Morphophysiological Responses of the Goat Mammary Gland to Water Scarcity in Arid and Semi-Arid Environments: Are They Enough to Generate Adaptation to New Climatic Challenges?
Abstract
Simple Summary
Abstract
1. Introduction
2. Review Methodology
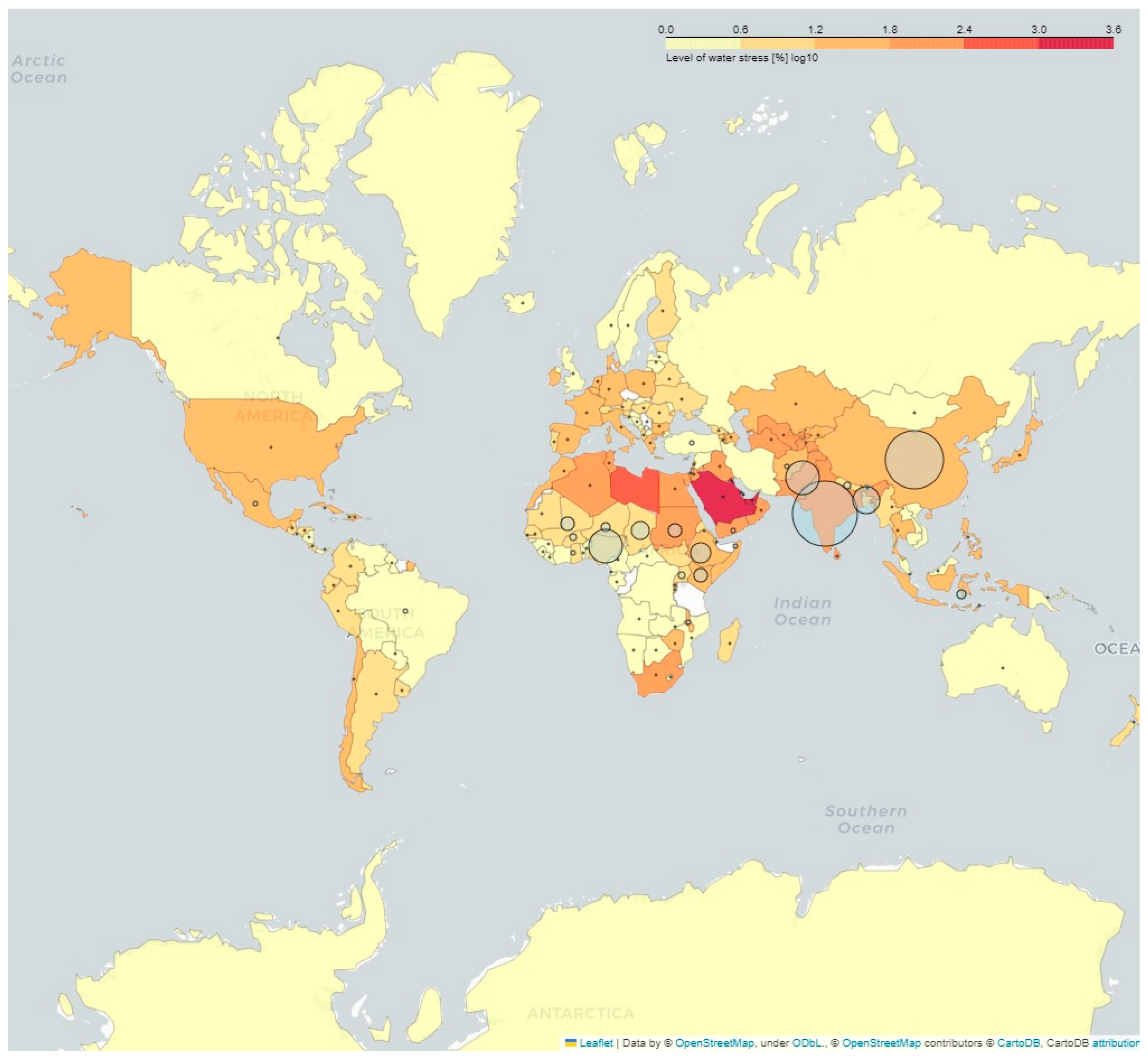
3. Goat Dairy Production Scenario Worldwide
4. Physiological Changes of the Goat Mammary Gland
4.1. Remodeling of Mammary Gland Tissue
4.2. Hormone Secretion and Hormone Receptor Expression
4.3. Mammary Gland Blood Flow
5. Physiological and Productive Changes in Goats under Water Intake Stress
5.1. Changes in Milk Production
5.2. Systemic Changes in Goats
5.3. Changes in Blood Flow on Mammary Gland
6. Perspectives and Future Research Gap
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naderi, S.; Rezaei, H.R.; Pompanon, F.; Blum, M.G.B.; Negrini, R.; Naghash, H.R.; Balkiz, Ö.; Mashkour, M.; Gaggiotti, O.E.; Ajmone-Marsan, P.; et al. The Goat Domestication Process Inferred from Large-Scale Mitochondrial DNA Analysis of Wild and Domestic Individuals. Proc. Natl. Acad. Sci. USA 2008, 105, 17659–17664. [Google Scholar] [CrossRef]
- Zeder, M.A.; Hesse, B. The Initial Domestication of Goats (Capra Hircus) in the Zagros Mountains 10,000 Years Ago. Science 2000, 287, 2254–2257. [Google Scholar] [CrossRef]
- Boyazoglu, J.; Hatziminaoglou, I.; Morand-Fehr, P. The Role of the Goat in Society: Past, Present and Perspectives for the Future. Small Rumin. Res. 2005, 60, 13–23. [Google Scholar] [CrossRef]
- Skapetas, B.; Bampidis, V. Goat Production in the World: Present Situation and Trends. Livest. Res. Rural. Dev. 2018, 28, 200. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 1 January 2023).
- Dubeuf, J.-P. Future Prospects on the Goat Activities for the Coming Decades in the Context of a World in Transition. In Goat Science Environment Health and Economy; Intechopen: London, UK, 2021. [Google Scholar] [CrossRef]
- Miller, B.A.; Lu, C.D. Special Issue-Current Status of Global Dairy Goat Production: An Overview. Asian-Australas. J. Anim. Sci. 2019, 32, 1219–1232. [Google Scholar] [CrossRef]
- Ruiz Morales Francisco Situation of Dairy Goats in the World. Available online: https://www.iga-goatworld.com/blog/situation-of-dairy-goats-in-the-world (accessed on 1 January 2023).
- Mazinani, M.; Rude, B. Population, World Production and Quality of Sheep and Goat Products. Am. J. Anim. Vet. Sci. 2020, 15, 291–299. [Google Scholar] [CrossRef]
- Ayuso Peraza, G.; Castillo León, M.T.; Ayuso Peraza, G.; Castillo León, M.T. Globalización y Nostalgia. Cambios En La Alimentación de Familias Yucatecas. Rev. Estud. Soc. 2017, 27, 1–18. [Google Scholar] [CrossRef][Green Version]
- Ngambi, J.W.; Alabi, O.J.; Norris, D. Role of Goats in Food Security, Poverty Alleviation and Prosperity with Special Reference to Sub-Saharan Africa: A Review. Indian J. Anim. Res. 2013, 47, 1–9. [Google Scholar]
- Ronda-Borzone, P.; Donoso, G.; Riveros, F.J.L. Food Security and Livelihood Challenges of Extensive Goat Production Systems in Desertification Areas. CABI Rev. 2022, 2022. [Google Scholar] [CrossRef]
- Lu, C.D.; Miller, B.A. Current Status, Challenges and Prospects for Dairy Goat Production in the Americas. Asian-Australas. J. Anim. Sci. 2019, 32, 1244–1255. [Google Scholar] [CrossRef]
- Turkmen, N. The Nutritional Value and Health Benefits of Goat Milk Components; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128097625. [Google Scholar]
- Dhasmana, S.; Das, S.; Shrivastava, S. Potential Nutraceuticals from the Casein Fraction of Goat’s Milk. J. Food Biochem. 2022, 46, e13982. [Google Scholar] [CrossRef]
- Akinyemi, M.O.; Ogunremi, O.R.; Adeleke, R.A.; Ezekiel, C.N. Probiotic Potentials of Lactic Acid Bacteria and Yeasts from Raw Goat Milk in Nigeria. Probiotics Antimicrob. Proteins 2022, 1–18. [Google Scholar] [CrossRef]
- Gallier, S.; Tolenaars, L.; Prosser, C. Whole Goat Milk as a Source of Fat and Milk Fat Globule Membrane in Infant Formula. Nutrients 2020, 12, 3486. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant Properties of Milk and Dairy Products: A Comprehensive Review of the Current Knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef]
- Riskó, T.C.; Csapó, Z. Goat Keeping and Goat Milk Products in Human Nutrition—Review. Apstract. Appl. Stud. Agribus. Commer. 2019, 13, 24–36. [Google Scholar] [CrossRef]
- Haenlein, G.F.W. Goat Milk in Human Nutrition. Small Rumin. Res. 2004, 51, 155–163. [Google Scholar] [CrossRef]
- Park, Y.W.; Juárez, M.; Ramos, M.; Haenlein, G.F.W. Physico-Chemical Characteristics of Goat and Sheep Milk. Small Rumin. Res. 2007, 68, 88–113. [Google Scholar] [CrossRef]
- Morand-Fehr, P. Goat Nutrition; Pudoc: Wageningen, The Netherlands, 1991; Volume 46, ISBN 90-220-1009-0. [Google Scholar]
- Gioffredo, J.J.; Pretryna, A. Caprinos: Generalidades, Nutrición, Reproducción e Instalaciones. Available online: www.produccion-animal.com.ar (accessed on 11 June 2022).
- Akinmoladun, O.F.; Muchenje, V.; Fon, F.N.; Mpendulo, C.T. Small Ruminants: Farmers’ Hope in a World Threatened by Water Scarcity. Animals 2019, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Faraz, A.; Ullah Khan, N.; Passantino, A.; Pugliese, M.; Eyduran, E.; Iglesias Pastrana, C.; Ismail, A.; Ali Tauqir, N.; Waheed, A.; Shahid Nabeel, M. Effect of Different Watering Regimes in Summer Season on Water Intake, Feed Intake, and Milk Production of Marecha She-Camel (Camelus dromedarius). Animals 2021, 11, 1342. [Google Scholar] [CrossRef]
- INE Producción Pecuaria. Available online: https://www.ine.cl/estadisticas/economia/agricultura-agroindustria-y-pesca/produccion-pecuaria (accessed on 11 June 2022).
- Maltz, E.; Shkolnik, A. Milk Production in the Desert: Lactation and Water Economy in the Black Bedouin Goat. Physiol. Zool. 1980, 53, 12–18. [Google Scholar] [CrossRef]
- Meneses, R. Manual de Producción Caprina. INIA 2017, 5, 63–64. [Google Scholar]
- Silanikove, N.; Leitner, G.; Merin, U.; Prosser, C.G. Recent Advances in Exploiting Goat’s Milk: Quality, Safety and Production Aspects. Small Rumin. Res. 2010, 89, 110–124. [Google Scholar] [CrossRef]
- Dahlborn, K. Effect of Temporary Food or Water Deprivation on Milk Secretion and Milk Composition in the Goat. J. Dairy Res. 1987, 54, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Mengistu, U.; Dahlborn, K.; Olsson, K. Mechanisms of Water Economy in Lactating Ethiopian Somali Goats during Repeated Cycles of Intermittent Watering. Animal 2007, 1, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Alamer, M. Effect of Water Restriction on Lactation Performance of Aardi Goats under Heat Stress Conditions. Small Rumin. Res. 2009, 84, 76–81. [Google Scholar] [CrossRef]
- Olsson, K. Fluid Balance in Ruminants: Adaptation to External and Internal Challenges. Ann. N. Y. Acad. Sci. 2005, 1040, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Casamassima, D.; Palazzo, M.; Nardoia, M.; D’Alessandro, A.G.; Vizzarri, F. Effect of Water Restriction on Milk Yield and Quality in Lacaune Breed Ewes. J. Anim. Physiol. Anim. Nutr. 2017, 102, e677–e685. [Google Scholar] [CrossRef] [PubMed]
- Little, W.; Sansom, B.F.; Manston, R.; Allen, W.M. Effects of Restricting the Water Intake of Dairy Cows upon Their Milk Yield, Body Weight and Blood Composition. Anim. Prod. 1976, 22, 329–339. [Google Scholar] [CrossRef]
- Cohick, W.S. The Role of the IGF System in Mammary Physiology of Ruminants. Domest. Anim. Endocrinol. 2022, 79, 106709. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Q.; Zhang, Y.; Liu, D.; Yang, D.; Han, L.; Chen, J.; Ding, Y. Regulation of Tight Junctions by Sex Hormones in Goat Mammary Epithelial Cells. Animals 2022, 12, 1404. [Google Scholar] [CrossRef]
- Cvek, K.; Dahlborn, K.; Riddestrale, Y. Localization of Carbonic Anhydrase in the Goat Mammary Gland during Involution and Lactogenesis. J. Dairy Res. 1998, 65, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kaliber, M.; Koluman, N.; Silanikove, N. Physiological and Behavioral Basis for the Successful Adaptation of Goats to Severe Water Restriction under Hot Environmental Conditions. Animal 2016, 10, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.; Benlamlih, S.; Dahlborn, K.; Fyhrquist, F. Effects of Water Deprivation and Hyperhydration in Pregnant and Lactating Goats. Acta Physiol. Scand. 1982, 115, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Olsson, K.; Maltz, E.; Glick, S.M.; Fyhrquist, F.; Shkolnik, A. On the Control of Water Balance in Lactating and Non-Lactating Bedouin Goats. Acta Physiol. Scand. 1983, 118, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.S.; Duvaux-Ponter, C.; Hamadeh, S.K.; Giger-Reverdin, S. Mild Heat Stress and Short Water Restriction Treatment in Lactating Alpine and Saanen Goats. Small Rumin. Res. 2019, 175, 46–51. [Google Scholar] [CrossRef]
- Mengistu, U.; Dahlborn, K.; Olsson, K. Effects of Intermittent Watering on Water Balance and Feed Intake in Male Ethiopian Somali Goats. Small Rumin. Res. 2007, 67, 45–54. [Google Scholar] [CrossRef]
- Utaaker, K.S.; Chaudhary, S.; Kifleyohannes, T.; Robertson, L.J. Global Goat! Is the Expanding Goat Population an Important Reservoir of Cryptosporidium? Front. Vet. Sci. 2021, 8, 648500. [Google Scholar] [CrossRef]
- Tajonar, K.; López Díaz, C.A.; Sánchez Ibarra, L.E.; Chay-Canul, A.J.; Gonzalez-Ronquillo, M.; Vargas-Bello-Pérez, E. A Brief Update on the Challenges and Prospects for Goat Production in Mexico. Animals 2022, 12, 837. [Google Scholar] [CrossRef]
- FAO. United Nations Progress on Level of Water Stress. In Global Status and Acceleration Needs for SDG Indicatoor 6.4.2; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Guevara, J.C.; Tonolli, A.J.; Paez Lama, S.A.; Allegretti, L.I.; Estevez, O.R. Determinant Factors of Economic Performance of Goat Meat Production Systems in Arid Rangelands of Mendoza, Argentina Factores Determinantes Del Rendimiento Económico de Los Sistemas de Producción de Caprinos Para Carne En Pastizales Naturales Áridos de Mendoza, Argentina. Multequina 2017, 26, 51–61. [Google Scholar]
- Fariña, S.R.; Chilibroste, P. Opportunities and Challenges for the Growth of Milk Production from Pasture: The Case of Farm Systems in Uruguay. Agric. Syst. 2019, 176, 102631. [Google Scholar] [CrossRef]
- Mahmood Conteh, A.; Kallon, S.; Nyandebo, J.P.; Sesay, M.E. Livestock Production: Purposes, Practices, and Challenges in Sierra Leone. Int. J. Anim. Sci. Husb. Livest. Prod. 2021, 7, 402–414. [Google Scholar]
- Liang, J.B.; Paengkoum, P. Current Status, Challenges and the Way Forward for Dairy Goat Production in Asia—Conference Summary of Dairy Goats in Asia. Asian-Australas. J. Anim. Sci. 2019, 32, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Hemme, T.; Garcia, O.; Khan, A.R. A Review of Milk Production in Bangladesh with Particular Emphasis on Small-Scale Producers; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar] [CrossRef]
- Daskiran, I.; Savas, T.; Koyuncu, M.; Koluman, N.; Keskin, M.; Esenbuga, N.; Konyali, A.; Cemal, İ.; Gül, S.; Elmaz, O.; et al. Goat Production Systems of Turkey: Nomadic to Industrial. Small Rumin. Res. 2018, 163, 15–20. [Google Scholar] [CrossRef]
- GIZ. The Dairy Sector in Burkina Faso the Capacity of the Sector and Impact of European Milk Powder Exports Sector Project Agricultural Trade and Value Chains. In Policy Brief 02; GIZ: Bonn, Germany, 2018. [Google Scholar]
- Khemiri, H.; Attia, K.; Darej, C.; M’hamdi, N.; Moujahed, N. Characterization of Goat Breeding Systems in Tunisia and In the Mediterranean Region. Genet. Biodivers. J. 2022, 6, 211–223. [Google Scholar] [CrossRef]
- Ouchene-Khelifi, N.A.; Ouchene, N.; Lafri, M. Characterization and Typology of Goat Production Systems in Algeria Based on Producers Survey. Bull. Natl. Res. Cent. 2021, 45, 22. [Google Scholar] [CrossRef]
- Patrick Baenyi, S.; Owino Junga, J.; Keambou Tiambo, C.; Bwihangane Birindwa, A.; Karume, K.; Mekuriaw Tarekegn, G.; Winyo Ochieng, J. Production Systems, Genetic Diversity and Genes Associated with Prolificacy and Milk Production in Indigenous Goats of Sub-Saharan Africa: A Review. Open J. Anim. Sci. 2020, 10, 735–749. [Google Scholar] [CrossRef]
- Peña-Avelino, L.Y.; Ceballos-Olvera, I.; Rosales-Martinez, G.N.; Hernández-Melendez, J.; Alva-Pérez, J. Milk Composition of Creole Goats Raised at Different Altitudes in an Extensive Production System in Northeast Mexico. Animals 2023, 13, 1738. [Google Scholar] [CrossRef]
- Mikulec, K.; Susic, V.; Mikulec, Z.; Bunta, V. Goat Production Development in the Republic of Croatia. In Analysis and Definition of the Objectives in Genetic Improvement Programmes in Sheep and Goats. An Economic Approach to Increase Their Profitability; Gabiña, D., Ed.; CIHEAM: Zaragoza, Spain, 2000; pp. 87–92. [Google Scholar]
- Morales, F.D.A.R.; Castel Genís, J.M.; Guerrero, Y.M. Current Status, Challenges and the Way Forward for Dairy Goat Production in Europe. Asian-Australas. J. Anim. Sci. 2019, 32, 1256–1265. [Google Scholar] [CrossRef]
- Smith, O.B.; Salla, A.; Bedane, B. Review of the Livestock/Meat and Milk Value Chains and Policy Influencing in Liberia; Food and Agriculture Organization of the United Nations and the Economic Community of West African States: Rome, Italy, 2017. [Google Scholar]
- Gebre, K.T.; Yfter, K.A.; Teweldemedhn, T.G.; Gebremariam, T. Optimization and Evaluation of Alternative Village-Based Breeding Plans: The Case of Goats in Afar Region, Ethiopia. Asian-Australas. J. Anim. Sci. 2019, 1–19. [Google Scholar] [CrossRef]
- Kukovics, S. Sustainable Goat Breeding and Goat Farming in the Central and Eastern European Countries; Food and Agriculture Organization of the United Nations: Rome, Italy, 2016. [Google Scholar]
- Choudhary, S.; Raheja, N.; Kamboj, M.L. Present Status and Proposed Breeding Strategies for Goat Production in India. Indian Dairym. 2018, 98–102. [Google Scholar]
- Strahsburger, E.; Scopinich-Cisternas, J. Goat Type Selection and Molecular Markers; A Solution for Milk Production in Recently Desertified Zones. In Goat Science—Environment, Health and Economy; IntechOpen: London, UK, 2021. [Google Scholar]
- Akraim, P. Goat Production in Libya Current State and Production Contraints. Krmiva 2012, 54, 189–194. [Google Scholar]
- Pantschenko, A.G.; Woodcock-Mitchell, J.; Bushmich, S.L.; Yang, T.J. Establishment and Characterization of a Caprine Mammary Epithelial Cell Line (CMEC). Vitr. Cell. Dev. Biol. Anim. 2000, 36, 26. [Google Scholar] [CrossRef]
- Bowden, C.E.; Plaut, K.; Maple, U.L.; Caler, W. Negative Effects of a High Level of Nutrient Intake on Mammary Gland Development of Prepubertal Goats. J. Dairy Sci. 1995, 78, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.R.; Harness, J.R.; Snead, A.F.; Salah, M.S. Mammary Growth Pattern in Goats during Pregnancy and Lactation. J. Dairy Sci. 1981, 64, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Prpar, S.; Martignani, E.; Dovc, P.; Baratta, M. Identification of Goat Mammary Stem/Progenitor Cells1. Biol. Reprod. 2012, 86, 117. [Google Scholar] [CrossRef]
- Safayi, S.; Theil, P.K.; Elbrønd, V.S.; Hou, L.; Engbæk, M.; Nørgaard, J.V.; Sejrsen, K.; Nielsen, M.O. Mammary Remodeling in Primiparous and Multiparous Dairy Goats during Lactation. J. Dairy Sci. 2010, 93, 1478–1490. [Google Scholar] [CrossRef]
- Weller, M.M.D.C.A.; Albino, R.L.; Marcondes, M.I.; Silva, W.; Daniels, K.M.; Campos, M.M.; Duarte, M.S.; Mescouto, M.L.; Silva, F.F.; Guimarães, S.E.F. Effects of Nutrient Intake Level on Mammary Parenchyma Growth and Gene Expression in Crossbred (Holstein × Gyr) Prepubertal Heifers. J. Dairy Sci. 2016, 99, 9962–9973. [Google Scholar] [CrossRef]
- Panzuti, C.; Duvaux-Ponter, C.; Bruckmaier, R.M.; Dessauge, F. Effect of Feeding Level during the Prepubertal Phase on Mammary Gland Development in Female Goat Kids. J. Dairy Res. 2019, 86, 267–271. [Google Scholar] [CrossRef]
- Li, P.; Rudland, P.S.; Fernin, D.G.; Finch, L.M.B.; Wilde, C.J. Modulation of Mammary Development and Programmed Cell Death by the Frequency of Milk Removal in Lactating Goats. J. Physiol. 1999, 519, 885–900. [Google Scholar] [CrossRef]
- Zhao, X.; Ponchon, B.; LanctÃ, S.; Lacasse, P. Invited Review: Accelerating Mammary Gland Involution after Drying-off in Dairy Cattle. J. Dairy Sci. 2019, 102, 6701–6717. [Google Scholar] [CrossRef]
- Wall, E.H.; McFadden, T.B. Triennial Lactation Symposium: A Local Affair: How the Mammary Gland Adapts to Changes in Milking Frequency1,2. J. Anim Sci. 2012, 90, 1695–1707. [Google Scholar] [CrossRef]
- Ben Chedly, H.; Boutinaud, M.; Bernier-Dodier, P.; Marnet, P.-G.; Lacasse, P. Disruption of Cell Junctions Induces Apoptosis and Reduces Synthetic Activity in Lactating Goat Mammary Gland. J. Dairy Sci. 2010, 93, 2938–2951. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, J.; Zhao, Y.; Zheng, Z.; Song, Y.; Wang, H.; Tong, D. The Inhibitory Effect of Melatonin on Mammary Function of Lactating Dairy Goats. Biol. Reprod. 2019, 100, 455–467. [Google Scholar] [CrossRef]
- Brisken, C.; Park, S.; Vass, T.; Lydon, J.P.; O’Malley, B.W.; Weinberg, R.A. A Paracrine Role for the Epithelial Progesterone Receptor in Mammary Gland Development. Proc. Natl. Acad. Sci. USA 1998, 95, 5076–5081. [Google Scholar] [CrossRef]
- Yart, L.; Finot, L.; Marnet, P.-G.; Dessauge, F. Suppression of Ovarian Secretions before Puberty Strongly Affects Mammogenesis in the Goat. J. Dairy Res. 2012, 79, 157–167. [Google Scholar] [CrossRef]
- Matsuda, M.; Imaoka, T.; Vomachka, A.J.; Gudelsky, G.A.; Hou, Z.; Mistry, M.; Bailey, J.P.; Nieport, K.M.; Walther, D.J.; Bader, M.; et al. Serotonin Regulates Mammary Gland Development via an Autocrine-Paracrine Loop. Dev. Cell 2004, 6, 193–203. [Google Scholar] [CrossRef]
- Lacasse, P.; Ollier, S.; Lollivier, V.; Boutinaud, M. New Insights into the Importance of Prolactin in Dairy Ruminants. J. Dairy Sci. 2016, 99, 864–874. [Google Scholar] [CrossRef]
- Flint, D.J.; Knight, C.H. Interactions of Prolactin and Growth Hormone (GH) in the Regulation of Mammary Gland Function and Epithelial Cell Survival. J. Mammary Gland. Biol. Neoplasia 1997, 2, 41–48. [Google Scholar] [CrossRef]
- Kornfeld, J.-W. The Different Functions of Stat5 and Chromatin Alteration through Stat5 Proteins. Front. Biosci. 2008, 13, 6237. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Trujillo, A.; Argüello, A.; Rivero, M.A.; Capote, J.; Castro, N. Short Communication: Differences in Distribution of Serotonin Receptor Subtypes in the Mammary Gland of Sheep, Goats, and Cows during Lactation and Involution. J. Dairy Sci. 2019, 102, 2703–2707. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Q.; Gao, X. Expression and Function of Leptin and Its Receptor in Dairy Goat Mammary Gland. J. Dairy Res. 2010, 77, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Linzell, J.L. Mammary-Gland Blood Flow and Oxygen, Glucose and Volatile Fatty Acid Uptake in the Conscious Goat. J. Physiol. 1960, 153, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Burvenich, C. Variations of Mammary Artery Blood Flow and Milk Yield under Normal Conditions and during the Oestrous Cycle of the Dairy Goat. Z. Tierphysiol. Tierernahr. Futtermittelkd. 1980, 43, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.O.; Cvek, K.; Dahlborn, K. Evolution of the Mammary Capillary Network and Carbonic Anhydrase Activity throughout Lactation and during Somatotropin Treatment in Goats. J. Dairy Res. 2010, 77, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Silanikove, N.; Koluman (Darcan), N. Impact of Climate Change on the Dairy Industry in Temperate Zones: Predications on the Overall Negative Impact and on the Positive Role of Dairy Goats in Adaptation to Earth Warming. Small Rumin. Res. 2015, 123, 27–34. [Google Scholar] [CrossRef]
- Nguyen, T.; Chaiyabutr, N.; Chanpongsang, S.; Thammacharoen, S. Dietary Cation and Anion Difference: Effects on Milk Production and Body Fluid Distribution in Lactating Dairy Goats under Tropical Conditions. J. Anim. Sci. 2018, 89, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.S.; Barbour, E.K.; Abi-Said, M.R.; Chedid, M.; Giger-Reverdin, S.; Duvaux-Ponter, C.; Morand-Fehr, P.; Hamadeh, S.K. Responses to Repeated Cycles of Water Restriction in Lactating Shami Goats. J. Appl. Anim. Res. 2015, 43, 39–45. [Google Scholar] [CrossRef]
- Alamer, M. Physiological Responses of Saudi Arabia Indigenous Goats to Water Deprivation. Small Rumin. Res. 2006, 63, 100–109. [Google Scholar] [CrossRef]
- Hossaini-Hilali, J.; Benlamlih, S.; Dahlborn, K. Effects of Dehydration, Rehydration, and Hyperhydration in the Lactating and Non-Lactating Black Moroccan Goat. Comp. Biochem. Physiol. A Physiol. 1994, 109, 1017–1026. [Google Scholar] [CrossRef]
- Maltz, E.; Olson, K.; Glrk, S.M.; Fyhrquist, F.; Silanikove, N.; Choshniak, I.; Shkolnik, A. Homeostatic Responses to Water Deprivation or Hemorrhage in Non-Lactating Bedouin Goats. Biochrm. Physiol. 1984, 77, 79–84. [Google Scholar] [CrossRef]
- Al-Ramamneh, D.; Riek, A.; Gerken, M. Effect of Water Restriction on Drinking Behaviour and Water Intake in German Black-Head Mutton Sheep and Boer Goats. Animal 2012, 6, 173–178. [Google Scholar] [CrossRef]
- Silanikove, N.; Tagari, H.; Shkolnik, A. Comparison of Rate of Passage, Fermentation Rate and Efficiency of Digestion of High Fiber Diet in Desert Bedouin Goats Compared to Swiss Saanen Goats. Small Rumin. Res. 1993, 12, 45–60. [Google Scholar] [CrossRef]
- Dickhoefer, U.; Ramadhan, M.R.; Apenburg, S.; Buerkert, A.; Schlecht, E. Effects of Mild Water Restriction on Nutrient Digestion and Protein Metabolism in Desert-Adapted Goats. Small Rumin. Res. 2021, 204, 106500. [Google Scholar] [CrossRef]
- Ramadhan, M.R.; Schlecht, E.; Dickhoefer, U.; Mahgoub, O.; Joergensen, R.G. Feed Digestibility, Digesta Passage and Faecal Microbial Biomass in Desert-adapted Goats Exposed to Mild Water Restriction. J. Anim. Physiol. Anim. Nutr. 2022, 106, 721–732. [Google Scholar] [CrossRef]
- Giger-Reverdin, S.; Domange, C.; Broudiscou, L.P.; Sauvant, D.; Berthelot, V. Rumen Function in Goats, an Example of Adaptive Capacity. J. Dairy Res. 2020, 87, 45–51. [Google Scholar] [CrossRef]
- Al-Tamimi, H.; Al-Atiyat, R.; Al-Majali, A.; Alameri, O. Renal Efficiency Underlies Adaptive Heterothermy of Heat-Stressed Hypohydrated Goats. Trop. Anim. Health Prod. 2019, 51, 2287–2295. [Google Scholar] [CrossRef]
- Gupta, M.; Mondal, T. Heat Stress and Thermoregulatory Responses of Goats: A Review. Biol. Rhythm. Res. 2021, 52, 407–433. [Google Scholar] [CrossRef]
- Chaiyabutr, N.; Faulkner, A.; Peaker, M. Effects of Starvation on the Cardiovascular System, Water Balance and Milk Secretion in Lactating Goats. Res. Vet. Sci. 1980, 28, 291–295. [Google Scholar] [CrossRef]
- Dahlborn, K.; Olaf Nielsen, M.; Hossaini-Hilali, J. Mechanisms Causing Decreased Milk Production in Water Deprived Goats. CIHEAM Opt. Mediterr. 1997, 34, 199–202. [Google Scholar]
- Olsson, K.; Carlsson, E. Cardiovascular Changes Associated with Dehydration and Drinking in Unrestrained, Lactating Goats. Exp. Physiol. 1999, 84, 571–578. [Google Scholar] [CrossRef]
- Sakanashi, T.M.; Brigham, H.E.; Rasmussen, K.M. Effect of Dietary Restriction during Lactation on Cardiac Output, Organ Blood Flow and Organ Weights of Rats. J. Nutr. 1987, 117, 1469–1474. [Google Scholar] [CrossRef]
- Stewart, K.W.; Cooper, G.J.S.; Davis, S.R. Coordination of Mammary Metabolism and Blood Flow after Refeeding in Rats. J. Dairy Sci. 2009, 92, 1543–1553. [Google Scholar] [CrossRef]
- Zucali, M.; Lovarelli, D.; Celozzi, S.; Bacenetti, J.; Sandrucci, A.; Bava, L. Management Options to Reduce the Environmental Impact of Dairy Goat Milk Production. Livest. Sci. 2020, 231, 103888. [Google Scholar] [CrossRef]
- Mattiello, S.; Battini, M.; Vieira, A.; Stilwell, G. AWIN Welfare Assessment Protocol for Goats; AWIN: Edinburgh, UK, 2015. [Google Scholar] [CrossRef]
- Scopinich-Cisternas, J.; Strahsburger, E. Goat Type: The Key Factor to Produce Goat Milk with Economic Profitable Purpose in Arid and Desert Zones. IDESIA 2019, 37, 123–128. [Google Scholar] [CrossRef]
- Menchaca, A.; Ungerfeld, R. Sustainable Goat Production in Adverse Environments: Volume I. In Sustainable Goat Production in Adverse Environments: Volume I; Simões, J., Gutiérrez, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 71–88. [Google Scholar]
- Kume, K.; Papa, L.; Hajno, L. Effects on Milk Production in F1 Crossbred of Alpine Goat Breed (♂) and Albanian Goat Breed (♀). Ital. J. Anim. Sci. 2012, 11, e47. [Google Scholar] [CrossRef][Green Version]
- Misra, R.K. Present Role and Future Potential of Goats in Semi-Arid Regions of India. Outlook Agric. 1987, 16, 136–139. [Google Scholar] [CrossRef]
- Koluman Darcan, N.; Silanikove, N. The Advantages of Goats for Future Adaptation to Climate Change: A Conceptual Overview. Small Rumin. Res. 2018, 163, 34–38. [Google Scholar] [CrossRef]
- Elbeltagy, A.R.; Aboul Naga, A.M.; Hassen, H.; Solouma, G.M.; Rischkowsky, B.; Mwacharo, J.M. Genetic Diversity and Structure of Goats within an Early Livestock Dispersal Area in Eastern North Africa. Afr. J. Biotechnol. 2016, 15, 431–441. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.-R.; Lv, F.-H.; He, S.-G.; Tian, S.-L.; Peng, W.-F.; Sun, Y.-W.; Zhao, Y.-X.; Tu, X.-L.; Zhang, M.; et al. Whole-Genome Sequencing of Native Sheep Provides Insights into Rapid Adaptations to Extreme Environments. Mol. Biol. Evol. 2016, 33, 2576–2592. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, X.; Li, X.; Wang, J.; Wang, C. Milk Compositional Changes of Laoshan Goat Milk from Partum up to 261 Days Postpartum. J. Anim. Sci. 2018, 89, 1355–1363. [Google Scholar] [CrossRef]
- Contreras-Jodar, A.; Nayan, N.H.; Hamzaoui, S.; Caja, G.; Salama, A.A.K. Heat Stress Modifies the Lactational Performances and the Urinary Metabolomic Profile Related to Gastrointestinal Microbiota of Dairy Goats. PLoS ONE 2019, 14, e0202457. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Samee, A.M.; Kamal, T.H.; Abu-Sinna, G.; Hagag, A.M. Alleviation of the Heat Load on Lactating Goats with the Use of Diuretics and Drinking Cool Water. Beitr. Trop. Landwirtsch. Veterinarmed. 1992, 30, 91–99. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geldsetzer-Mendoza, C.; Riveros, J.L. Morphophysiological Responses of the Goat Mammary Gland to Water Scarcity in Arid and Semi-Arid Environments: Are They Enough to Generate Adaptation to New Climatic Challenges? Animals 2023, 13, 3825. https://doi.org/10.3390/ani13243825
Geldsetzer-Mendoza C, Riveros JL. Morphophysiological Responses of the Goat Mammary Gland to Water Scarcity in Arid and Semi-Arid Environments: Are They Enough to Generate Adaptation to New Climatic Challenges? Animals. 2023; 13(24):3825. https://doi.org/10.3390/ani13243825
Chicago/Turabian StyleGeldsetzer-Mendoza, Carolina, and José Luis Riveros. 2023. "Morphophysiological Responses of the Goat Mammary Gland to Water Scarcity in Arid and Semi-Arid Environments: Are They Enough to Generate Adaptation to New Climatic Challenges?" Animals 13, no. 24: 3825. https://doi.org/10.3390/ani13243825
APA StyleGeldsetzer-Mendoza, C., & Riveros, J. L. (2023). Morphophysiological Responses of the Goat Mammary Gland to Water Scarcity in Arid and Semi-Arid Environments: Are They Enough to Generate Adaptation to New Climatic Challenges? Animals, 13(24), 3825. https://doi.org/10.3390/ani13243825





