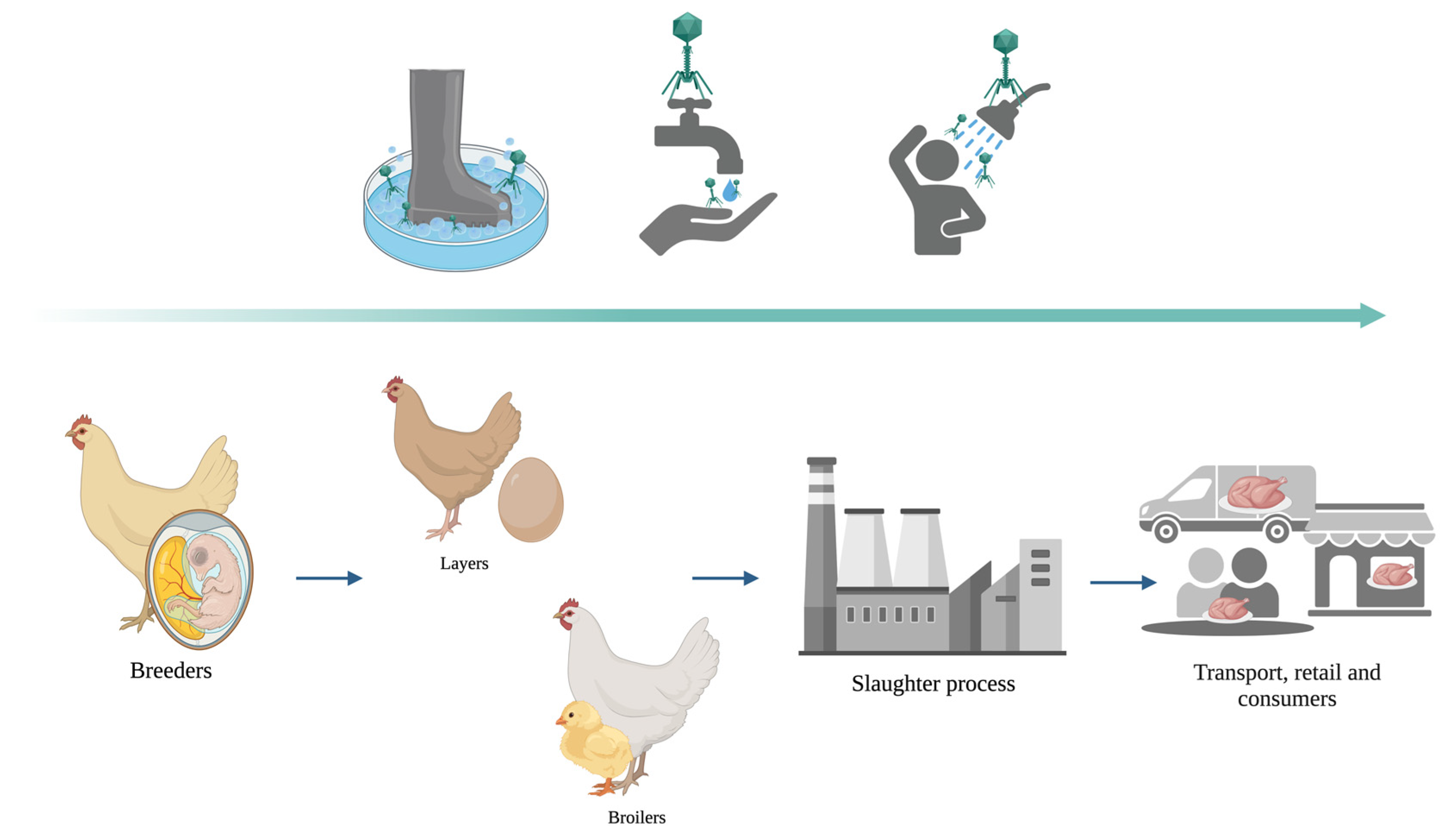
6. The Role of on-Farm Use of Bacteriophages as Disinfectants
Bacteriophages as Disinfectants in Poultry Pre-Harvest Stages
7. Bacteriophages as Disinfectants in Poultry Post-Harvest Stages
8. Main Limitations to the Use of Bacteriophages
8.1. Limitation Due to the Phage of Choice
8.2. Lysogenous Forms
8.3. Legal Limitations
8.4. Need for Purification and Stabilization
8.5. Dosage
8.6. Terms of Use
8.7. Resistance Mechanisms
8.8. Effectiveness
- Food matrix: In solid foods, it will depend on the ability of the food to adsorb the phage suspension and that it is not diluted [193]. One solution that would avoid this problem would be phage immobilization. In plant compounds, substances such as organic acids and tannins would inactivate bacteriophages [193,194,195].
- Combination with other control measures: Although phages are a good alternative for biocontrol, they do not usually achieve a complete elimination of the pathogen, so it is always recommended that they be applied together with other measures [202].
9. Key Insights into Bacteriophage Application in Poultry Farming
9.1. Pathogen Threat
9.2. Emerging Phage Technology
9.3. Persistent Bacteria and Phage Solutions
9.4. Biofilm Challenge
9.5. Multifaceted Phage Approach
9.6. Effective Phage-Based Treatment
9.7. Main Phage Limitations
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA; ECDC. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2019–2020. EFSA J. 2022, 20, e07209. [Google Scholar] [CrossRef]
- World Health Organization. Food Safety. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 1 August 2023).
- Sosnowski, M.; Osek, J. Microbiological safety of food of animal origin from organic farms. J. Vet. Res. 2021, 65, 87–92. [Google Scholar] [CrossRef]
- Stewart, J.; Pavic, A. Advances in enteropathogen control throughout the meat chicken production chain. Compr. Rev. Food Sci. Food Saf. 2023, 22, 2346–2407. [Google Scholar] [CrossRef] [PubMed]
- Venkitanarayanan, K.; Thakur, S.; Ricke, S.C. Salmonella in Poultry Meat Production. In Food Safety in Poultry Meat Production; Springer International Publishing AG: Cham, Switzerland, 2019; pp. 1–24. [Google Scholar]
- Liu, B.; Zhang, X.; Ding, X.; Bin, P.; Zhu, G. The vertical transmission of Salmonella Enteritidis in a One-Health context. One Health 2023, 16, 100469. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The Global Burden of Nontyphoidal Salmonella Gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Andino, A.; Hanning, I. Salmonella enterica: Survival, Colonization, and Virulence Differences among Serovars. Sci. World J. 2015, 2015, 520179. [Google Scholar] [CrossRef] [PubMed]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Salmonella infection–prevention and treatment by antibiotics and probiotic yeasts: A review. Microbiology 2018, 164, 1327–1344. [Google Scholar] [CrossRef]
- Teklemariam, A.D.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Alghamdi, M.A.; Filimban, A.A.R.; Al Mutiri, A.S.; Al-Alyani, A.M.; Alseghayer, M.S.; Almaneea, A.M.; et al. Human Salmonellosis: A Continuous Global Threat in the Farm-to-Fork Food Safety Continuum. Foods 2023, 12, 1756. [Google Scholar] [CrossRef]
- Montoro-Dasi, L.; Lorenzo-Rebenaque, L.; Marco-Fuertes, A.; Vega, S.; Marin, C. Holistic Strategies to Control Salmonella Infantis: An Emerging Challenge in the European Broiler Sector. Microorganisms 2023, 11, 1765. [Google Scholar] [CrossRef]
- Gast, R.K.; Dittoe, D.K.; Ricke, S.C. Salmonella in eggs and egg-laying chickens: Pathways to effective control. Crit. Rev. Microbiol. 2022, 1–25, ahead-of-print. [Google Scholar] [CrossRef]
- Altekruse, S.; Koehler, J.; Hickman-Brenner, F.; Tauxe, R.V.; Ferris, K. A comparison of Salmonella enteritidis phage types from egg-associated outbreaks and implicated laying flocks. Epidemiol. Infect. 1993, 110, 17–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. Campylobacter. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/campylobacter (accessed on 1 August 2023).
- Taha-Abdelaziz, K.; Singh, M.; Sharif, S.; Sharma, S.; Kulkarni, R.R.; Alizadeh, M.; Yitbarek, A.; Helmy, Y.A. Intervention Strategies to Control Campylobacter at Different Stages of the Food Chain. Microorganisms 2023, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.E.; Corcoran, D.; Dooley, J.S.G.; Fanning, S.; Lucey, B.; Matsuda, M.; McDowell, D.A.; Mégraud, F.; Millar, B.C.; O’Mahony, R.; et al. Campylobacter. Vet. Res. 2005, 36, 351–382. [Google Scholar] [CrossRef]
- Hermans, D.; Pasmans, F.; Messens, W.; Martel, A.; Van Immerseel, F.; Rasschaert, G.; Heyndrickx, M.; Van Deun, K.; Haesebrouck, F. Poultry as a Host for the Zoonotic Pathogen Campylobacter jejuni. Vector Borne Zoonotic Dis. 2012, 12, 89–98. [Google Scholar] [CrossRef]
- Lee, M.D.; Newell, D.G. Campylobacter in Poultry: Filling an Ecological Niche. Avian Dis. 2006, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, J.A.; French, N.P.; Havelaar, A.H. Preventing Campylobacter at the Source: Why Is It So Difficult? Clin. Infect. Dis. 2013, 57, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Balasch, S.; Vega, S.; Lainez, M. Sources of Salmonella contamination during broiler production in Eastern Spain. Prev. Vet. Med. 2011, 98, 39–45. [Google Scholar] [CrossRef]
- Thames, H.T.; Theradiyil Sukumaran, A. A Review of Salmonella and Campylobacter in Broiler Meat: Emerging Challenges and Food Safety Measures. Foods 2020, 9, 776. [Google Scholar] [CrossRef]
- Davies, R.H.; Wray, C. Observations on Disinfection Regimens Used on Salmonella enteritidis Infected Poultry Units. Poult. Sci. 1995, 74, 638–647. [Google Scholar] [CrossRef]
- Jassim, S.A.A.; Limoges, R.G. Bacteriophages: Practical Applications for Nature’s Biocontrol; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Gildea, L.; Ayariga, J.A.; Robertson, B.K. Bacteriophages as Biocontrol Agents in Livestock Food Production. Microorganisms 2022, 10, 2126. [Google Scholar] [CrossRef]
- Andreatti Filho, R.L.; Higgins, J.P.; Higgins, S.E.; Gaona, G.; Wolfenden, A.D.; Tellez, G.; Hargis, B.M. Ability of Bacteriophages Isolated from Different Sources to Reduce Salmonella enterica Serovar Enteritidis In Vitro and In Vivo. Poult. Sci. 2007, 86, 1904–1909. [Google Scholar] [CrossRef]
- Borie, C.; Sánchez, M.L.; Navarro, C.; Ramírez, S.; Morales, M.A.; Retamales, J.; Robeson, J. Aerosol Spray Treatment with Bacteriophages and Competitive Exclusion Reduces Salmonella Enteritidis Infection in Chickens. Avian Dis. 2009, 53, 250–254. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.W.; Shin, H.S.; Kim, M.C.; Lee, J.H.; Kim, G.-B.; Kil, D.Y. Effect of dietary supplementation of bacteriophage on performance, egg quality and caecal bacterial populations in laying hens. Br. Poult. Sci. 2015, 56, 132–136. [Google Scholar] [CrossRef]
- Grabowski, Ł.; Węgrzyn, G.; Węgrzyn, A.; Podlacha, M. Highly different effects of phage therapy and antibiotic therapy on immunological responses of chickens infected with Salmonella enterica serovar Typhimurium. Front. Immunol. 2022, 13, 956833. [Google Scholar] [CrossRef]
- Lorenzo-Rebenaque, L.; Malik, D.J.; Catalá-Gregori, P.; Torres-Boncompte, J.; Marin, C.; Sevilla-Navarro, S. Microencapsulated bacteriophages incorporated in feed for Salmonella control in broilers. Vet. Microbiol. 2022, 274, 109579. [Google Scholar] [CrossRef] [PubMed]
- Peh, E.; Szott, V.; Reichelt, B.; Friese, A.; Rösler, U.; Plötz, M.; Kittler, S. Bacteriophage cocktail application for Campylobacter mitigation—From in vitro to in vivo. BMC Microbiol. 2023, 23, 209. [Google Scholar] [CrossRef] [PubMed]
- Sarrami, Z.; Sedghi, M.; Mohammadi, I.; Bedford, M.; Miranzadeh, H.; Ghasemi, R. Effects of bacteriophage on Salmonella Enteritidis infection in broilers. Sci. Rep. 2023, 13, 12198. [Google Scholar] [CrossRef]
- Thanki, A.M.; Hooton, S.; Whenham, N.; Salter, M.G.; Bedford, M.R.; O’Neill, H.V.M.; Clokie, M.R.J. A bacteriophage cocktail delivered in feed significantly reduced Salmonella colonization in challenged broiler chickens. Emerg. Microbes Infect. 2023, 12, 2217947. [Google Scholar] [CrossRef] [PubMed]
- Bordet, J.; Ciuca, M. Remarques sur l’historique de recherches concernant la lyse microbienne transmisible. Compt. Rend. Soc. Biol. 1921, 745–747. [Google Scholar]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Weinbauer, M.G. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 2004, 28, 127–181. [Google Scholar] [CrossRef] [PubMed]
- Skurnik, M.; Strauch, E. Phage therapy: Facts and fiction. Int. J. Med. Microbiol. 2006, 296, 5–14. [Google Scholar] [CrossRef]
- Favrin, S.J.; Jassim, S.A.; Griffiths, M.W. Development and Optimization of a Novel Immunomagnetic Separation- Bacteriophage Assay for Detection of Salmonella enterica Serovar Enteritidis in Broth. Appl. Environ. Microbiol. 2001, 67, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Proença, D.; Cantante, C.; Silva, F.A.; Leandro, C.; Lourenço, S.; Milheiriço, C.; de Lencastre, H.; Cavaco-Silva, P.; Pimentel, M.; et al. Novel Chimerical Endolysins with Broad Antimicrobial Activity Against Methicillin-Resistant Staphylococcus aureus. Microb. Drug Resist. 2012, 18, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Ross, R.P.; Coffey, A. Bacteriophage and their lysins for elimination of infectious bacteria. FEMS Microbiol. Rev. 2009, 33, 801–819. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Annapure, U.S. Bacteriophage biocontrol of foodborne pathogens. J. Food Sci. Technol. 2016, 53, 1355–1362. [Google Scholar] [CrossRef]
- Domingo-Calap, P.; Georgel, P.; Bahram, S. Back to the future: Bacteriophages as promising therapeutic tools. HLA 2016, 87, 133–140. [Google Scholar] [CrossRef]
- Cisek, A.A.; Dąbrowska, I.; Gregorczyk, K.P.; Wyżewski, Z. Phage Therapy in Bacterial Infections Treatment: One Hundred Years after the Discovery of Bacteriophages. Curr. Microbiol. 2017, 74, 277–283. [Google Scholar] [CrossRef]
- Grabowski, Ł.; Łepek, K.; Stasiłojć, M.; Kosznik-Kwaśnicka, K.; Zdrojewska, K.; Maciąg-Dorszyńska, M.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage-encoded enzymes destroying bacterial cell membranes and walls, and their potential use as antimicrobial agents. Microbiol. Res. 2021, 248, 126746. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, W.; Zhang, Z.; Gu, Y.; Huang, A.; Wang, J.; Hao, H.; Huang, Y.; Wang, W.; Zhang, Z.; et al. Phage Products for Fighting Antimicrobial Resistance. Microorganisms 2022, 10, 1324. [Google Scholar] [CrossRef] [PubMed]
- Endersen, L.; O’Mahony, J.; Hill, C.; Ross, R.P.; McAuliffe, O.; Coffey, A. Phage therapy in the food industry. Annu. Rev. Food Sci. Technol. 2014, 5, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, Z.; Abedon, S.T. Diversity of phage infection types and associated terminology: The problem with ‘Lytic or lysogenic’. FEMS Microbiol. Lett. 2016, 363, fnw047. [Google Scholar] [CrossRef] [PubMed]
- Brix, A.; Cafora, M.; Aureli, M.; Pistocchi, A. Animal Models to Translate Phage Therapy to Human Medicine. Int. J. Mol. Sci. 2020, 21, 3715. [Google Scholar] [CrossRef] [PubMed]
- Verheust, C.; Pauwels, K.; Mahillon, J.; Helinski, D.R.; Herman, P. Contained use of Bacteriophages: Risk Assessment and Biosafety Recommendations. Appl. Biosaf. 2010, 15, 32–44. [Google Scholar] [CrossRef]
- Jamal, M.; Bukhari, S.M.A.U.S.; Andleeb, S.; Ali, M.; Raza, S.; Nawaz, M.A.; Hussain, T.; Rahman, S.U.; Shah, S.S.A. Bacteriophages: An overview of the control strategies against multiple bacterial infections in different fields. J. Basic Microbiol. 2019, 59, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Sklar, I.B.; Joerger, R.D. Attempts to utilize bacteriophage to combat Salmonella enterica serovar enteritidis infection in chickens. J. Food Saf. 2001, 21, 15–29. [Google Scholar] [CrossRef]
- Fiorentin, L.; Vieira, N.D.; Barioni, W.J. Oral treatment with bacteriophages reduces the concentration of Salmonella Enteritidis PT4 in caecal contents of broilers. Avian Pathol. 2005, 34, 258–263. [Google Scholar] [CrossRef]
- Sorour, H.K.; Gaber, A.F.; Hosny, R.A. Evaluation of the efficiency of using Salmonella Kentucky and Escherichia coli O119 bacteriophages in the treatment and prevention of salmonellosis and colibacillosis in broiler chickens. Lett. Appl. Microbiol. 2020, 71, 345–350. [Google Scholar] [CrossRef]
- Loc Carrillo, C.; Atterbury, R.J.; El-Shibiny, A.; Connerton, P.L.; Dillon, E.; Scott, A.; Connerton, I.F. Bacteriophage Therapy to Reduce Campylobacter jejuni Colonization of Broiler Chickens. Appl. Environ. Microbiol. 2005, 71, 6554–6563. [Google Scholar] [CrossRef]
- Wagenaar, J.A.; Bergen, M.A.P.V.; Mueller, M.A.; Wassenaar, T.M.; Carlton, R.M. Phage therapy reduces Campylobacter jejuni colonization in broilers. Vet. Microbiol. 2005, 109, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Gannon, B.W.; Halfhide, D.E.; Santos, S.B.; Hayes, C.M.; Roe, J.M.; Azeredo, J. The in vivo efficacy of two administration routes of a phage cocktail to reduce numbers of Campylobacter coli and Campylobacter jejuni in chickens. BMC Microbiol. 2010, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Kittler, S.; Fischer, S.; Abdulmawjood, A.; Glünder, G.; Klein, G. Effect of Bacteriophage Application on Campylobacter jejuni Loads in Commercial Broiler Flocks. Appl. Environ. Microbiol. 2013, 79, 7525–7533. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.J.; Connerton, P.L.; Connerton, I.F. Phage Biocontrol of Campylobacter jejuni in Chickens Does Not Produce Collateral Effects on the Gut Microbiota. Front. Microbiol. 2019, 10, 476. [Google Scholar] [CrossRef]
- Barrow, P.; Lovell, M.; Berchieri, J. A Use of Lytic Bacteriophage for Control of Experimental Escherichia coli Septicemia and Meningitis in Chickens and Calves. Clin. Diagn. Lab. Immunol. 1998, 5, 294–298. [Google Scholar] [CrossRef]
- Huff, W.E.; Huff, G.R.; Rath, N.C.; Balog, J.M.; Xie, H.; Moore, P.A., Jr.; Donoghue, A.M. Prevention of Escherichia coli Respiratory Infection in Broiler Chickens with Bacteriophage (SPR02). Poult. Sci. 2002, 81, 437–441. [Google Scholar] [CrossRef]
- El-Gohary, F.A.; Huff, W.E.; Huff, G.R.; Rath, N.C.; Zhou, Z.Y.; Donoghue, A.M. Environmental augmentation with bacteriophage prevents colibacillosis in broiler chickens. Poult. Sci. 2014, 93, 2788–2792. [Google Scholar] [CrossRef]
- Miller, R.W.; Skinner, J.; Sulakvelidze, A.; Mathis, G.F.; Hofacre, C.L. Bacteriophage Therapy for Control of Necrotic Enteritis of Broiler Chickens Experimentally Infected with Clostridium perfringens. Avian Dis. 2010, 54, 33–40. [Google Scholar] [CrossRef]
- Bae, D.; Lee, J.W.; Chae, J.P.; Kim, J.W.; Eun, J.S.; Lee, K.W.; Seo, K.H. Characterization of a novel bacteriophage φCJ22 and its prophylactic and inhibitory effects on necrotic enteritis and Clostridium perfringens in broilers. Poult. Sci. 2021, 100, 302. [Google Scholar] [CrossRef]
- Keerqin, C.; McGlashan, K.; Van, T.T.H.; Chinivasagam, H.N.; Moore, R.J.; Choct, M.; Wu, S.B. A lytic bacteriophage isolate reduced Clostridium perfringens induced lesions in necrotic enteritis challenged broilers. Front. Vet. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Adhikari, P.A.; Cosby, D.E.; Cox, N.A.; Lee, J.H.; Kim, W.K. Effect of dietary bacteriophage supplementation on internal organs, fecal excretion, and ileal immune response in laying hens challenged by Salmonella Enteritidis. Poult. Sci. 2017, 96, 3264–3271. [Google Scholar] [CrossRef]
- Sevilla-Navarro, S.; Marín, C.; Cortés, V.; García, C.; Vega, S.; Catalá-Gregori, P. Autophage as a control measure for Salmonella in laying hens. Poult. Sci. 2018, 97, 4367–4373. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Hosseindoust, A.; Oh, S.M.; Ko, H.S.; Cho, E.S.; Sa, S.J.; Kim, Y.I.; Choi, J.W.; Kim, J.S. Impact of an anti-Salmonella. Typhimurium Bacteriophage on intestinal microbiota and immunity status of laying hens. J. Anim. Physiol. Anim. Nutr. 2021, 105, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Evran, S.; Tayyarcan, E.K.; Acar-Soykut, E.; Boyaci, I.H. Applications of Bacteriophage Cocktails to Reduce Salmonella Contamination in Poultry Farms. Food Environ. Virol. 2022, 14. [Google Scholar] [CrossRef]
- Duc, H.M.; Son, H.M.; Honjoh, K.; Miyamoto, T. Isolation and application of bacteriophages to reduce Salmonella contamination in raw chicken meat. Food Sci. Technol. 2018, 91, 353–360. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.J.; Jung, S.J.; Mizan, M.F.R.; Park, S.H.; Ha, S.D. Characterization of Salmonella spp.-specific bacteriophages and their biocontrol application in chicken breast meat. J. Food Sci. 2020, 85, 526–534. [Google Scholar] [CrossRef]
- Noor Mohammadi, T.; Shen, C.; Li, Y.; Zayda, M.G.; Sato, J.; Masuda, Y.; Honjoh, K.i.; Miyamoto, T. Characterization of Clostridium perfringens bacteriophages and their application in chicken meat and milk. Int. J. Food Microbiol. 2022, 361. [Google Scholar] [CrossRef]
- Tian, R.; Xu, S.; Li, P.; Li, M.; Liu, Y.; Wang, K.; Liu, G.; Li, Y.; Dai, L.; Zhang, W. Characterization of G-type Clostridium perfringens bacteriophages and their disinfection effect on chicken meat. Anaerobe 2023, 81, 102736. [Google Scholar] [CrossRef]
- Sharma, C.S.; Dhakal, J.; Nannapaneni, R. Efficacy of Lytic Bacteriophage Preparation in Reducing Salmonella In Vitro, on Turkey Breast Cutlets, and on Ground Turkey. J. Food Prot. 2015, 78, 1357–1362. [Google Scholar] [CrossRef]
- Sonalika, J.; Srujana, A.S.; Akhila, D.S.; Juliet, M.R.; Santhosh, K.S. Application of bacteriophages to control Salmonella Enteritidis in raw eggs. Iran. J. Vet. Res. 2020, 21, 221. [Google Scholar] [CrossRef]
- Azari, R.; Yousefi, M.H.; Taghipour, Z.; Wagemans, J.; Lavigne, R.; Hosseinzadeh, S.; Mazloomi, S.M.; Vallino, M.; Khalatbari-Limaki, S.; Berizi, E. Application of the lytic bacteriophage Rostam to control Salmonella enteritidis in eggs. Int. J. Food Microbiol. 2023, 389, 110097. [Google Scholar] [CrossRef] [PubMed]
- Abd-El Wahab, A.; Basiouni, S.; El-Seedi, H.R.; Ahmed, M.F.E.; Bielke, L.R.; Hargis, B.; Tellez-Isaias, G.; Eisenreich, W.; Lehnherr, H.; Kittler, S.; et al. An overview of the use of bacteriophages in the poultry industry: Successes, challenges, and possibilities for overcoming breakdowns. Front. Microbiol. 2023, 14, 1136638. [Google Scholar] [CrossRef] [PubMed]
- Verbeken, G.; Pirnay, J.P.; De Vos, D.; Jennes, S.; Zizi, M.; Lavigne, R.; Casteels, M.; Huys, I. Optimizing the European regulatory framework for sustainable bacteriophage therapy in human medicine. Arch. Immunol. Ther. Exp. 2012, 60, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Naureen, Z.; Malacarne, D.; Anpilogov, K.; Dautaj, A.; Camilleri, G.; Cecchin, S.; Bressan, S.; Casadei, A.; Albion, E.; Sorrentino, E.; et al. Comparison between American and European legislation in the therapeutical and alimentary bacteriophage usage. Acta Bio Medica Atenei Parm. 2020, 91, 2020023. [Google Scholar] [CrossRef]
- Diallo, K.; Dublanchet, A. A Century of Clinical Use of Phages: A Literature Review. Antibiotics 2023, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.J.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Bacteriophage Delivery Systems for Food Applications: Opportunities and Perspectives. Viruses 2023, 15, 1271. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, H.; Ji, X.; Yan, G.; Lei, L.; Han, W.; Gu, J.; Huang, J. Therapeutic applications of lytic phages in human medicine. Microb. Pathog. 2020, 142, 104048. [Google Scholar] [CrossRef]
- Efsa, P.B.H. Evaluation of the safety and efficacy of Listex™ P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA J. 2016, 14, e04565. [Google Scholar] [CrossRef]
- Winfield, M.D.; Groisman, E.A. Role of Nonhost Environments in the Lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 2003, 69, 3687–3694. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, e07666. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA J. 2018, 16, e05182. [Google Scholar] [CrossRef]
- Bezek, K.; Avberšek, J.; Zorman Rojs, O.; Barlič-Maganja, D. Antimicrobial and Antibiofilm Effect of Commonly Used Disinfectants on Salmonella Infantis Isolates. Microorganisms 2023, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Aviv, G.; Tsyba, K.; Steck, N.; Salmon-Divon, M.; Cornelius, A.; Rahav, G.; Grassl, G.A.; Gal-Mor, O. Unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent Salmonella enterica serovar Infantis strain. Environ. Microbiol. 2014, 16, 977–994. [Google Scholar] [CrossRef] [PubMed]
- Aviv, G.; Rahav, G.; Gal-Mor, O. Horizontal Transfer of the Salmonella enterica Serovar Infantis Resistance and Virulence Plasmid pESI to the Gut Microbiota of Warm-Blooded Hosts. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Leekitcharoenphon, P.; Feltrin, F.; Alba, P.; Cordaro, G.; Iurescia, M.; Tolli, R.; D’Incau, M.; Staffolani, M.; Di Giannatale, E.; et al. Emergence of a clonal lineage of multidrug-resistant ESBL-producing Salmonella infantis transmitted from broilers and broiler meat to humans in Italy between 2011 and 2014. PLoS ONE 2015, 10, e0144802. [Google Scholar] [CrossRef] [PubMed]
- Bogomazova, A.N.; Gordeeva, V.D.; Krylova, E.V.; Soltynskaya, I.V.; Davydova, E.E.; Ivanova, O.E.; Komarov, A.A. Mega-plasmid found worldwide confers multiple antimicrobial resistance in Salmonella Infantis of broiler origin in Russia. Int. J. Food Microbiol. 2020, 319, 108497. [Google Scholar] [CrossRef]
- Davies, R.; Breslin, M. Observations on Salmonella contamination of commercial laying farms before and after cleaning and disinfection. Vet. Rec. 2003, 152, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Ruiz, F.M.; Romero, A.; Martínez, J.L. The Binding of Triclosan to SmeT, the Repressor of the Multidrug Efflux Pump SmeDEF, Induces Antibiotic Resistance in Stenotrophomonas maltophilia. PLoS Pathog. 2011, 7, e1002103. [Google Scholar] [CrossRef]
- Rodriguez, A.; Gutierrez, D.; Garcia, P.; Fernandez, L. Application of Bacteriophages in the Agro-Food Sector: A Long Way toward Approval. Front. Cell. Infect. Microbiol. 2018, 8, 296. [Google Scholar] [CrossRef]
- Fotheringham, V.J.C. Disinfection of livestock production premises. Rev. Sci. Tech. (Int. Off. Epizoot.) 1995, 14, 191–205. [Google Scholar] [CrossRef]
- Münster, P.; Pöppel, L.; Antakli, A.; Müller-Doblies, D.; Radko, D.; Kemper, N. The Detection of Salmonella Enteritidis on German Layer Farms after Cleaning and Disinfection. Animals 2023, 13, 2588. [Google Scholar] [CrossRef]
- Gray, J.A.; Chandry, P.S.; Kaur, M.; Kocharunchitt, C.; Bowman, J.P.; Fox, E.M. Novel Biocontrol Methods for Listeria monocytogenes Biofilms in Food Production Facilities. Front. Microbiol. 2018, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Suárez, J.V.; Ortiz, S.; López-Alonso, V. Potential Impact of the Resistance to Quaternary Ammonium Disinfectants on the Persistence of Listeria monocytogenes in Food Processing Environments. Front. Microbiol. 2016, 7, 638. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Schirmer, B.C.T.; Heir, E.; Fagerlund, A.; Hjemli, P.; Langsrud, S. Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry. Int. J. Food Microbiol. 2017, 241, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Overney, A.; Jacques-André-Coquin, J.; Ng, P.; Carpentier, B.; Guillier, L.; Firmesse, O. Impact of environmental factors on the culturability and viability of Listeria monocytogenes under conditions encountered in food processing plants. Int. J. Food Microbiol. 2017, 244, 74–81. [Google Scholar] [CrossRef]
- Gottardi, W.; Nagl, M. Chlorine covers on living bacteria: The initial step in antimicrobial action of active chlorine compounds. J. Antimicrob. Chemother. 2005, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ren, T.; Qiao, M.; Huang, T.; Xia, X. The reduction of Salmonella on chicken skin by the combination of sodium dodecyl sulfate with antimicrobial chemicals and coating wax microemulsions. Poult. Sci. 2019, 98, 2615–2621. [Google Scholar] [CrossRef] [PubMed]
- Byun, K.; Han, S.H.; Yoon, J.; Park, S.H.; Ha, S. Efficacy of chlorine-based disinfectants (sodium hypochlorite and chlorine dioxide) on Salmonella Enteritidis planktonic cells, biofilms on food contact surfaces and chicken skin. Food Control 2021, 123, 107838. [Google Scholar] [CrossRef]
- LeChevallier, M.W.; Au, K. Water Treatment and Pathogen Control; IWA Publishing: Geneva, Switzerland, 2004. [Google Scholar]
- Kim, J.; Pitts, B.; Stewart, P.S.; Camper, A.; Yoon, J. Comparison of the Antimicrobial Effects of Chlorine, Silver Ion, and Tobramycin on Biofilm. Antimicrob. Agents Chemother. 2008, 52, 1446–1453. [Google Scholar] [CrossRef]
- Ran, Y.; Qingmin, C.; Maorun, F. Chlorine Dioxide Generation Method and Its Action Mechanism for Removing Harmful Substances and Maintaining Quality Attributes of Agricultural Products. Food Bioprocess Technol. 2019, 12, 1110–1122. [Google Scholar] [CrossRef]
- Villarreal, M.E.; Baker, R.C.; Regenstein, J.M. The incidence of Salmonella on poultry carcasses following the use of slow release chlorine dioxide (Alcide). J. Food Prot. 1990, 53, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Purnell, G.; James, C.; James, S.J.; Howell, M.; Corry, J.E.L. Comparison of Acidified Sodium Chlorite, Chlorine Dioxide, Peroxyacetic Acid and Tri-Sodium Phosphate Spray Washes for Decontamination of Chicken Carcasses. Food Bioprocess Technol. 2014, 7, 2093–2101. [Google Scholar] [CrossRef]
- Mani-Lopez, E.; Garcia, H.S.; Lopez-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Ricke, S.C. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2003, 82, 632–639. [Google Scholar] [CrossRef]
- Madushanka, D.N.N.; Jayaweera, T.S.P.; Jayasinghe, J.M.C.S.; Yasawathie, D.G.; Ruwandeepika, H.A.D. Decontaminating Effect of Organic Acids and Natural Compounds on Broiler Chicken Meat Contaminated with Salmonella typhimurium. Asian Food Sci. J. 2018, 3, 1–9. [Google Scholar] [CrossRef]
- Yeh, Y.; de Moura, F.H.; Van Den Broek, K.; de Mello, A.S. Effect of ultraviolet light, organic acids, and bacteriophage on Salmonella populations in ground beef. Meat Sci. 2018, 139, 44–48. [Google Scholar] [CrossRef]
- Fernández, M.; Rodríguez, A.; Fulco, M.; Soteras, T.; Mozgovoj, M.; Cap, M. Effects of lactic, malic and fumaric acids on Salmonella spp. counts and on chicken meat quality and sensory characteristics. J. Food Sci. Technol. 2021, 58, 3817–3824. [Google Scholar] [CrossRef]
- Radkowski, M.; Zdrodowska, B.; Gomółka-Pawlicka, M. Effect of Succinic Acid on Elimination of Salmonella in Chicken Meat. J. Food Prot. 2018, 81, 1491–1495. [Google Scholar] [CrossRef]
- Cosansu, S.; Ayhan, K. Effects of Lactic and Acetic Acid on Survival of Salmonella enteritidis during Refrigerated and Frozen Storage of Chicken Meats. Food Bioprocess Technol. 2012, 5, 372–377. [Google Scholar] [CrossRef]
- Abdul-Rahiman, U.A.; Nordin, N.; Abdul-Mutalib, N.A.; Sanny, M. Holistic Approaches to Reducing Salmonella Contamination in Poultry Industry. Pertanika J. Trop. Agric. Sci. 2021, 44. [Google Scholar] [CrossRef]
- Bilgill, S.F.; Conner, D.E.; Pinion, J.L.; Tamblyn, K.C. Broiler skin color as affected by organic acids: Influence of concentration and method of application. Poult. Sci. 1998, 77, 752–757. [Google Scholar] [CrossRef]
- Hajati, H. Application of organic acids in poultry nutrition. Int. J. Avian Wildl. Biol. 2018, 3, 324–329. [Google Scholar] [CrossRef]
- Ben Braïek, O.; Smaoui, S. Chemistry, Safety, and Challenges of the Use of Organic Acids and Their Derivative Salts in Meat Preservation. J. Food Qual. 2021, 2021, 6653190. [Google Scholar] [CrossRef]
- Ramirez-Hernandez, A.; Brashears, M.M.; Sanchez-Plata, M.X. Efficacy of Lactic Acid, Lactic Acid–Acetic Acid Blends, and Peracetic Acid To Reduce Salmonella on Chicken Parts under Simulated Commercial Processing Conditions. J. Food Prot. 2018, 81, 17–24. [Google Scholar] [CrossRef]
- Wessels, K.; Rip, D.; Gouws, P. Salmonella in Chicken Meat: Consumption, Outbreaks, Characteristics, Current Control Methods and the Potential of Bacteriophage Use. Foods 2021, 10, 1742. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Listeria monocytogenes: Biofilm formation and persistence in food-processing environments. Biofilms 2004, 1, 107–121. [Google Scholar] [CrossRef]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.-C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N. Biofilm formation and persistence on abiotic surfaces in the context of food and medical environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Lazazzera, B.A. Lessons from DNA microarray analysis: The gene expression profile of biofilms. Curr. Opin. Microbiol. 2005, 8, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, D.; Rodriguez-Rubio, L.; Martinez, B.; Rodriguez, A.; Garcia, P. Bacteriophages as Weapons Against Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 825. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yu, X.; Guo, W.; Guo, C.; Guo, X.; Li, Q.; Zhu, Y. Bacteriophage-Mediated Control of Biofilm: A Promising New Dawn for the Future. Front. Microbiol. 2022, 13, 825828. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, P.; Baliou, S.; Samonis, G. Bacteriophages in Infectious Diseases and Beyond-A Narrative Review. Antibiotics 2023, 12, 1012. [Google Scholar] [CrossRef] [PubMed]
- Hosny, R.A.; Shalaby, A.G.; Nasef, S.A.; Sorour, H.K. Antibiofilm activity of a lytic Salmonella phage on different Salmonella enterica serovars isolated from broiler farms. Int. Microbiol. 2023, 26, 205–217. [Google Scholar] [CrossRef]
- Marin, C.; Hernandiz, A.; Lainez, M. Biofilm development capacity of Salmonella strains isolated in poultry risk factors and their resistance against disinfectants. Poult. Sci. 2009, 88, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Soro, A.B.; Whyte, P.; Bolton, D.J.; Tiwari, B.K. Strategies and novel technologies to control Campylobacter in the poultry chain: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1353–1377. [Google Scholar] [CrossRef]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef]
- Yan, J.; Mao, J.; Xie, J. Bacteriophage Polysaccharide Depolymerases and Biomedical Applications. BioDrugs 2014, 28, 265–274. [Google Scholar] [CrossRef]
- Harper, D.; Parracho, H.; Walker, J.; Sharp, R.; Hughes, G.; Werthén, M.; Lehman, S.; Morales, S. Bacteriophages and Biofilms. Antibiotics 2014, 3, 270–284. [Google Scholar] [CrossRef]
- Domingo-Calap, P.; Delgado-Martínez, J. Bacteriophages: Protagonists of a Post-Antibiotic Era. Antibiotics 2018, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Abedon, S.T. Bacteriophages and their enzymes in biofilm control. Curr. Pharm. Des. 2015, 21, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Young, R. Bacteriophage lysis: Mechanism and regulation. Microbiol. Rev. 1992, 56, 430–481. [Google Scholar] [CrossRef]
- Fernandes, S.; São-José, C. Enzymes and Mechanisms Employed by Tailed Bacteriophages to Breach the Bacterial Cell Barriers. Viruses 2018, 10, 396. [Google Scholar] [CrossRef]
- Davies, R.H.; Breslin, M. Persistence of Salmonella Enteritidis Phage Type 4 in the environment and arthropod vectors on an empty free-range chicken farm. Environ. Microbiol. 2003, 5, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Gosling, B.; Rabie, A.; Davies, R. Field investigations of multidrug-resistant Salmonella Infantis epidemic strain incursions into broiler flocks in England and Wales. Avian Pathol. 2020, 49, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Wales, A.; Breslin, M.; Carter, B.; Sayers, R.; Davies, R. A longitudinal study of environmental salmonella contamination in caged and free-range layer flocks. Avian Pathol. 2007, 36, 187–197. [Google Scholar] [CrossRef]
- Ethèves, M.A.; Choisis, N.; Alvarez, S.; Dalleau, F.; Hascoat, J.; Gallard, V.; Cardinale, E. Risk factors for Salmonella enterica subsp. enterica persistence in broiler-chicken flocks on Reunion Island. Heliyon 2021, 7, e06278. [Google Scholar] [CrossRef]
- Korzeniowski, P.; Śliwka, P.; Kuczkowski, M.; Mišić, D.; Milcarz, A.; Kuźmińska-Bajor, M. Bacteriophage Cocktail Can Effectively Control Salmonella Biofilm in Poultry Housing. Front. Microbiol. 2022, 13, 901770. [Google Scholar] [CrossRef]
- Ning, Z.; Zhang, L.; Cai, L.; Xu, X.; Chen, Y.; Wang, H. Biofilm removal mediated by Salmonella phages from chicken-related sources. Food Sci. Hum. Wellness 2023, 12, 1799–1808. [Google Scholar] [CrossRef]
- Ge, H.; Lin, C.; Xu, Y.; Hu, M.; Xu, Z.; Geng, S.; Jiao, X.; Chen, X. A phage for the controlling of Salmonella in poultry and reducing biofilms. Vet. Microbiol. 2022, 269, 109432. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Jiang, X.; Wang, J. Application of bacteriophages to reduce Salmonella contamination on workers’ boots in rendering-processing environment. Poult. Sci. 2017, 96, 3700–3708. [Google Scholar] [CrossRef] [PubMed]
- Al Kandari, S. Characterization and Comparison of Campylobacter Bacteriophages. Doctoral Dissertation, University of Nottingham, Nottingham, UK, 2013. [Google Scholar]
- Zeng, H.; De Reu, K.; Gabriël, S.; Mattheus, W.; De Zutter, L.; Rasschaert, G. Salmonella prevalence and persistence in industrialized poultry slaughterhouses. Poult. Sci. 2021, 100, 100991. [Google Scholar] [CrossRef] [PubMed]
- Pintar, K.; Cook, A.; Pollari, F.; Ravel, A.; Lee, S.; Odumeru, J.A. Quantitative Effect of Refrigerated Storage Time on the Enumeration of Campylobacter, Listeria, and Salmonella on Artificially Inoculated Raw Chicken Meat. J. Food Prot. 2007, 70, 739–743. [Google Scholar] [CrossRef]
- Maziero, M.T.; Oliveira, T.C.R.M.d. Effect of refrigeration and frozen storage on the Campylobacter jejuni recovery from naturally contaminated broiler carcasses. Braz. J. Microbiol. 2010, 41, 501–505. [Google Scholar] [CrossRef]
- Blankenship, L.C.; Craven, S.E. Campylobacter jejuni survival in chicken meat as a function of temperature [Gastroenteritis]. Appl. Environ. Microbiol. 1982, 44, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Whiley, H.; Ross, K. Salmonella and Eggs: From Production to Plate. Int. J. Environ. Res. Public Health 2015, 12, 2543–2556. [Google Scholar] [CrossRef]
- de Ornellas Dutka Garcia, K.C.; de Oliveira Corrêa, I.M.; Pereira, L.Q.; Silva, T.M.; de Souza Ribeiro Mioni, M.; de Moraes Izidoro, A.C.; Vellano Bastos, I.H.; Marietto Gonçalves, G.A.; Okamoto, A.S.; Andreatti Filho, R.L. Bacteriophage use to control Salmonella biofilm on surfaces present in chicken slaughterhouses. Poult. Sci. 2017, 96, 3392–3398. [Google Scholar] [CrossRef]
- Harada, L.K.; Silva, E.C.; Campos, W.F.; Del Fiol, F.S.; Vila, M.; Dąbrowska, K.; Krylov, V.N.; Balcão, V.M. Biotechnological applications of bacteriophages: State of the art. Microbiol. Res. 2018, 212–213, 38–58. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Food Res. Int. 2019, 119, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Siringan, P.; Connerton, P.L.; Cummings, N.J.; Connerton, I.F. Alternative bacteriophage life cycles: The carrier state of Campylobacter jejuni. Open Biol. 2014, 4, 130200. [Google Scholar] [CrossRef] [PubMed]
- D’Accolti, M.; Soffritti, I.; Mazzacane, S.; Caselli, E. Bacteriophages as a Potential 360-Degree Pathogen Control Strategy. Microorganisms 2021, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Hagens, S.; Loessner, M.J. Bacteriophage for Biocontrol of Foodborne Pathogens: Calculations and Considerations. Curr. Pharm. Biotechnol. 2010, 11, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Davison, M.R.; Nelson, W.C.; Smith, M.L.; Lipton, M.S.; Jansson, J.K.; McClure, R.S.; McDermott, J.E.; Hofmockel, K.S. Hi-C metagenome sequencing reveals soil phage–host interactions. Nat. Commun. 2023, 14, 7666. [Google Scholar] [CrossRef] [PubMed]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B.; Delattre, A.; Lavigne, R. Learning from Bacteriophages-Advantages and Limitations of Phage and Phage-Encoded Protein Applications. Curr. Protein Pept. Sci. 2012, 13, 699–722. [Google Scholar] [CrossRef]
- Goh, S. Phage Transduction. In Clostridium Difficile; Springer: New York, NY, USA, 2016; Volume 1476, pp. 177–185. [Google Scholar]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Brown, P.; Chen, Y.; Parsons, C.; Brown, E.; Loessner, M.J.; Shen, Y.; Kathariou, S. Whole Genome Sequence Analysis of Phage-Resistant Listeria monocytogenes Serotype 1/2a Strains from Turkey Processing Plants. Pathogens 2021, 10, 199. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectiveness of Phage-Based Inhibition of Listeria monocytogenes in Food Products and Food Processing Environments. Microorganisms 2020, 8, 1764. [Google Scholar] [CrossRef]
- Aprea, G.; Zocchi, L.; Di Fabio, M.; De Santis, S.; Prencipe, V.A.; Migliorati, G. The applications of bacteriophages and their lysins as biocontrol agents against the foodborne pathogens Listeria monocytogenes and Campylobacter: An updated look. Vet. Ital. 2018, 54, 293–303. [Google Scholar] [CrossRef]
- EFSA BIOHAZ Panel; Koutsoumanis, K.; Allende, A.; Alvarez-Ordonez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Updated list of QPS-recommended microorganisms for safety risk assessments carried out by EFSA. 2023. Available online: https://zenodo.org/records/8124409 (accessed on 1 August 2023).
- Boulanger, P. Purification of Bacteriophages and SDS-PAGE Analysis of Phage Structural Proteins from Ghost Particles. In Bacteriophages; Humana Press: Totowa, NJ, USA, 2009; Volume 502, pp. 227–238. [Google Scholar]
- Gill, J.J.; Hyman, P. Phage Choice, Isolation, and Preparation for Phage Therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar] [CrossRef]
- Manohar, P.; Ramesh, N. Improved lyophilization conditions for long-term storage of bacteriophages. Sci. Rep. 2019, 9, 15242. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, M.; Scherer, S.; Loessner, M.J. Genomic Analysis of Clostridium perfringens Bacteriophage φ3626, Which Integrates into guaA and Possibly Affects Sporulation. J. Bacteriol. 2002, 184, 4359–4368. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.; Kim, M.; Lee, D.; Lee, Y.; Park, J.; Youn, H.; Lee, H.; Yang, S.; Cho, Y.; Lee, J.; et al. Use of bacteriophage for biological control of Salmonella Enteritidis infection in chicken. Res. Vet. Sci. 2012, 93, 1173–1178. [Google Scholar] [CrossRef]
- Berchieri, A.; Lovell, M.A.; Barrow, P.A. The activity in the chicken alimentary tract of bacteriophages lytic for Salmonella typhimurium. Res. Microbiol. 1991, 142, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Bigot, B.; Lee, W.-J.; McIntyre, L.; Wilson, T.; Hudson, J.A.; Billington, C.; Heinemann, J.A. Control of Listeria monocytogenes growth in a ready-to-eat poultry product using a bacteriophage. Food Microbiol. 2011, 28, 1448–1452. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the maintenance of the list of QPS microorganisms intentionally added to food or feed (2009 update). EFSA J. 2009, 7, 1431. [Google Scholar] [CrossRef]
- Goodridge, L.D.; Bisha, B. Phage-based biocontrol strategies to reduce foodborne pathogens in foods. Bacteriophage 2011, 1, 130–137. [Google Scholar] [CrossRef]
- Moineau, S.; Labrie, S.J.; Samson, J.E. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Shabbir, M.A.B.; Hao, H.; Shabbir, M.Z.; Wu, Q.; Sattar, A.; Yuan, Z. Bacteria vs. Bacteriophages: Parallel Evolution of Immune Arsenals. Front. Microbiol. 2016, 7, 1292. [Google Scholar] [CrossRef]
- Sorek, R.; Kunin, V.; Hugenholtz, P. CRISPR—A widespread system that provides acquired resistance against phages in bacteria and archaea. Nat. Rev. Microbiol. 2008, 6, 181–186. [Google Scholar] [CrossRef]
- Li, K.; Barksdale, L.; Garmise, L. Phenotypic Alterations Associated with the Bacteriophage Carrier State of Shigella dysenteriae. J. Gen. Microbiol. 1961, 24, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Jarling, M.; Bartkowiak, K.; Robenek, H.; Pape, H.; Meinhardt, F. Isolation of phages infecting Actinoplanes SN223 and characterization of two of these viruses. Appl. Microbiol. Biotechnol. 2004, 64, 250–254. [Google Scholar] [CrossRef]
- Bastías, R.; Higuera, G.; Sierralta, W.; Espejo, R.T. new group of cosmopolitan bacteriophages induce a carrier state in the pandemic strain of Vibrio parahaemolyticus. Environ. Microbiol. 2010, 12, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.E.; Timms, A.R.; Connerton, P.L.; Loc Carrillo, C.; Adzfa Radzum, K.; Connerton, I.F. Genome Dynamics of Campylobacter jejuni in Response to Bacteriophage Predation. PLoS Pathog. 2007, 3, e119. [Google Scholar] [CrossRef] [PubMed]
- Hooton, S.P.T.; Connerton, I.F. Campylobacter jejuni acquire new host-derived CRISPR spacers when in association with bacteriophages harboring a CRISPR-like Cas4 protein. Front. Microbiol. 2015, 5, 744. [Google Scholar] [CrossRef]
- Lis, L.; Connerton, I.F. The Minor Flagellin of Campylobacter jejuni (FlaB) Confers Defensive Properties against Bacteriophage Infection. Front. Microbiol. 2016, 7, 1908. [Google Scholar] [CrossRef]
- Gencay, Y.E.; Sørensen, M.C.H.; Wenzel, C.Q.; Szymanski, C.M.; Brøndsted, L. Phase Variable Expression of a Single Phage Receptor in Campylobacter jejuni NCTC12662 Influences Sensitivity toward Several Diverse CPS-Dependent Phages. Front. Microbiol. 2018, 9, 82. [Google Scholar] [CrossRef]
- Olson, E.G.; Micciche, A.C.; Rothrock, M.J.; Yang, Y.; Ricke, S.C. Application of Bacteriophages to Limit Campylobacter in Poultry Production. Front. Microbiol. 2021, 12, 458721. [Google Scholar] [CrossRef]
- Piel, D.; Bruto, M.; Labreuche, Y.; Blanquart, F.; Goudenège, D.; Barcia-Cruz, R.; Chenivesse, S.; Le Panse, S.; James, A.; Dubert, J.; et al. Phage–host coevolution in natural populations. Nat. Microbiol. 2022, 7, 1075–1086. [Google Scholar] [CrossRef]
- Burmeister, A.R.; Fortier, A.; Roush, C.; Lessing, A.J.; Bender, R.G.; Barahman, R.; Grant, R.; Chan, B.K.; Turner, P.E. Pleiotropy complicates a trade-off between phage resistance and antibiotic resistance. Proc. Natl. Acad. Sci. USA 2020, 117, 11207–11216. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Herzig, O.; Fieseler, L.; Klumpp, J.; Loessner, M.J. Biocontrol of Salmonella Typhimurium in RTE foods with the virulent bacteriophage FO1-E2. Int. J. Food Microbiol. 2012, 154, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Viñas, I.; Colàs, P.; Anguera, M.; Usall, J.; Abadias, M. Effectiveness of a bacteriophage in reducing Listeria monocytogenes on fresh-cut fruits and fruit juices. Food Microbiol. 2014, 38, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent Bacteriophage for Efficient Biocontrol of Listeria monocytogenes in Ready-To-Eat Foods. Appl. Environ. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Anany, H.; Chen, W.; Pelton, R.; Griffiths, M.W. Biocontrol of Listeria monocytogenes and Escherichia coli O157:H7 in Meat by Using Phages Immobilized on Modified Cellulose Membranes. Appl. Environ. Microbiol. 2011, 77, 6379–6387. [Google Scholar] [CrossRef]
- Wójcicki, M.; Świder, O.; Gientka, I.; Błażejak, S.; Średnicka, P.; Shymialevich, D.; Cieślak, H.; Wardaszka, A.; Emanowicz, P.; Sokołowska, B.; et al. Effectiveness of a Phage Cocktail as a Potential Biocontrol Agent against Saprophytic Bacteria in Ready-To-Eat Plant-Based Food. Viruses 2023, 15, 172. [Google Scholar] [CrossRef]
- Hudson, J.A.; Billington, C.; Carey-Smith, G.; Greening, G. Bacteriophages as biocontrol agents in food. J. Food Prot. 2005, 68, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Leverentz, B.; Conway, W.S.; Alavidze, Z.; Janisiewicz, W.J.; Fuchs, Y.; Camp, M.J.; Chighladze, E.; Sulakvelidze, A. Examination of Bacteriophage as a Biocontrol Method for Salmonella on Fresh-Cut Fruit: A Model Study. J. Food Prot. 2001, 64, 1116–1121. [Google Scholar] [CrossRef]
- Sharma, M.; Patel, J.R.; Conway, W.S.; Ferguson, S.; Sulakvelidze, A. Effectiveness of Bacteriophages in Reducing Escherichia coli O157:H7 on Fresh-Cut Cantaloupes and Lettuce. J. Food Prot. 2009, 72, 1481–1485. [Google Scholar] [CrossRef]
- Atterbury, R.J.; Connerton, P.L.; Dodd, C.E.R.; Rees, C.E.D.; Connerton, I.F. Application of host-specific bacteriophages to the surface of chicken skin leads to a reduction in recovery of Campylobacter jejuni. Appl. Environ. Microbiol. 2003, 69, 6302–6306. [Google Scholar] [CrossRef]
- Goode, D.; Allen, V.M.; Barrow, P.A. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl. Environ. Microbiol. 2003, 69, 5032–5036. [Google Scholar] [CrossRef]
- Rendueles, O.; de Sousa, J.A.M.; Rocha, E.P.C. Competition between lysogenic and sensitive bacteria is determined by the fitness costs of the different emerging phage-resistance strategies. eLife 2023, 12. [Google Scholar] [CrossRef]
- Jorquera, D.; Galarce, N.; Borie, C. The challenge of controlling foodborne diseases: Bacteriophages as a new biotechnological tool. Rev. Chil. Infectol. 2015, 32, 678–688. [Google Scholar] [CrossRef]
| Limitation | Description |
|---|---|
| Phage of Choice | Individual phages are insufficient for broad-spectrum infections; complex identification is needed. Elimination by the reticuloendothelial system reduces half-life, limiting efficacy. |
| Lysogenic Forms | Lysogenic phages confer poor results due to acquired immunity. Transduction of bacterial genome and potential transmission of harmful genes are concerns. Using multiple phages is often more effective. |
| Legal Limitations | Global regulatory variation in phage utilization. Permitted as a processing aid in certain countries, limited in the EU. |
| Purification and Stabilization | Phage characterization is essential for toxicity removal. Purification by ultracentrifugation or chromatography. Stability is crucial but varies among phages. |
| Dosage | High phage concentrations are needed for bacterial removal; lower doses are ineffective. Timing and delivery are critical, with potential for induced antibodies. |
| Terms of Use | Bacteriophage persistence varies with type, application conditions, and environmental factors. Refrigeration enhances persistence. |
| Resistance Mechanisms | Increased phage application may lead to bacterial resistance mechanisms. Coevolution cycles involve various resistance strategies. |
| Effectiveness | Efficacy is a major limitation; initial reduction is observed, but complete eradication is challenging. Factors influencing effectiveness include food matrix, pH, temperature, MOI, phagoresistance, and combination with other measures. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jordá, J.; Lorenzo-Rebenaque, L.; Montoro-Dasi, L.; Marco-Fuertes, A.; Vega, S.; Marin, C. Phage-Based Biosanitation Strategies for Minimizing Persistent Salmonella and Campylobacter Bacteria in Poultry. Animals 2023, 13, 3826. https://doi.org/10.3390/ani13243826
Jordá J, Lorenzo-Rebenaque L, Montoro-Dasi L, Marco-Fuertes A, Vega S, Marin C. Phage-Based Biosanitation Strategies for Minimizing Persistent Salmonella and Campylobacter Bacteria in Poultry. Animals. 2023; 13(24):3826. https://doi.org/10.3390/ani13243826
Chicago/Turabian StyleJordá, Jaume, Laura Lorenzo-Rebenaque, Laura Montoro-Dasi, Ana Marco-Fuertes, Santiago Vega, and Clara Marin. 2023. "Phage-Based Biosanitation Strategies for Minimizing Persistent Salmonella and Campylobacter Bacteria in Poultry" Animals 13, no. 24: 3826. https://doi.org/10.3390/ani13243826
APA StyleJordá, J., Lorenzo-Rebenaque, L., Montoro-Dasi, L., Marco-Fuertes, A., Vega, S., & Marin, C. (2023). Phage-Based Biosanitation Strategies for Minimizing Persistent Salmonella and Campylobacter Bacteria in Poultry. Animals, 13(24), 3826. https://doi.org/10.3390/ani13243826








