1. Introduction
Increasing numbers of livestock and aquaculture are resulting in insufficient feed. Therefore, the development and utilization of unconventional raw materials are important for the health and sustainability of animal husbandry.
Unconventional raw materials are usually by-products of the agricultural and food industries. Corn bran (CB) is a by-product of the corn starch obtained via wet milling [
1], with a production of about 60 to 70 g per kg of corn [
2]. In 2008, 25.6 × 10
6 t of corn was processed through wet milling, producing about 0.341 × 10
6 t of CB in America [
3]. Globally, however, CB production is projected to be even larger. Traditional uses of CB include animal feed because of its low price. In truth, the amount of CB used in animal feed is limited because of its high fiber content, which has a negative impact on nutrient utilization and has been recognized as an anti-nutritional factor in the past few decades [
4]. However, in recent years, fiber feedstuffs have received particular attention due to their functional value in improving digestive organ development, regulating gut health, promoting growth performance, and maintaining animal overall health in monogastric animals [
5,
6,
7]. Currently, research into fiber feedstuffs in poultry mainly focuses on broilers [
8] and meat ducks [
9,
10]. CB is an important source of fiber, including 10~20% cellulose and 30~40% hemicellulose, and mainly comprises arabinoxylan [
11]. Arabinoxylan is good for animal health because it serves as a microbial fermentation substrate in the gut. Previous studies revealed that a post-weaning diet containing 5% CB altered intestinal microbial diversity [
12,
13], increased butyrate and total short-chain fatty acid (SCFA) production [
13], and promoted an anti-inflammatory response to some extent in piglets [
12]. To our knowledge, the application of CB as a fiber feedstuff in animal feed is lacking, especially in laying ducks. This leads to a lack of data to support CB’s use in the laying duck industry.
Considering the literature and the application potential of CB in the laying duck industry, the present study hypothesized that incremental dietary CB levels (0%, 3%, 6%, 9%, and 12%) would have no adverse effects on laying performance in ducks because of its beneficial impacts on intestinal organ development, intestinal microbial communities, and intestinal volatile fatty acid (VFA) profiles. In this study, we set a maximum CB level of 12%, which is double its maximum level in the laying duck industry. This study aimed to explore how CB level impacts production performance, digestive organ development, and the cecal microbial communities of laying ducks and finally find the optimal CB level for laying ducks.
2. Materials and Methods
2.1. Animals, Diets, and Management
All experimental procedures involved in this study were approved by the Institutional Animal Care and Use Committee of Sichuan Agricultural University (Chengdu, China; Ethic Approval Code: SICAUAC201910-1).
A total of 600 commercial laying ducks (Shendan-II, a new variety of laying ducks in China) at 49 weeks of age were randomly assigned to 5 dietary treatments based on their initial laying performance. Each treatment included 6 replicates, and each replicate had 10 cages (2 ducks/cage, 35 cm × 30 cm × 35 cm). The control group had 0% CB in the basal diet, and the other four groups included 3%, 6%, 9%, and 12% CB, respectively. The analyzed composition of corn bran used in this experiment is given in
Table 1. The duration of this experiment was 11 weeks.
The diets were formulated to meet the nutrient requirements of laying ducks following the recommendation of Chinese laying ducks’ nutrient requirements (2021) [
16]. The diet composition and nutrient levels are shown in
Table 2. All diets were iso-nitrogen and iso-energy diets by switching the soybean oil with increased CB. All the diets were processed into pellets with a diameter of 4.5 mm. Ducks were reared in a controlled environment with a 16 h constant-light schedule (04:00–20:00) and free access to water and feed.
2.2. Production Performance of Laying Ducks
The number of eggs and ducks and egg weight were recorded daily, while the feed intake was recorded weekly for each replicate. The laying rate, egg weight, egg mass (egg weight per bird per day), average daily feed intake (ADFI), feed conversion ratio (FCR; g feed/g egg), mortality, and elimination rate were calculated.
2.3. Egg Quality
During the 4th, 8th, and 11th weeks, three eggs with an average weight in each replicate were selected for the egg quality analysis. Firstly, all eggs were examined to determine the shell quality characteristics, such as shell-breaking strength and shell thickness. Shell-breaking strength was measured using a shell force gauge model II (Robotmation Co., Ltd., Tokyo, Japan). The shell thickness was measured at the small end, equatorial region, and large end using a shell thickness gauge (Robotmation Co., Ltd., Tokyo, Japan). Secondly, the albumen height, Haugh unit, and yolk color were evaluated using an egg multi-tester (EMT-5200; Robotmation, Co., Ltd., Tokyo, Japan).
2.4. Relative Digestive Organ Weight and Length
After 11 weeks of rearing, 1 laying duck was randomly selected for each replicate, individually weighed, stunned, and exsanguinated (n = 6, 6 ducks per treatment). After dissection, the proventriculus, gizzard, small intestine, and cecum were instantly removed. After removing the contents, the weights of the proventriculus, gizzard, small intestine, and cecum and the length of intestinal segments were recorded. Based on the previous data, the relative weight (g/100 g body weight) and length (cm/100 g body weight) of intestinal segments, including the duodenum (from the gizzard to the bile duct), jejunum (from the bile duct to the Meckel’s diverticulum), ileum (from the Meckel’s diverticulum to the ileocecal junction), and cecum, were calculated.
2.5. Cecum Digesta Sample Collection and Measurement
The cecum digesta was collected, immediately frozen in liquid nitrogen, and stored at −80 °C until analysis. Subsequently, the cecum digesta samples were used for 16S rRNA, VFA, and metabolite analysis.
2.5.1. Volatile Fatty Acid
VFA concentrations in cecal digesta were analyzed using the previously described method [
17]. Approximately 0.6 g of sample was diluted in 1.5 mL of ultrapure water and homogenized via ultrasonic oscillation. All samples were stood for 30 min and then centrifuged at 20,000×
g for 15 min. Then, 1 mL of supernatant was mixed with reagents (0.2 mL of 25% metaphosphoric acid and 23.3 μL of 210 mmol/L crotonic acid). After being mixed homogeneously and incubated at 4 °C for 30 min, the tube was centrifuged at 20,000×
g for 10 min. Then, 0.3 mL of supernatant was collected and mixed with 0.9 mL of methanol, centrifuged at 10,000×
g for 5 min, and filtered through a 0.22 μm filter. The supernatant was quantified using a gas chromatographic system (VARIAN CP-3800, San Diego, CA, USA). The VFA concentrations were expressed as μmol/g of digesta in the cecum.
2.5.2. 16S-rRNA-Based Microbial Community Analysis
Cecal digesta samples were analyzed for the microbial community by Biotree Biomedical Technology Co., Ltd. (Shanghai, China) Total genomic DNA was extracted from cecal digesta using the TIANamp Soil DNA Kit (Tiangen BiotECH (Beijing) Co., Ltd., Beijing, China). Then, the V3-V4 variable regions of the bacterial 16S rRNA gene were amplified with universal primers (341F 5′-CCTACGGGNGGCWGCAG-3′ and 806R 5′-GGACTACHVGGGTWTCTAAT-3′) with barcodes unique to each sample. The amplicon sequencing with the Illumina HiSeq 2500 platform and bioinformatics were carried out. Quantitative Insights Into Microbial Ecology (QIIME, v1.9.1) was used for data analysis. Demultiplexing and quality filtering of the raw reads were performed with the FLASH, Trimmomatic, and UCHIME programs. The high-quality sequences were clustered into the operational taxonomic unit (OTU) using UCLUST at 97% similarity. OTU were then taxonomically classified using an RDP Classifier against a curated Green Genes database with a bootstrap cutoff of 80%. The weighted UniFrac distance metrics-based principal coordinate analysis (PCoA) plots showing microbial β-diversity were generated in EMPeror. Additionally, the analysis of microbial α- and β-diversity was performed with QIIME 2 (Version 1.7.0), and Tax4Fun predicted the microbial community functional profile.
2.5.3. Cecal Digesta Metabolomics
The metabolomics analysis of cecal digesta was based on liquid chromatography mass spectrometry (LC-MS) and performed by Biotree Biomedical Technology Co., Ltd. (Shanghai, China). About 50 mg of digesta was mixed with 1000 μL of extract solution in an EP tube, homogenized at 35 Hz for 4 min, and sonicated for 5 min. The homogenization and sonication were repeated three times in an ice-water bath. Then, those samples were incubated at −40 °C for 1 h and subsequently centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatant was used for analysis. In addition, quality control samples were also prepared for all samples. The UHPLC system (Vanquish, Thermo Fisher Scientific, Waltham, MA, USA) was used for LC-MS/MS analyses with a UPLC BEH Amide column (2.1 mm × 100 mm, 1.7 μm) coupled to a Q Exactive HFX mass spectrometer to acquire MS/MS spectra in an information-dependent acquisition mode. Finally, the data were processed using R 4.0.5 after the raw data were converted to the mzXML format using ProteoWizard.
2.6. Statistical Analysis
All data were analyzed using SAS statistical software (SAS 9.4, Inst. Inc., Cary, NC, USA). The experimental unit was the replicate (n = 6). The effect of treatment was analyzed using the MIXED procedure. Meanwhile, the analysis of linear and quadratic CB levels was conducted using orthogonal polynomial contrasts with the command CONTRAST. An independent two-sample t-test was used for microbial diversity analysis. The PROC GLIMMIX with gamma transformation was used when the residuals were nonnormal. p < 0.05 was considered significant; meanwhile, 0.05 ≤ p < 0.10 was considered a tendency. Additionally, function prediction was analyzed using a bootstrap Mann–Whitney U test based on gene distribution, with cutoffs of p < 0.01, false discovery rate < 0.1, and mean counts > 10.
4. Discussion
This study evaluated the effects of dietary CB levels on laying performance, organ development, cecum microbial communities, VFA profiles in cecal digesta, and egg quality in laying ducks. To our knowledge, this study is the first trial to explore whether CB can be used in laying ducks’ diet and its maximum level, which will guide the use of CB in the laying duck industry.
Corn bran is an insoluble fiber source. A previous study indicated that based on a typical corn–soybean meal diet for laying ducks, the substitution of soybean meal and wheat bran by different levels of the distiller dried grains with solubles (6%, 12%, 18%, 24%, and 30%) under iso-energy and iso-nitrogen diets showed no effect on laying rate [
18]. However, an energy- and nutrient-reduced diet containing 10% lignocellulose in laying hens displayed a higher laying rate than the control group in 23–52 weeks [
19]. In the present study, there were no significant differences in indexes relative to laying performance except for ADFI among groups. However, the 12% CB group numerically increased duck-housed egg production by 2.46% and duck-housed egg number by two eggs compared with the control (0% CB) group, suggesting that the inclusion of 12% CB in the diet had a positive impact on the laying performance. Usually, egg production is driven by dietary energy and nutrient ingestion. The results showed that there were no differences in ADFI between the 0% CB and 12% CB groups in each stage, which suggested that feed intake was not the only factor that drove laying performance when ducks consumed a diet that included high crude fiber by adding 12% CB. In this study, the incremental CB level (from 0% to 3%, 6%, 9%, and 12%) gradually increased feed cost (2.57, 2.58, 2.60, 2.61, and 2.63 RMB/kg), displaying an increased net profit of RMB −2.31, −0.73, −0.94, and 1.31 in the 3%, 6%, 9%, and 12% CB groups, respectively, which indicated that only the 12% CB group had a positive effect on economic benefit. In conclusion, the 12% CB group displayed a beneficial effect rather than an anti-nutrition effect on laying performance in laying ducks.
Corn bran is a fiber source for animal feed. The CB used in the current study contained 12.4% crude fiber. However, the effect of CB on egg quality in laying ducks is rarely reported. In the current study, as the dietary CB level increased, the eggshell thickness quadratically changed in weeks 4, 8, and 11. The 12% CB group illustrated the lowest eggshell thickness on week 8, which was lower than the control (0% CB) group (0.34 vs. 0.38 mm). This result was consistent with the previous studies that were conducted with other fiber sources in laying hens, in which adding incremental sugar beet pulp (0%, 5%, 10%, 15%, and 20%) to the laying hen diet during 24 to 27 weeks of age linearly decreased the shell thickness [
20]. However, the slightly decreased shell thickness in the 12% CB group had no influence on shell-breaking strength compared to the control group in week 8, despite thinner shells always being accompanied by lower shell-breaking strength in theory [
21]. Additionally, the decreased shell thickness (0.37 vs. 0.42 mm) in the 6% CB group led to a decreased tendency of shell-breaking strength compared with the control (0% CB) group in week 11. The results of the 0% CB and 6% CB groups agreed with a previous study about fiber source feedstuffs, in which a laying hen diet that contained 5% sunflower hull decreased shell thickness compared with the control [
22]. However, the mechanism behind the dosage-dependent effect of CB on shell thickness and shell-breaking strength is still unknown. As is known, the higher the albumen height and Haugh unit, the better the egg quality. The 12% CB group had the highest albumen height and Haugh unit in week 8, indicating better egg quality in this group. In this study, the yolk color was improved as the CB level increased in week 4, and the yolk color was greatly enhanced in the 12% CB group compared with the control (0% CB) group in weeks 4 and 8. These observations were expected because CB contains relatively high xanthophyll content, which is an important contributor to yolk pigmentation [
23]. In conclusion, the inclusion of 12% CB in a duck diet showed positive effects on albumen height, Haugh unite, and yolk color and had no adverse effects on shell-breaking strength compared with the 0% CB group.
The proventriculus and gizzard are the characteristic components of the digestive tract in poultry [
8]. As is known, the proventriculus, gizzard, and intestine play an important role in the digestion and absorption of nutrients and are, thus, the organs most influenced by dietary changes [
24]. Fiber type and feed particle size are the determining factors that stimulate the gizzard’s muscular activity, resulting in the altered size of those organs [
25]. In the present study, the relative weights of the proventriculus and gizzard linearly increased as the CF level increased, and the highest relative weight was found in the 12% CB group, which was significantly higher than other groups. These results were consistent with previous studies about other fiber sources, in which the inclusion of 6% wood shavings, 3% oat hulls, or 3% soy hulls in the diet led to an increased size of proventriculus and gizzard in broilers as a logical result of an increased volume due to the slower passage rate of the almost-intact feed particles [
26,
27]. Additionally, the fiber source also impacts intestinal development in poultry, although the results are not always consistent. The inclusion of 3% oat hull in a broiler diet led to a lower relative length of the small intestine and a higher relative weight of the cecum; meanwhile, the inclusion of 3% soy hull had no impact on intestine development [
27]. Chickens fed 3% wheat bran increased the relative weight of the small intestine [
28]. In this study, there was no difference in the relative weight and length of the intestinal segments between the 12% and 0% CB groups. Still, the relative weight of the ileum was lower in the remaining groups than in the 0% CB and 12% CB groups, illustrating a fiber-level-dependent effect. The results suggested that the inclusion of 12% CB in the laying duck diet improved the development of digestive organs.
Dietary fiber, as the primary substance used for bacterial fermentation in the intestine, was reported to support the growth and establishment of beneficial microbes by providing extra fuel for their metabolism [
6], which ultimately enhanced the production of SCFA [
29,
30,
31,
32]. In this study, the CB raw material contained 40.46%, 5.16%, and 35.30% of total dietary fiber, soluble dietary fiber, and insoluble dietary fiber, respectively. As a result, the total SCFA, acetic acid, propionic acid, and butyric acid concentrations and the BCFA-to-SCFA ratio quadratically changed as the CB level increased. The 0% CB and 12% CB groups had higher contents of total SCFA, acetic acid, and propionic acid than other groups, reflecting a better fermentation effect in these two groups. Notably, the 12% CB group also showed a higher butyric acid concentration and proportion than the 0% CB group, indicating a CB level dependence in VFA production in the cecum. A previous study indicated that SCFA can provide up to 5% to 15% of daily metabolizable energy for the maintenance energy requirements of birds [
33]. Meanwhile, butyrate has the functions of anti-inflammatory activity, immune response modulation, and growth performance enhancement [
34]. These functions of SCFA and butyrate partly explained how the 12% CB group maintained or numerically improved the laying performance compared with the control group.
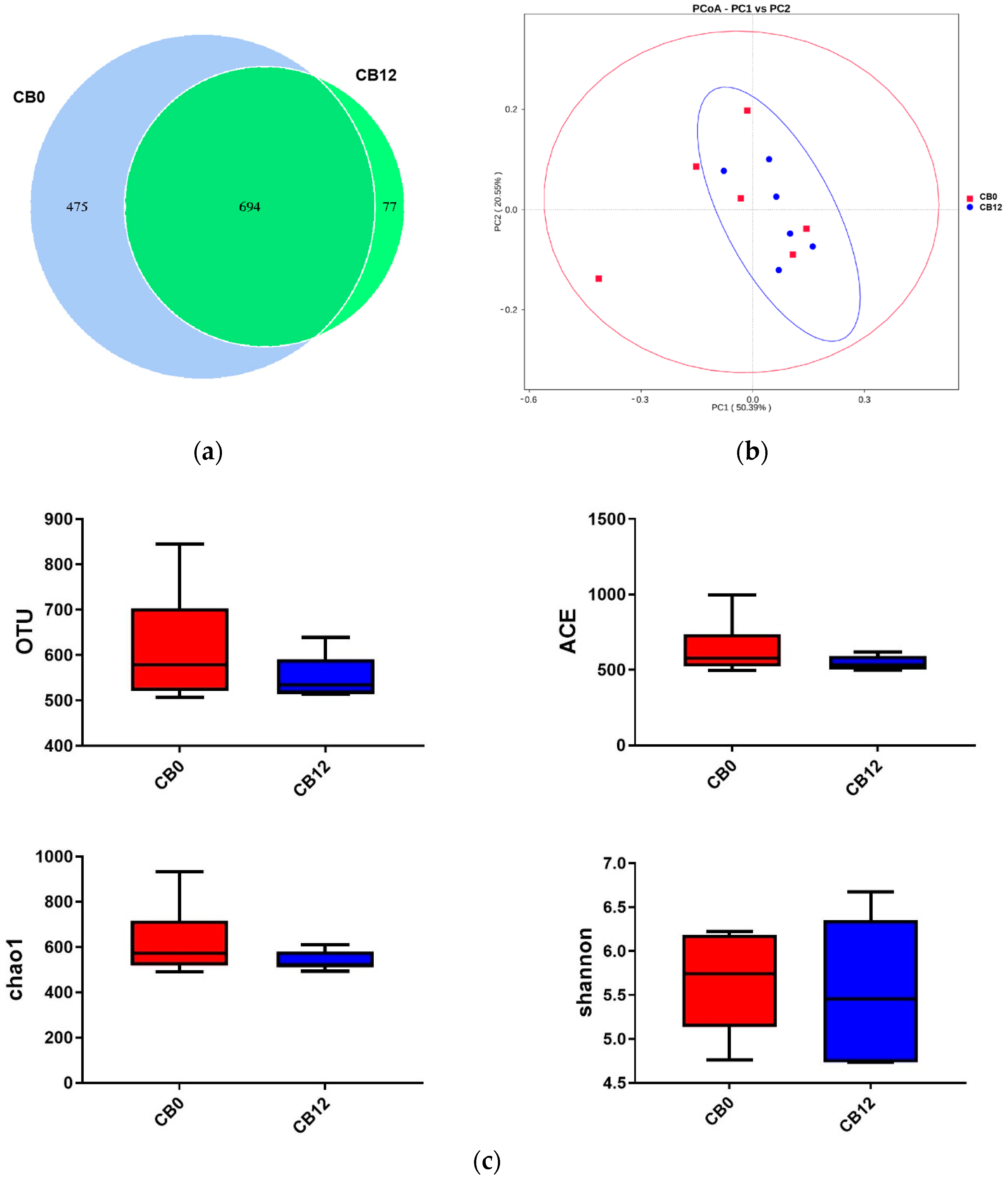
The above results showed that the 12% CB group demonstrated beneficial effects on digestive organ development and cecal VFA production without adverse effects on laying performance. To further explore the mechanism behind this phenomenon, we further explored the cecal microbial communities and metabolites in the 0% CB and 12% CB groups. Intestinal microbial communities result from their adaptation to the environment and nutrients [
35], showing a crucial role in manipulating host health and growth performance by regulating metabolism [
36,
37]. In this study, the PCoA showed an extensive range in the 0% CB group, although there was no difference in α-diversity, illustrating a distinct change in microbial composition between the 0% CB and 12% CB groups. In the present study, the three dominant phyla were Firmicutes, Bacteroidetes, and Actinobacteriota, which is consistent with the previous report on Shaoxing laying ducks [
38]. Higher levels of Firmicutes but lower levels of Bacteroidetes were found when the ducks consumed a diet containing 12% CB, which was in line with the altered cecum microbial communities with incremental fiber in geese [
12]. Deferribacteres, a harmful taxon [
39], was proven to have a positive correlation with IL-1β expression [
40]. In this study, ducks that were fed a diet including 12% CB resulted in lower Deferribacteres. Additionally, Spriochaetota and Fusobacteriota, phyla containing many pathogenic microorganisms [
41,
42], declined when ducks were fed a diet including 12% CB. These observations suggest the presence of better gut health when the ducks were fed the diet of the 12% CB group compared with that of the control (0% CB) group.
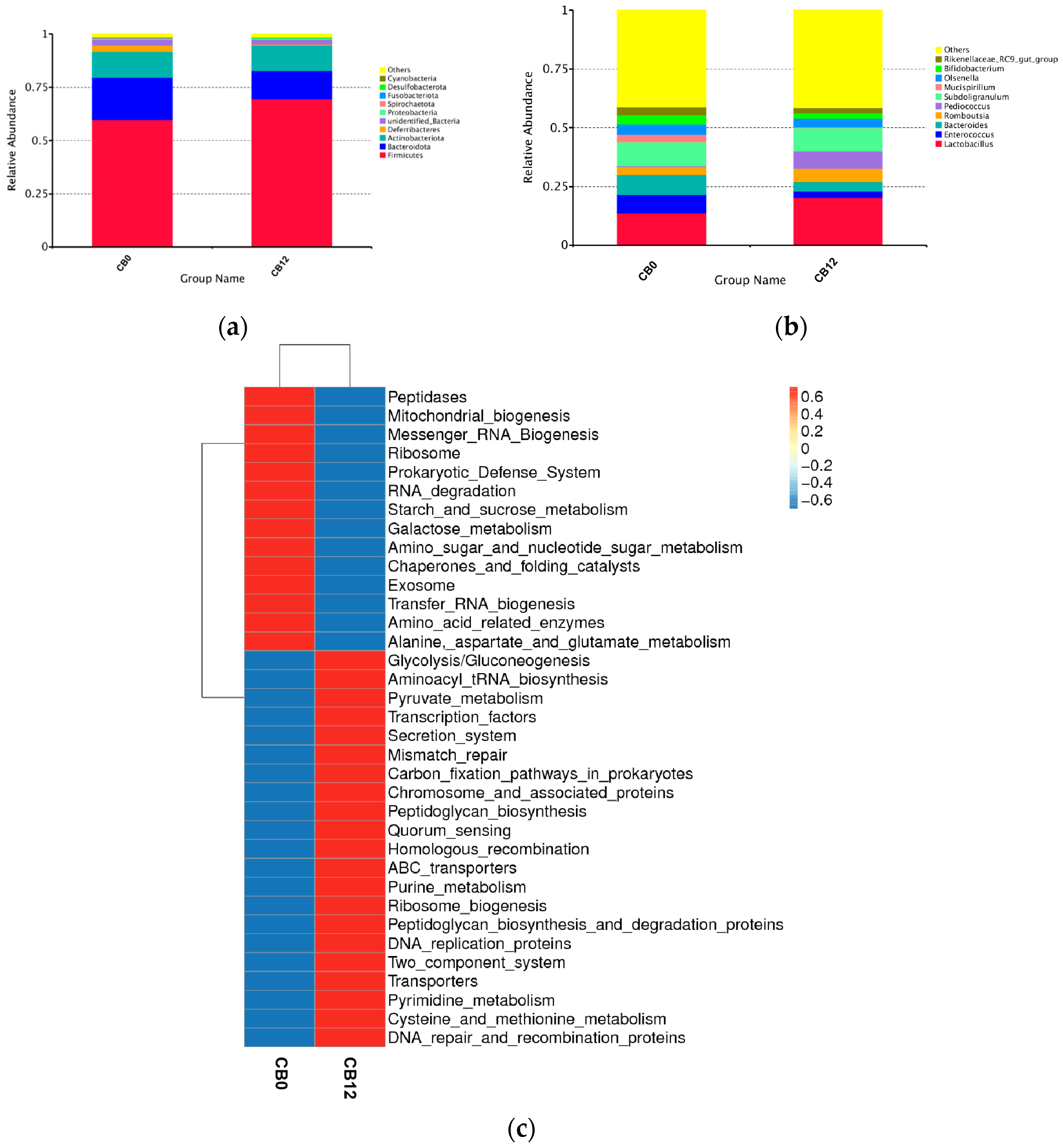
At the genus level,
Lactobacillus was the dominant genus, illustrating 6.5% higher relative abundance in the 12% CB group than in the 0% CB group. Likewise, the genus of
Pediococcus was also higher in the 12% CB group (7.42% vs. 0.54%). As is known, both
Lactobacillus and
Pediococcus are lactic acid bacteria with the function of producing lactate [
43,
44,
45], which is beneficial for gut health by inhibiting the growth of putrefying bacteria [
17,
46]. Meanwhile, both
Lactobacillus and
Pediococcus have the function of improving intestinal VFA production [
33,
47]. The higher relative abundance of
Lactobacillus and
Pediococcus in the 12% CB group explained its higher cecal butyric acid production compared to the 0% CB group. Furthermore,
Bacteroides, positively related to pro-inflammatory cytokines [
48], exhibited a declining trend as the crude fiber increased. However,
Bifidobacterium, which was set as a probiotic [
49], was higher in the 0% CB group. As a whole, the 12% CB group had a higher level of beneficial bacteria. Interestingly, we observed an increased level of the
Rikenellaceae_RC9_gut_group in the 0% CB group. Previous studies reported that the
Rikenellaceae_RC9_gut_group widely participated in the metabolism of bile acid, protein, fat, and carbohydrates [
50], and propionate, acetate, and succinate were the fermentation end products of this process [
51]. It partly explained the higher predictive function of the metabolism of peptidases, starch, sucrose, and amino acids in the 0% CB group. In addition, the distinct predictive function between those two groups indicated that the 12% CB group had a robust predictive function of glycolysis and pyruvate metabolism related to fiber fermentation in the gut [
52], which was reported to result in increased acetate, propionate, and butyrate concentrations [
53] and subsequently benefit the health of the host. Notably, the 12% CB group displayed enhanced quorum sensing. This density-dependent cell-to-cell signaling occurs when the bacterial population reaches a threshold density [
54]. The same phenomenon was observed in piglets due to the enhanced gut
Lactobacillus [
17], providing evidence for a link between higher
Lactobacillus and the robust quorum sensing in the 12% CB group.
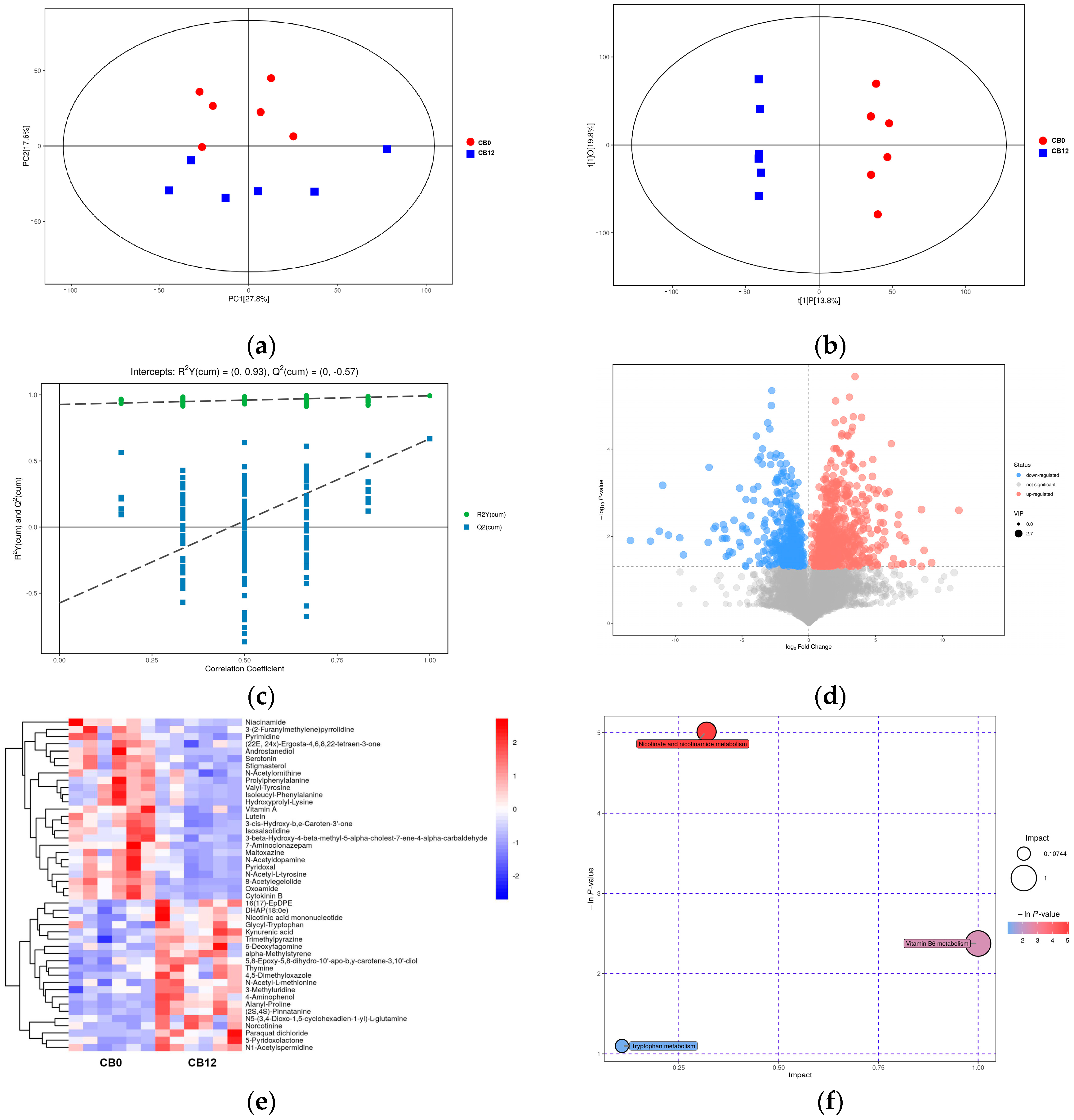
The cecal digesta metabolomics results revealed changes in cecal metabolites between the 0% CB and the 12% CB groups. Niacin is a vitamin B
3 compound with anti-inflammatory and antioxidant effects [
47]. Pyridoxal is a coenzyme of more than 260 enzymes and has antioxidant effects [
55]. Therefore, the increased concentrations of niacinamide and pyridoxal in the 12% CB group indicated that the diet in this group was more beneficial for laying ducks’ health, which lays a foundation for its positive impact rather than an anti-nutrition effect on laying performance. Previous studies have shown that increasing dietary fiber content can enhance serotonin production by affecting microbial metabolites, such as increasing butyric acid content [
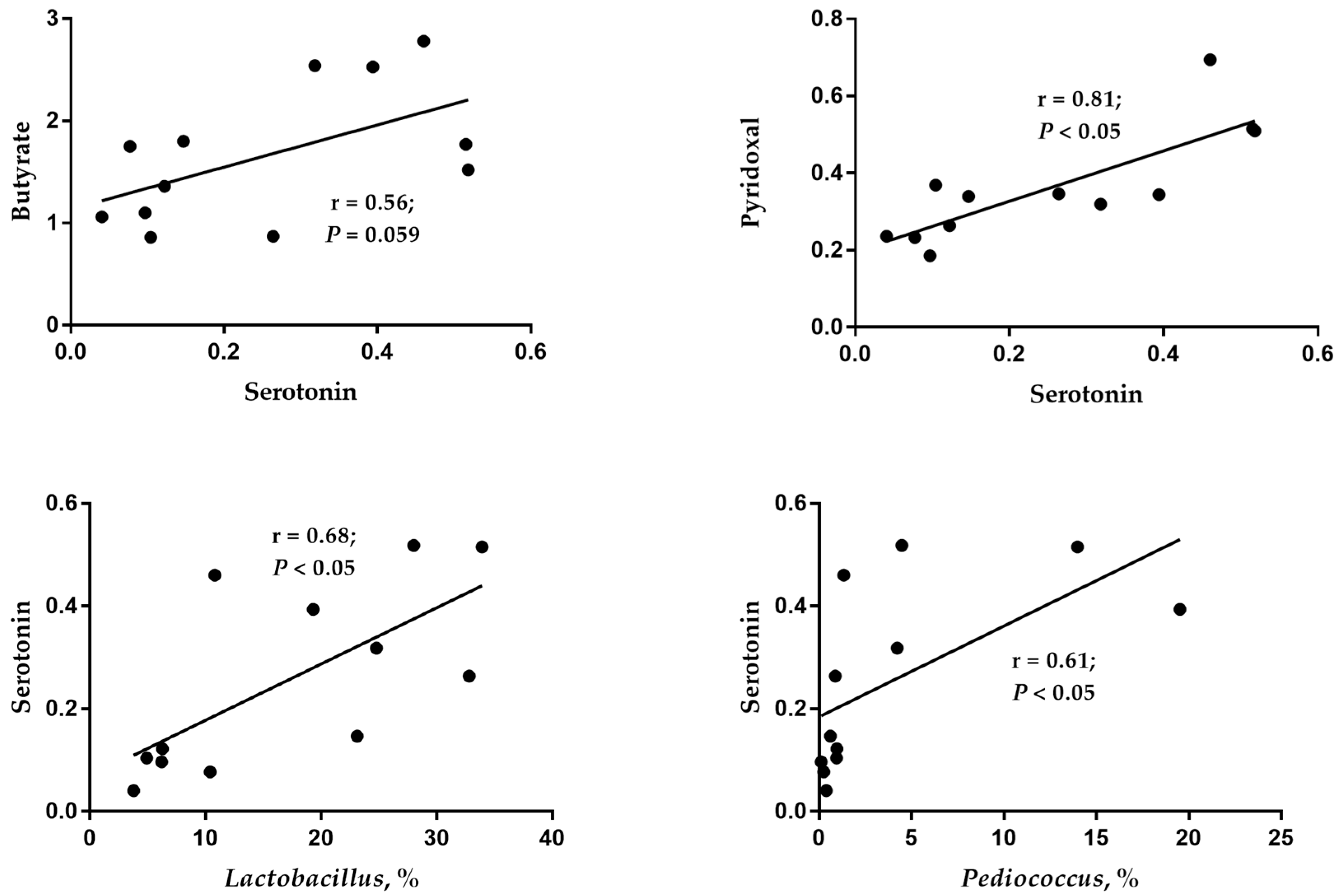
56,
57]. Likewise, when the crude fiber level increased from 2.84% to 3.94% by adding 12% CB, the serotonin content in cecal digesta increased in this study, and the serotonin content showed a positive correlation with the abundance of
Lactobacillus and
Pediococcus and the content of butyric acid in cecal digesta. In addition, as pyridoxal is a coenzyme in the process of serotonin synthesis, an increase in its content might be more conducive to the synthesis of serotonin. As a result, the current study observed a significant positive correlation between pyridoxal and serotonin. In conclusion, there were different impacts on the intestinal microbial composition and microbial derivative metabolites between the 0% CB and 12% CB groups, suggesting a beneficial effect on the intestinal health of ducks in the 12% CB group.