Effects of LED Light Colors on the Growth Performance, Intestinal Morphology, Cecal Short-Chain Fatty Acid Concentrations and Microbiota in Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Growth Performance
2.3. Intestinal Histomorphology
2.4. Cecal Sampling and Short-Chain Fatty Acid Measurement
2.5. Cecal Microbiota
2.6. Statistical Analysis
3. Results
3.1. Effects of LED Light Colors on the Growth Performance
3.2. Effects of LED Light Colors on the Intestinal Morphology
3.3. Effects of LED Light Colors on the Cecal SCFAs Concentrations
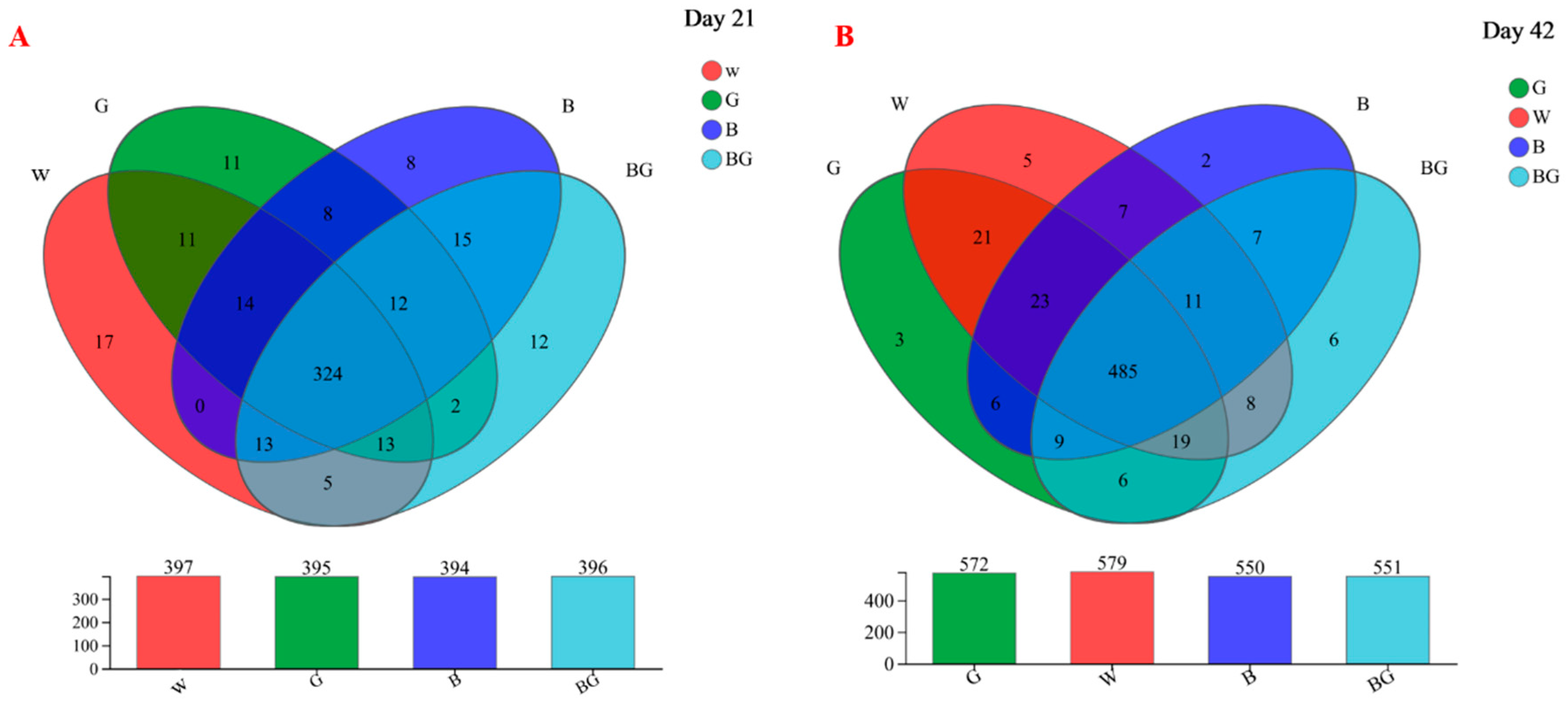
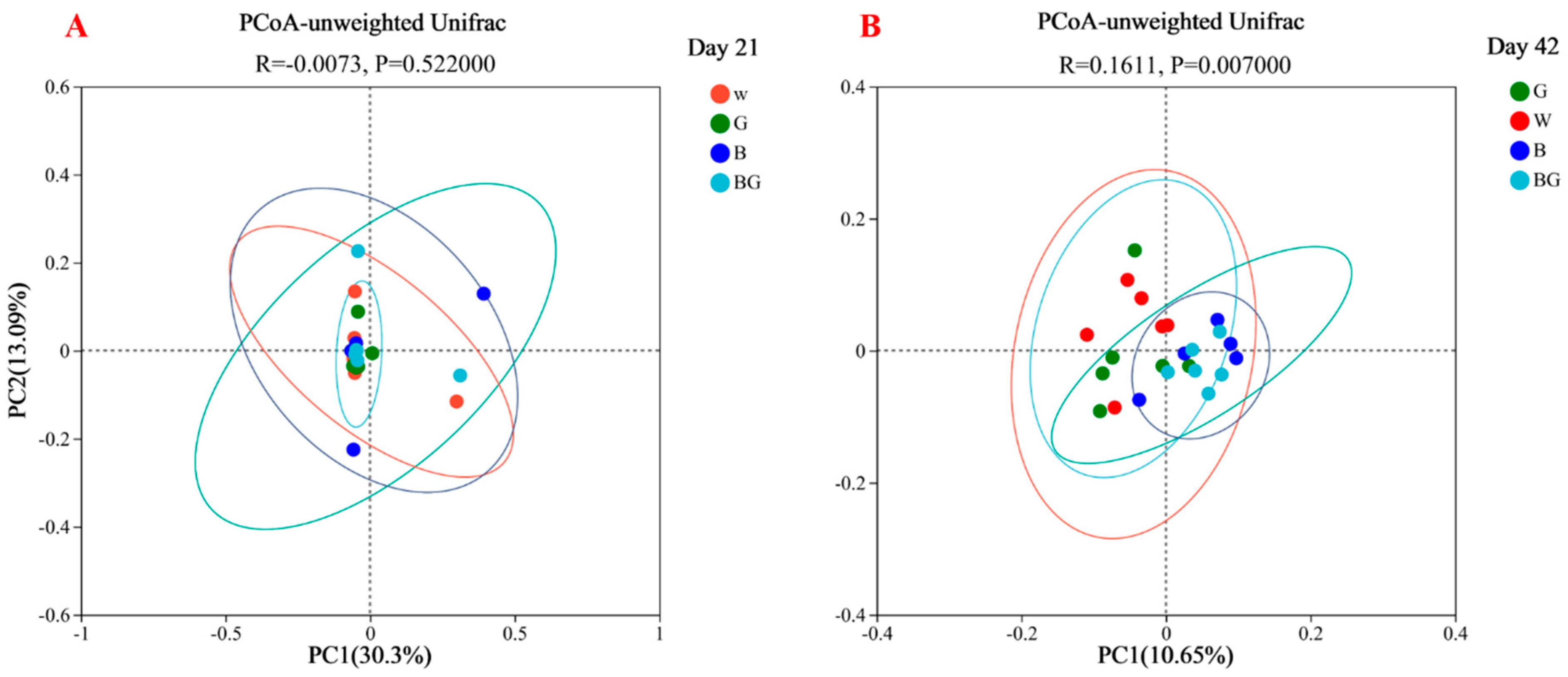
3.4. Species Annotation and Assessment in Cecal Microbiota
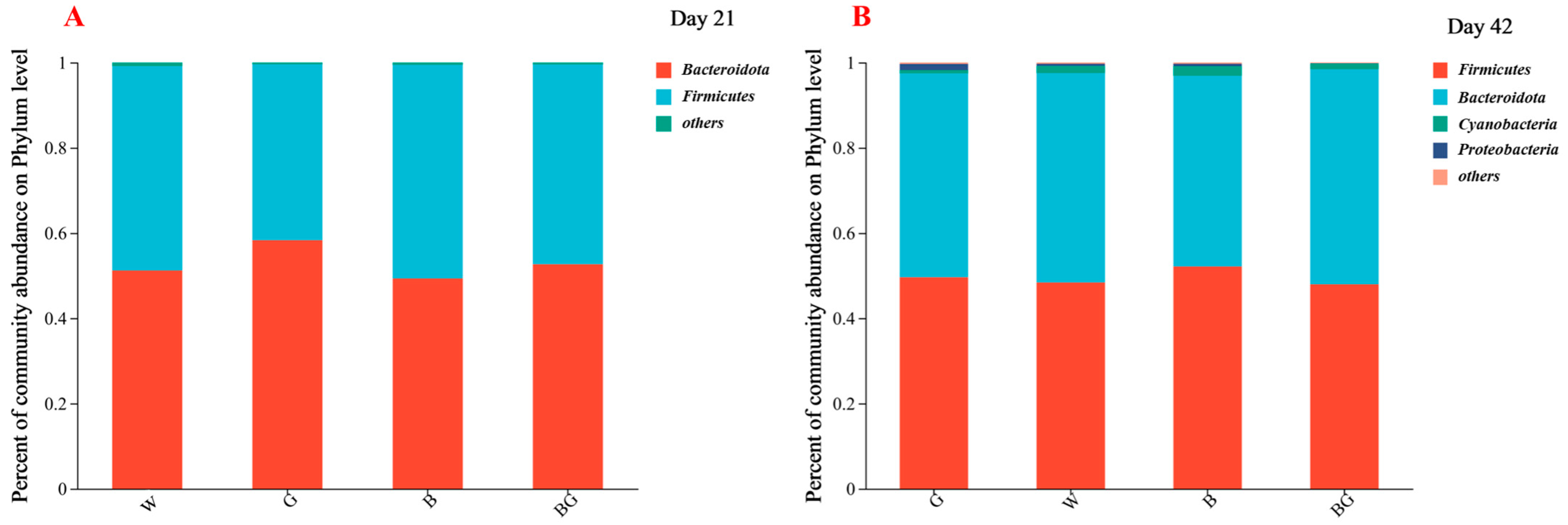
3.5. Effects of LED Light Colors on the Cecal Microbiota at the Phylum Level
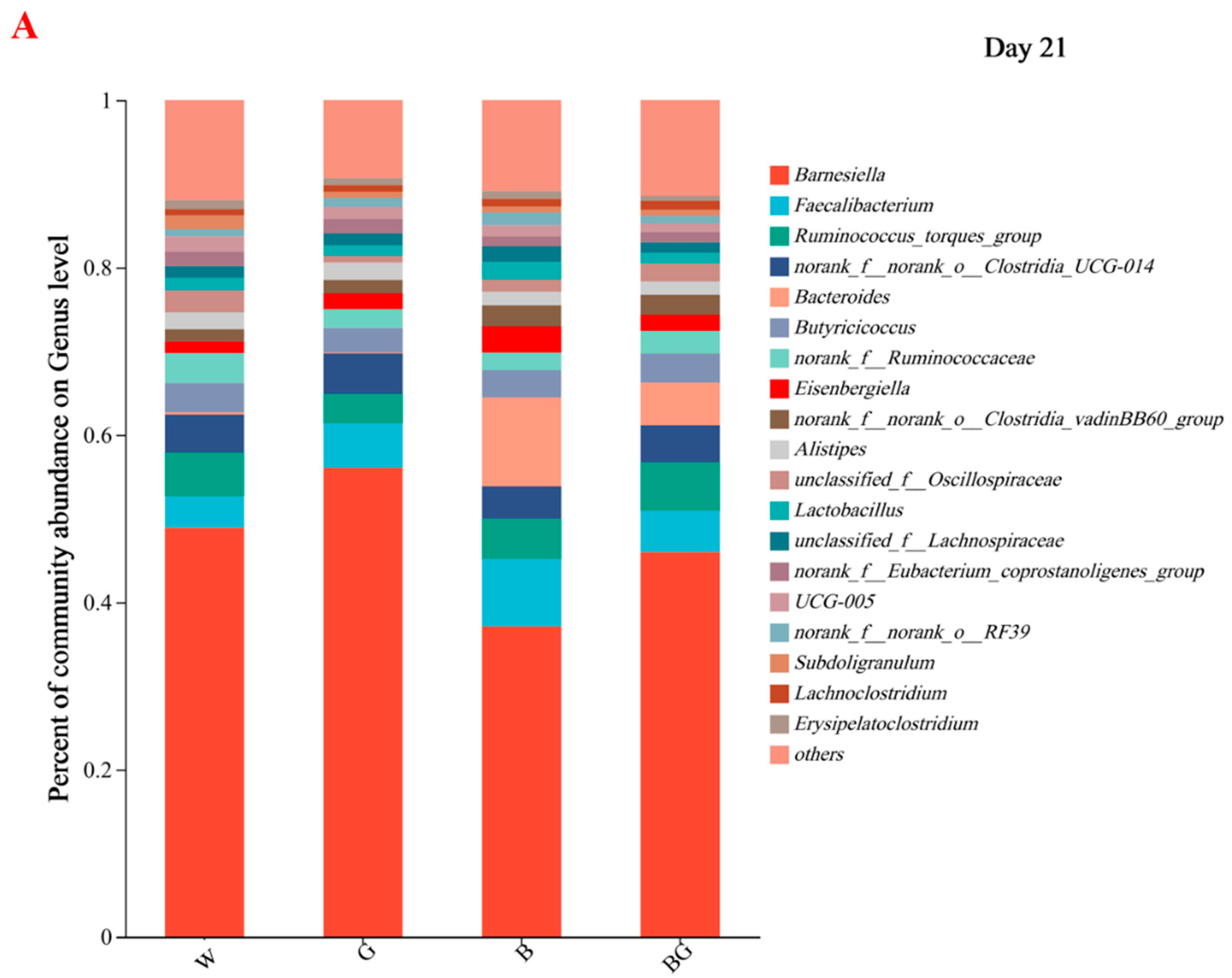
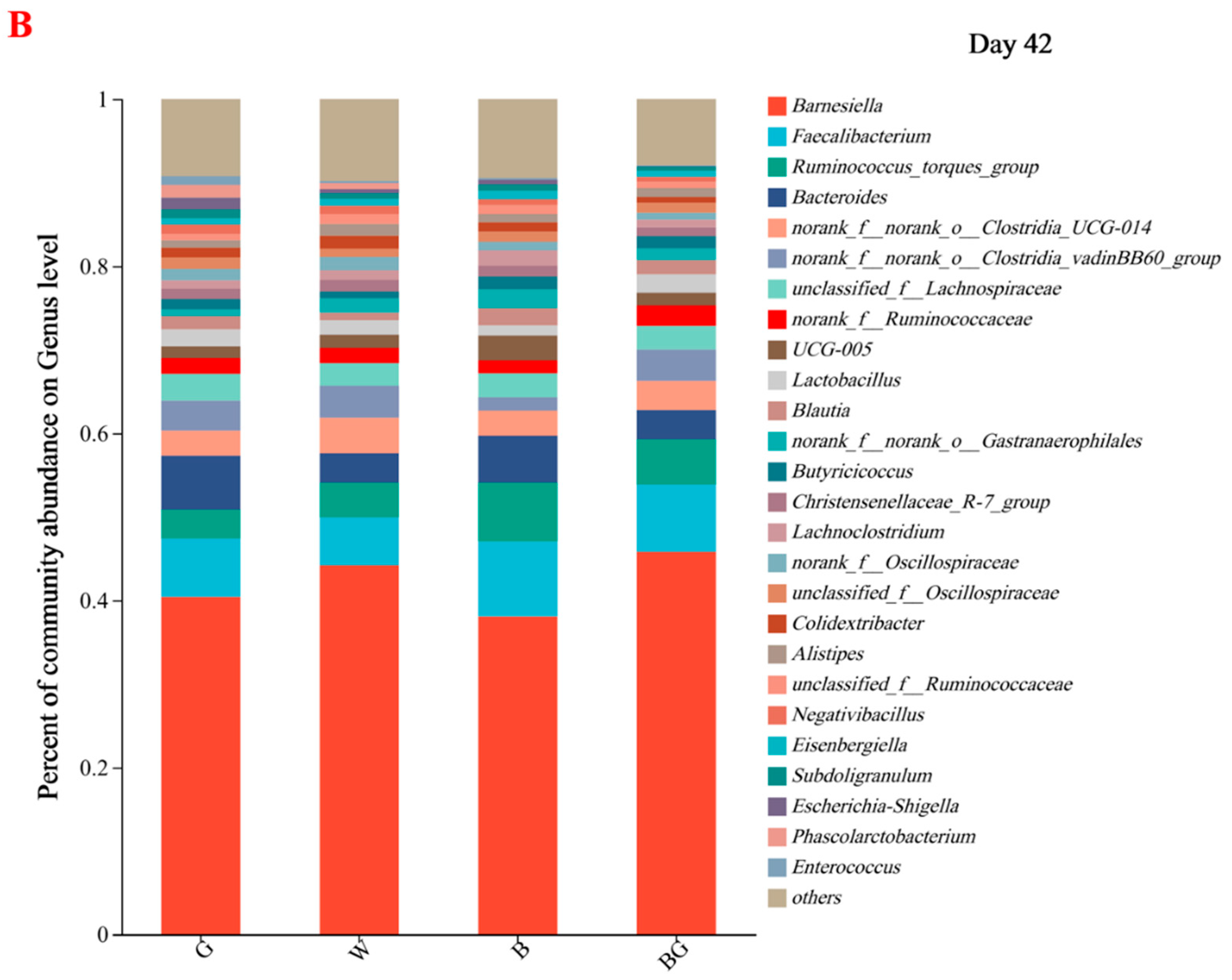
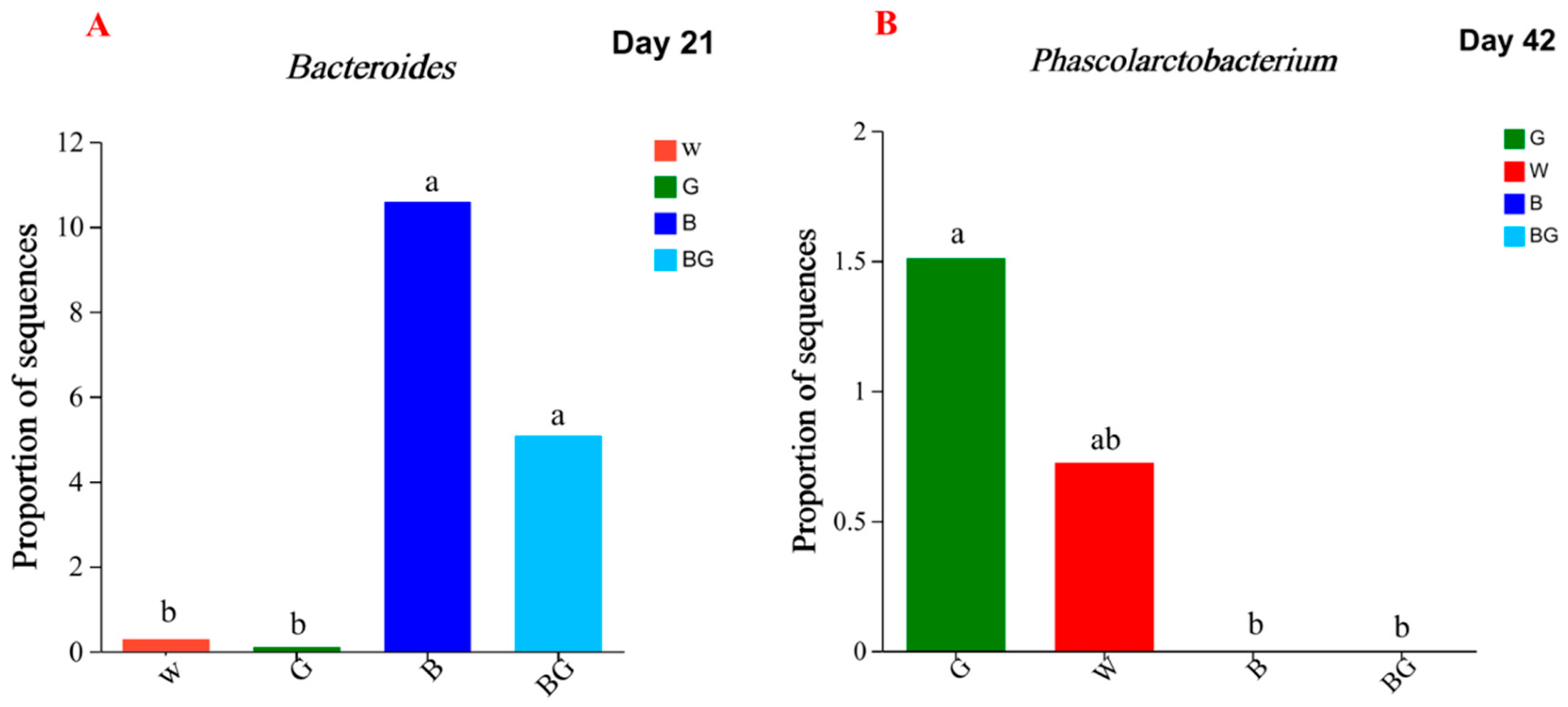
3.6. Effects of LED Light Colors on the Cecal Microbiota at the Genera Level
3.7. Correlations between Cecal Microbiota and SCFAs
4. Discussion
4.1. Growth Performance
4.2. Intestinal Morphology
4.3. Cecal SCFAs Concentrations
4.4. Cecal Microbiota
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemmer, B. The Circadian Clock is Now Ticking Even in Stockholm—Nobel Prize in Medicine 2017. Dtsch. Med. Wochenschr. 2017, 142, 1897–1900. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Okimura, K.; Yoshimura, T. Light and Hormones in Seasonal Regulation of Reproduction and Mood. Endocrinology 2020, 161, bqaa130. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Kamagata, M.; Hirao, M.; Yasuda, S.; Iwami, S.; Sasaki, H.; Tsubosaka, M.; Hattori, Y.; Todoh, A.; Tamura, K.; et al. Glucagon and/or IGF-1 Production Regulates Resetting of the Liver Circadian Clock in Response to a Protein or Amino Acid-only Diet. EBioMedicine 2018, 28, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Yadlapalli, S.; Jiang, C.; Bahle, A.; Reddy, P.; Meyhofer, E.; Shafer, O.T. Circadian clock neurons constantly monitor environmental temperature to set sleep timing. Nature 2018, 555, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.; Lazar, M.A. Circadian time signatures of fitness and disease. Science 2016, 354, 994–999. [Google Scholar] [CrossRef]
- Surbhi; Kumar, V. Avian photoreceptors and their role in the regulation of daily and seasonal physiology. Gen. Comp. Endocrinol. 2015, 220, 13–22. [Google Scholar] [CrossRef]
- Golombek, D.A.; Rosenstein, R.E. Physiology of circadian entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef]
- Viets, K.; Eldred, K.; Johnston, R.J., Jr. Mechanisms of Photoreceptor Patterning in Vertebrates and Invertebrates. Trends Genet. 2016, 32, 638–659. [Google Scholar] [CrossRef]
- Cao, J.; Liu, W.; Wang, Z.; Xie, D.; Jia, L.; Chen, Y. Green and blue monochromatic lights promote growth and development of broilers via stimulating testosterone secretion and myofiber growth. J. Appl. Poult. Res. 2008, 17, 211–218. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z.; Chen, Y. Effects of monochromatic light on developmental changes in satellite cell population of pectoral muscle in broilers during early posthatch period. Anat. Rec. 2010, 293, 1315–1324. [Google Scholar] [CrossRef]
- Nelson, J.R.; Bray, J.L.; Delabbio, J.; Archer, G.S. Light emitting diode (LED) color and broiler growth: Effect of supplementing blue/green LED to white LED light on broiler growth, stress, and welfare. Poult. Sci. 2020, 99, 3519–3524. [Google Scholar] [CrossRef] [PubMed]
- Rozenboim, I.; Biran, I.; Chaiseha, Y.; Yahav, S.; Rosenstrauch, A.; Sklan, D.; Halevy, O. The effect of a green and blue monochromatic light combination on broiler growth and development. Poult. Sci. 2004, 83, 842–845. [Google Scholar] [CrossRef] [PubMed]
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Rosselli, M.; Bellini, M.; Laffi, G.; Milani, S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: The top-down and the bottom-up model. J. Gastroenterol. 2012, 47, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, J.; Ling, Z. Short-chain fatty acids-producing probiotics: A novel source of psychobiotics. Crit. Rev. Food Sci. Nutr. 2022, 62, 7929–7959. [Google Scholar] [CrossRef] [PubMed]
- Frazier, K.; Chang, E.B. Intersection of the Gut Microbiome and Circadian Rhythms in Metabolism. Trends Endocrinol. Metab. 2020, 31, 25–36. [Google Scholar] [CrossRef]
- Wu, G.; Tang, W.; He, Y.; Hu, J.; Gong, S.; He, Z.; Wei, G.; Lv, L.; Jiang, Y.; Zhou, H.; et al. Light exposure influences the diurnal oscillation of gut microbiota in mice. Biochem. Biophys. Res. Commun. 2018, 501, 16–23. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, H.; Wang, X.; Xia, W.; Lv, W.; Xiao, Y.; Zou, X. Early Intervention with Cecal Fermentation Broth Regulates the Colonization and Development of Gut Microbiota in Broiler Chickens. Front. Microbiol. 2019, 10, 1422. [Google Scholar] [CrossRef]
- Seo, H.S.; Kang, M.; Yoon, R.H.; Roh, J.H.; Wei, B.; Ryu, K.S.; Cha, S.Y.; Jang, H.K. Effects of Various LED Light Colors on Growth and Immune Response in Broilers. J. Poult. Sci. 2016, 53, 76–81. [Google Scholar] [CrossRef]
- Retes, P.L.; Espósito, M.; das Neves, D.G.; Viana, A.G.; Coelho, L.M.; Bobadilla-Mendez, M.F.; Alvarenga, R.R.; Fassani, E.J.; Peixoto, J.V.; Zangeronimo, M.G. Influence of different types of lamps on the reproductive development of male Japanese quail (Coturnix coturnix japonica). Theriogenology 2017, 94, 59–63. [Google Scholar] [CrossRef]
- Oke, O.E.; Oni, A.I.; Adebambo, P.O.; Oso, O.M.; Adeoye, M.M.; Lawal, T.G.; Afolayan, T.R.; Ogunbajo, O.E.; Ojelade, D.I.; Bakre, O.A.; et al. Evaluation of light colour manipulation on physiological response and growth performance of broiler chickens. Trop. Anim. Health Prod. 2020, 53, 6. [Google Scholar] [CrossRef]
- Elnesr, S.S.; Alagawany, M.; Elwan, H.A.M.; Fathi, M.A.; Farag, M.R. Effect of sodium butyrate on intestinal health of poultry—A review. Ann. Anim. Sci. 2020, 20, 29–41. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Chen, K.; Zhao, X.; Geng, Z. Effect of l-theanine on growth performance, intestinal development and health, and peptide and amino acid transporters expression of broilers. J. Sci. Food Agric. 2020, 100, 1718–1725. [Google Scholar] [CrossRef]
- Naser, N.M.; Mohsen, S.S.; Reza, K.; Hamideh, M. Histomorphometric analysis of the small intestine of broiler chick embryos injected in ovo with methionine. Anim. Prod. Sci. 2017, 59, 133–139. [Google Scholar] [CrossRef]
- Cheled-Shoval, S.L.; Amit-Romach, E.; Barbakov, M.; Uni, Z. The effect of in ovo administration of mannan oligosaccharide on small intestine development during the pre- and posthatch periods in chickens. Poult. Sci. 2011, 90, 2301–2310. [Google Scholar] [CrossRef]
- Liao, X.; Shao, Y.; Sun, G.; Yang, Y.; Zhang, L.; Guo, Y.; Luo, X.; Lu, L. The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers. Poult. Sci. 2020, 99, 5883–5895. [Google Scholar] [CrossRef]
- Xie, D.; Li, J.; Wang, Z.X.; Cao, J.; Li, T.T.; Chen, J.L.; Chen, Y.X. Effects of monochromatic light on mucosal mechanical and immunological barriers in the small intestine of broilers. Poult. Sci. 2011, 90, 2697–2704. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, C.; Zhong, R.; Pan, J. The quantitative models for broiler chicken response to monochromatic, combined, and mixed light-emitting diode light: A meta-analysis. Poult. Sci. 2018, 97, 1980–1989. [Google Scholar] [CrossRef]
- Mackie, A. The Digestive Tract: A Complex System. In Interdisciplinary Approaches to Food Digestion; Gouseti, O., Bornhorst, G., Bakalis, S., Mackie, A., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Choi, S.I.; Son, J.H.; Kim, N.; Kim, Y.S.; Nam, R.H.; Park, J.H.; Song, C.H.; Yu, J.E.; Lee, D.H.; Yoon, K.; et al. Changes in Cecal Microbiota and Short-chain Fatty Acid During Lifespan of the Rat. J. Neurogastroenterol. Motil. 2021, 27, 134–146. [Google Scholar] [CrossRef]
- Salazar, N.; Arboleya, S.; Fernández-Navarro, T.; de Los Reyes-Gavilán, C.G.; Gonzalez, S.; Gueimonde, M. Age-Associated Changes in Gut Microbiota and Dietary Components Related with the Immune System in Adulthood and Old Age: A Cross-Sectional Study. Nutrients 2019, 11, 1765. [Google Scholar] [CrossRef]
- Smith, E.A.; Macfarlane, G.T. Dissimilatory amino Acid metabolism inhuman colonic bacteria. Anaerobe 1997, 3, 327–337. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Howard, A.G.; Meyer, K.A.; Tsilimigras, M.C.B.; Avery, C.L.; Sha, W.; Sun, S.; Zhang, J.; Su, C.; et al. Circulating Short-Chain Fatty Acids Are Positively Associated with Adiposity Measures in Chinese Adults. Nutrients 2020, 12, 2127. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Z.; Greenwald, J.; Kothapalli, K.S.; Park, H.G.; Liu, R.; Mendralla, E.; Lawrence, P.; Wang, X.; Brenna, J.T. BCFA suppresses LPS induced IL-8 mRNA expression in human intestinal epithelial cells. Prostaglandins Leukot. Essent. Fat. Acids 2017, 116, 27–31. [Google Scholar] [CrossRef]
- Tognini, P.; Murakami, M.; Sassone-Corsi, P. Interplay between Microbes and the Circadian Clock. Cold Spring Harb. Perspect. Biol. 2018, 10, a028365. [Google Scholar] [CrossRef]
- Zhu, Y.; Shi, X.; Lin, X.; Ye, K.; Xu, X.; Li, C.; Zhou, G. Beef, Chicken, and Soy Proteins in Diets Induce Different Gut Microbiota and Metabolites in Rats. Front. Microbiol. 2017, 8, 1395. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Lawley, B.; Tannock, G.; Engberg, R.M. Bacterial Succession in the Broiler Gastrointestinal Tract. Appl. Environ. Microbiol. 2016, 82, 2399–2410. [Google Scholar] [CrossRef]
- Zhou, Q.; Lan, F.; Li, X.; Yan, W.; Sun, C.; Li, J.; Yang, N.; Wen, C. The Spatial and Temporal Characterization of Gut Microbiota in Broilers. Front. Vet. Sci. 2021, 8, 712226. [Google Scholar] [CrossRef]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef]
- Barcena, C.; Valdés-Mas, R.; Mayoral, P.; Garabaya, C.; Durand, S.; Rodríguez, F.; Fernández-García, M.T.; Salazar, N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 2019, 25, 1234–1242. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, K.; Chen, H.; Su, Y.; Zhu, W. Caecal infusion of the short-chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Microb. Biotechnol. 2018, 11, 859–868. [Google Scholar] [CrossRef]
- Ogata, Y.; Suda, W.; Ikeyama, N.; Hattori, M.; Ohkuma, M.; Sakamoto, M. Complete Genome Sequence of Phascolarctobacterium faecium JCM 30894, a Succinate-Utilizing Bacterium Isolated from Human Feces. Microbiol. Resour. Announc. 2019, 8, e01487-18. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nagai, F.; Morotomi, M. Characterization of Phascolarctobacterium succinatutens sp. nov.; an asaccharolytic, succinate-utilizing bacterium isolated from human feces. Appl. Environ. Microbiol. 2012, 78, 511–518. [Google Scholar] [CrossRef]
- Sakaridis, I.; Ellis, R.J.; Cawthraw, S.A.; van Vliet, A.H.M.; Stekel, D.J.; Penell, J.; Chambers, M.; La Ragione, R.M.; Cook, A.J. Investigating the Association Between the Caecal Microbiomes of Broilers and Campylobacter Burden. Front. Inmicrobiol. 2018, 9, 927. [Google Scholar] [CrossRef]
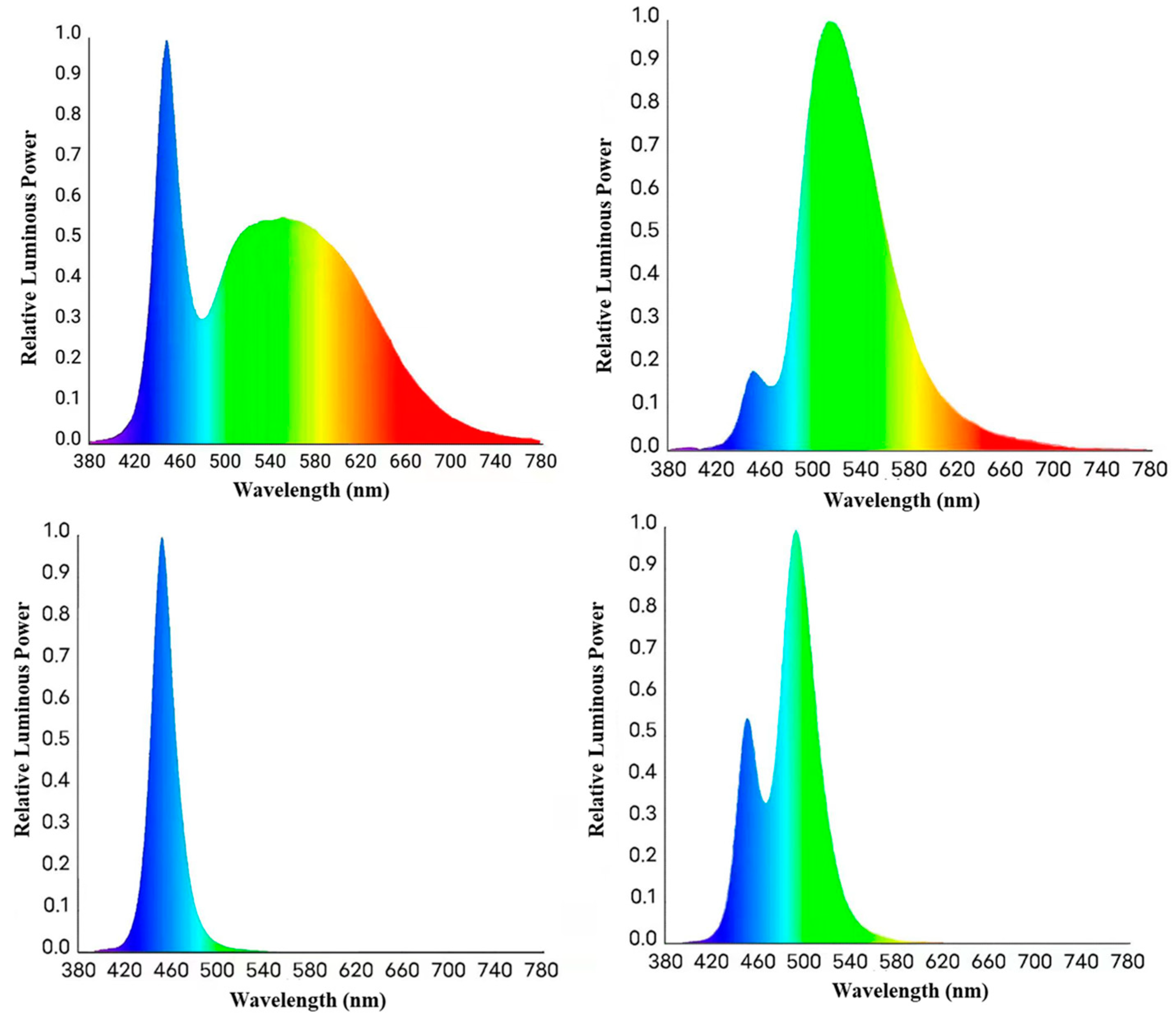


| Items | White Light | Green Light | Blue Light | Blue-Green Light | p-Value | ||
|---|---|---|---|---|---|---|---|
| Age | Light Color | Age × Light Color | |||||
| BW 1/g | |||||||
| 21 d | 806.03 ± 27.57 Bab | 779.92 ± 23.34 Bb | 822.97 ± 5.59 Ba | 796.29 ± 16.80 Bab | <0.001 | <0.001 | <0.001 |
| 42 d | 2338.89 ± 75.78 Ab | 2212.68 ± 29.61 Ac | 2492.53 ± 74.23 Aa | 2456.06 ± 119.67 Aa | |||
| ADFI 2/g | |||||||
| 21 d | 48.73 ± 1.30 Bb | 48.13 ± 1.08 Bb | 51.06 ± 0.68 Ba | 48.19 ± 2.25 Bb | <0.001 | <0.001 | 0.111 |
| 42 d | 82.72 ± 2.16 Aab | 77.45 ± 4.94 Ab | 86.28 ± 4.11 Aa | 78.83 ± 5.20 Ab | |||
| ADG 3/g | |||||||
| 21 d | 36.20 ± 1.22 Bab | 34.95 ± 1.56 Bc | 37.19 ± 0.48 Ba | 35.48 ± 0.43 Bbc | <0.001 | <0.001 | <0.001 |
| 42 d | 54.59 ± 1.78 Ab | 51.59 ± 0.69 Ac | 58.25 ± 1.78 Aa | 57.38 ± 2.81 Aa | |||
| FCR 4 | |||||||
| 21 d | 1.37 ± 0.21 B | 1.34 ± 0.05 B | 1.37 ± 0.12 B | 1.35 ± 0.06 B | <0.001 | 0.028 | 0.079 |
| 42 d | 1.52 ± 0.06 Aa | 1.50 ± 0.09 Aa | 1.48 ± 0.10 Aa | 1.38 ± 0.09 Ab | |||
| Items | Stage | White Light | Green Light | Blue Light | Blue–Green Light | p-Value | ||
|---|---|---|---|---|---|---|---|---|
| Age | Light Color | Age × Light Color | ||||||
| Duodenum | ||||||||
| Villus Height/μm | 21 d | 1016.82 ± 163.83 B | 957.12 ± 140.73 B | 1023.87 ± 133.83 B | 1056.44 ± 26.92 B | <0.001 | 0.559 | 0.949 |
| 42 d | 1300.90 ± 219.90 A | 1258.40 ± 71.89 A | 1380.93 ± 219.18 A | 1343.38 ± 216.58 A | ||||
| Crypt Depth/μm | 21 d | 96.77 ± 4.62 | 83.14 ± 6.45 | 94.59 ± 16.54 | 102.06 ± 22.79 | 0.428 | 0.063 | 0.521 |
| 42 d | 99.36 ± 17.77 | 86.12 ± 10.23 | 85.76 ± 10.32 | 92.72 ± 5.61 | ||||
| V/C 1 | 21 d | 10.51 ± 1.19 B | 12.62 ± 3.00 B | 11.46 ± 2.54 B | 10.78 ± 2.74 B | <0.001 | 0.008 | 0.146 |
| 42 d | 12.14 ± 1.00 Ac | 15.45 ± 1.17 Aab | 17.40 ± 2.70 Aa | 14.18 ± 0.45 Abc | ||||
| Jejunum | ||||||||
| Villus Height/μm | 21 d | 957.39 ± 95.65 Bab | 809.95 ± 91.03 Bb | 1103.88 ± 174.57 Ba | 992.64 ± 168.35 Ba | <0.001 | 0.038 | 0.363 |
| 42 d | 1301.59 ± 279.39 A | 1076.72 ± 230.86 A | 1178.98 ± 57.39 A | 1244.10 ± 117.57 A | ||||
| Crypt Depth/μm | 21 d | 75.76 ± 5.94 B | 76.13 ± 7.49 B | 83.84 ± 9.59 B | 75.38 ± 7.06 B | 0.006 | 0.037 | 0.989 |
| 42 d | 81.71 ± 2.13 A | 83.30 ± 5.99 A | 89.00 ± 7.78 A | 81.70 ± 6.12 A | ||||
| V/C | 21 d | 12.74 ± 1.93 | 11.61 ± 2.33 | 13.39 ± 2.99 | 13.36 ± 3.12 | 0.193 | 0.266 | 0.416 |
| 42 d | 16.12 ± 3.88 | 12.61 ± 3.53 | 12.64 ± 2.71 | 14.28 ± 2.90 | ||||
| Ileum | ||||||||
| Villus Height/μm | 21 d | 805.18 ± 64.25 Bab | 744.07 ± 40.46 Bb | 836.21 ± 75.90 Ba | 873.58 ± 40.13 Ba | 0.004 | 0.184 | 0.255 |
| 42 d | 879.85 ± 224.98 A | 971.87 ± 134.63 A | 857.75 ± 62.91 A | 1017.64 ± 150.49 A | ||||
| Crypt Depth/μm | 21 d | 100.64 ± 16.21 a | 80.05 ± 11.41 bc | 87.90 ± 9.39 ab | 72.09 ± 3.07 c | 0.614 | 0.001 | 0.002 |
| 42 d | 82.08 ± 6.00 b | 83.18 ± 11.27 b | 98.32 ± 3.80 a | 82.98 ± 5.08 b | ||||
| V/C | 21 d | 8.55 ± 1.47 b | 9.43 ± 1.20 b | 10.04 ± 2.04 ab | 11.83 ± 0.67 a | 0.076 | 0.002 | 0.044 |
| 42 d | 10.72 ± 2.66 ab | 11.73 ± 1.34 a | 8.69 ± 0.55 b | 12.31 ± 2.00 a | ||||
| Items | White Light | Green Light | Blue Light | Blue–Green Light | p-Value | ||
|---|---|---|---|---|---|---|---|
| Age | Light Color | Age × Light Color | |||||
| Acetate, mmol/L | |||||||
| 21 d | 61.85 ± 8.51 A | 66.64 ± 5.09 A | 64.79 ± 13.67 A | 79.93 ± 25.72 A | <0.001 | 0.174 | 0.308 |
| 42 d | 16.24 ± 6.20 B | 26.59 ± 10.64 B | 17.69 ± 0.42 B | 19.99 ± 9.02 B | |||
| Propionate, mmol/L | |||||||
| 21 d | 15.23 ± 1.04 Ab | 17.49 ± 1.26 Ab | 17.21 ± 5.31 Ab | 26.66 ± 9.13 Aa | <0.001 | 0.009 | 0.002 |
| 42 d | 3.31 ± 1.85 B | 3.77 ± 2.18 B | 2.48 ± 0.35 B | 2.59 ± 1.43 B | |||
| Isobutyrate, mmol/L | |||||||
| 21 d | 2.93 ± 0.25 Ab | 3.35 ± 0.12 Ab | 3.04 ± 0.24 Ab | 5.05 ± 1.52 Aa | <0.001 | <0.001 | 0.004 |
| 42 d | 0.37 ± 0.18 Bb | 0.53 ± 0.21 Bb | 0.38 ± 0.05 Bb | 0.88 ± 0.24 Ba | |||
| Butyrate, mmol/L | |||||||
| 21 d | 6.60 ± 0.59 Ab | 7.48 ± 0.85 Ab | 6.61 ± 1.10 Ab | 10.09 ± 3.04 Aa | <0.001 | 0.006 | 0042 |
| 42 d | 1.85 ± 1.02 B | 2.14 ± 1.02 B | 2.21 ± 0.40 B | 2.44 ± 1.12 B | |||
| Isovalerate, mmol/L | |||||||
| 21 d | 2.55 ± 0.25 Ab | 3.01 ± 0.16 Ab | 2.82 ± 0.49 Ab | 4.12 ± 1.22 Aa | <0.001 | <0.001 | 0.051 |
| 42 d | 0.35 ± 0.16 B | 0.37 ± 0.18 B | 0.43 ± 0.07 B | 0.71 ± 0.46 B | |||
| Valerate, mmol/L | |||||||
| 21 d | 2.36 ± 0.19 Abc | 2.57 ± 0.14 Aab | 2.29 ± 0.18 Ac | 2.70 ± 0.06 Aa | <0.001 | 0.077 | 0.014 |
| 42 d | 0.33 ± 0.10 B | 0.27 ± 0.15 B | 0.30 ± 0.02 B | 0.28 ± 0.13 B | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; He, Y.; Fan, S.; Gong, X.; Zhou, Y.; Jian, Y.; Ouyang, J.; Jiang, Q.; Zhang, P. Effects of LED Light Colors on the Growth Performance, Intestinal Morphology, Cecal Short-Chain Fatty Acid Concentrations and Microbiota in Broilers. Animals 2023, 13, 3731. https://doi.org/10.3390/ani13233731
Liu Y, He Y, Fan S, Gong X, Zhou Y, Jian Y, Ouyang J, Jiang Q, Zhang P. Effects of LED Light Colors on the Growth Performance, Intestinal Morphology, Cecal Short-Chain Fatty Acid Concentrations and Microbiota in Broilers. Animals. 2023; 13(23):3731. https://doi.org/10.3390/ani13233731
Chicago/Turabian StyleLiu, Yihui, Youkuan He, Siqin Fan, Xinyu Gong, Yuqiao Zhou, Yaowei Jian, Jiuyi Ouyang, Qianming Jiang, and Peihua Zhang. 2023. "Effects of LED Light Colors on the Growth Performance, Intestinal Morphology, Cecal Short-Chain Fatty Acid Concentrations and Microbiota in Broilers" Animals 13, no. 23: 3731. https://doi.org/10.3390/ani13233731
APA StyleLiu, Y., He, Y., Fan, S., Gong, X., Zhou, Y., Jian, Y., Ouyang, J., Jiang, Q., & Zhang, P. (2023). Effects of LED Light Colors on the Growth Performance, Intestinal Morphology, Cecal Short-Chain Fatty Acid Concentrations and Microbiota in Broilers. Animals, 13(23), 3731. https://doi.org/10.3390/ani13233731





