Staphylococcus haemolyticus and Providencia vermicola Infections Occurring in Farmed Tilapia: Two Potentially Emerging Pathogens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Animals
2.2. Bacteriological Analysis and Phenotypic Characterization of Isolates
2.3. Molecular Identification of Bacteria
2.4. Virulence Assay
2.5. Histopathological Examination
3. Results
3.1. Clinical-Lesional Picture of the Sampled Organisms
3.2. Bacteriological Analysis and Phenotypic Characterization of Isolates
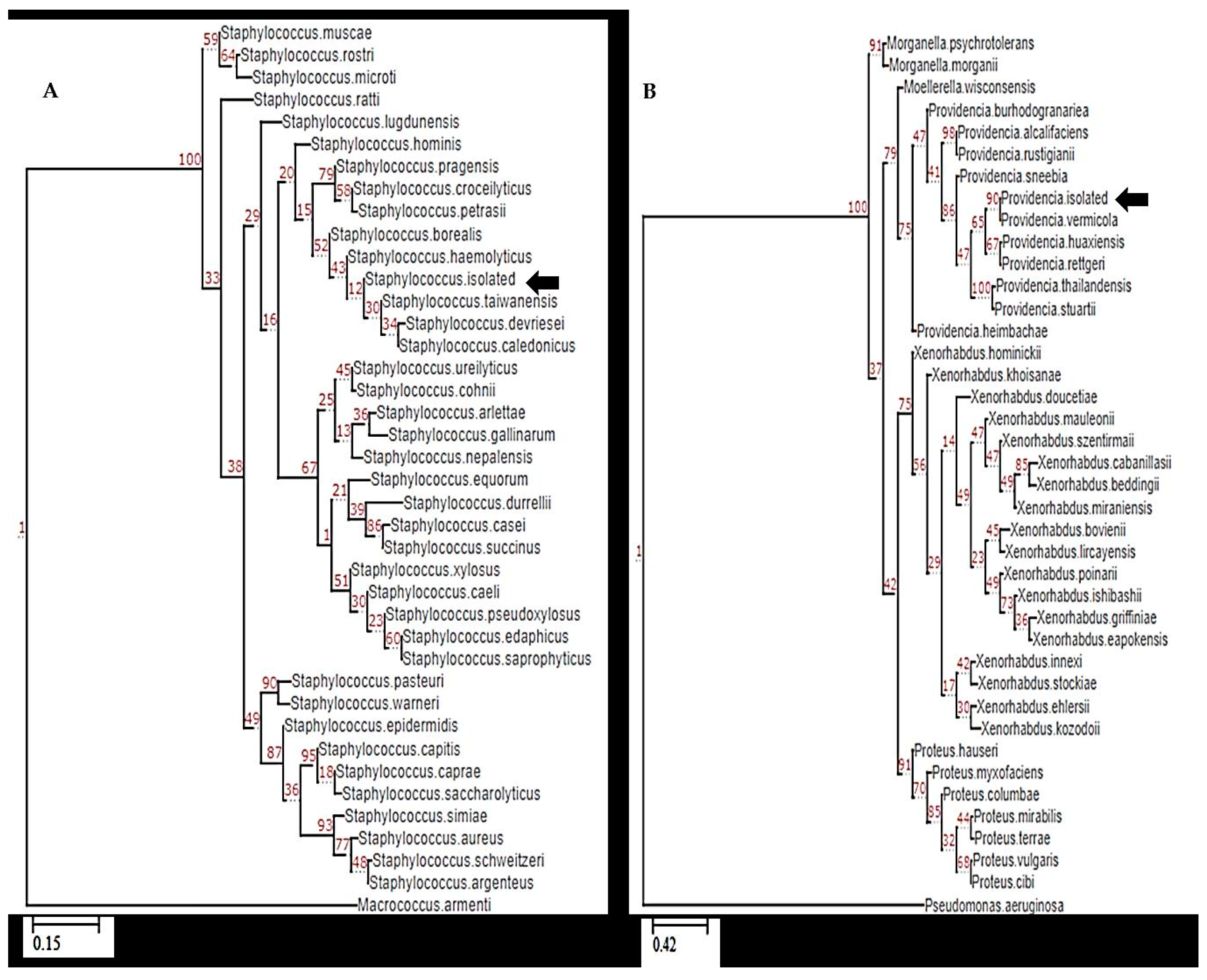
3.3. Molecular Identification Based on 16S rRNA
3.4. Virulence Assay
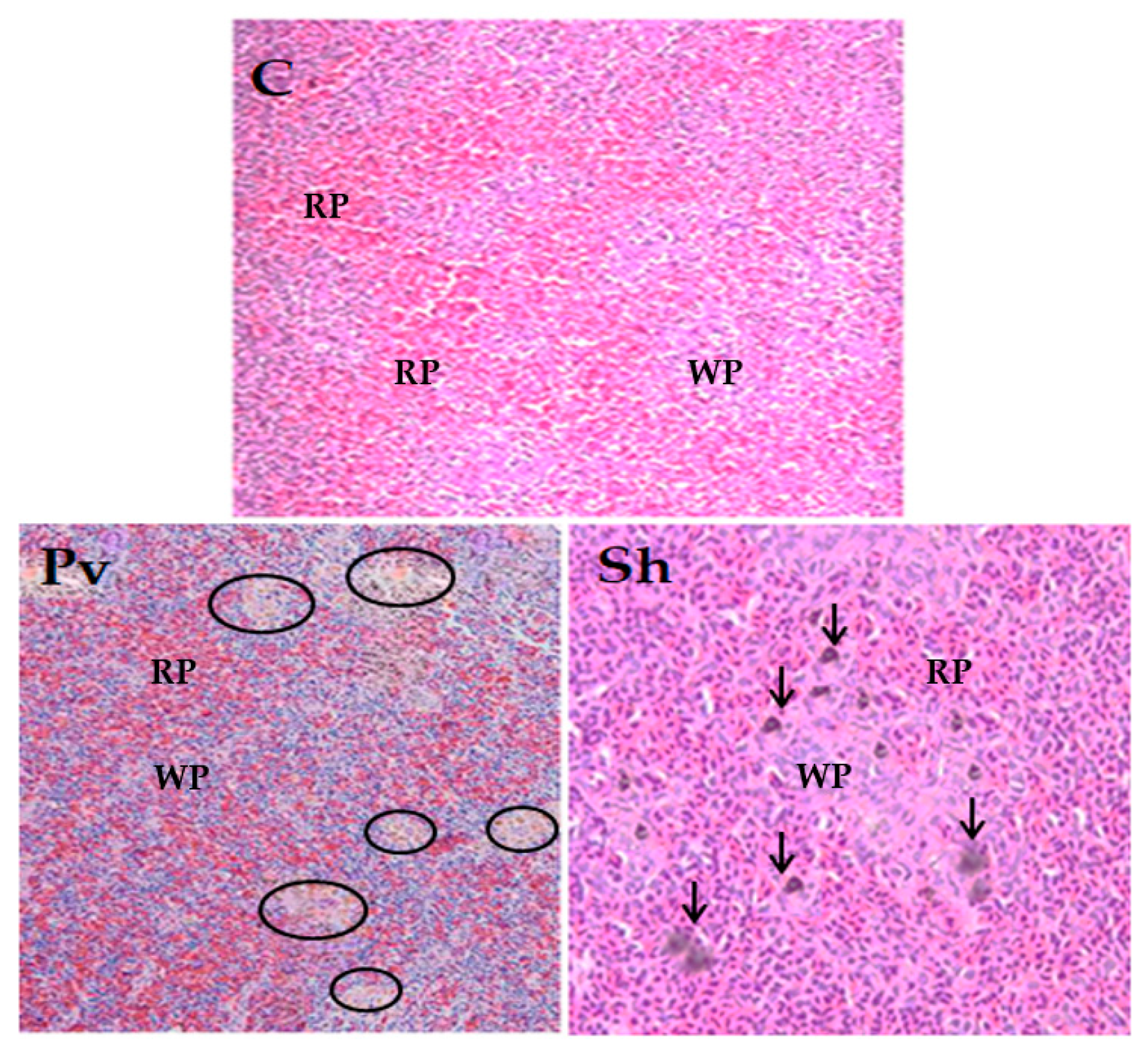
3.5. Histopathological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
- >Staphylococcus haemolyticus isolated
- >Providencia vermicola isolated
References
- Fitzsimmons, K. Future trends of tilapia aquaculture in the Americas. Tilapia Aquac. Am. 2000, 2, 252–264. [Google Scholar]
- Ornelas-Luna, R.; Aguilar-Palomino, B.; Hernández-Díaz, A.; Hinojosa-Larios, J.Á.; Godínez-Siordia, D.E. Un enfoque sustentable al cultivo de tilapia. Acta Univ. 2017, 27, 19–25. [Google Scholar] [CrossRef]
- CONAPESCA. Comisión Nacional de Acuacultura y Pesca. Anuario Estadístico de Pesca 2014. Available online: www.conapesca.gob.mx (accessed on 1 October 2023).
- Platas-Rosado, D. Importancia económico y social del sector acuícola en México. Agro Product. 2017, 10, 19–24. [Google Scholar]
- Dong, H.T.; Nguyen, V.V.; Le, H.D.; Sangsuriya, P.; Jitrakorn, S.; Saksmerprome, V.; Senapin, S.; Rodkhum, C. Naturally concurrent infections of bacterial and viral pathogens in disease outbreaks in cultured Nile tilapia (Oreochromis niloticus) farms. Aquaculture 2015, 448, 427–435. [Google Scholar] [CrossRef]
- El-Sayed, A.-F.M. Tilapia Culture; CABI Publishing: Alexandria, Egypt, 2006; p. 277. [Google Scholar]
- Kayansamruaj, P.; Pirarat, N.; Hirono, I.; Rodkhum, C. Increasing of temperature induces pathogenicity of Streptococcus agalactiae and the up-regulation of inflammatory related genes in infected Nile tilapia (Oreochromis niloticus). Vet. Microbiol. 2014, 172, 265–271. [Google Scholar] [CrossRef]
- Kayansamruaj, P.; Pirarat, N.; Katagiri, T.; Hirono, I.; Rodkhum, C. Molecular characterization and virulence gene profiling of pathogenic Streptococcus agalactiae populations from tilapia (Oreochromis sp.) farms in Thailand. J. Vet. Diagn. Investig. 2014, 26, 488–495. [Google Scholar] [CrossRef]
- Meyburgh, C.; Bragg, R.; Boucher, C. Lactococcus garvieae: An emerging bacterial pathogen of fish. Dis. Aquat. Org. 2017, 123, 67–79. [Google Scholar] [CrossRef]
- Luo, W.; Gan, X.; Zhu, J.; Ao, Q.; Tan, Y.; Chen, M.; Luo, Y. A pathological study of GIFT strain of Nile Tilapia (Oreochromis niloticus) infected by Streptococcus agalactiae. Agric. Biotechnol. 2018, 7, 136–142. [Google Scholar]
- Leal, C.; Tavares, G.; Figueiredo, H. Outbreaks and genetic diversity of Francisella noatunensis subsp orientalis isolated from farm-raised Nile tilapia (Oreochromis niloticus) in Brazil. Genet. Mol. Res. 2014, 13, 5704–5712. [Google Scholar] [CrossRef]
- Jantrakajorn, S.; Wongtavatchai, J. Francisella infection in cultured tilapia in Thailand and the inflammatory cytokine response. J. Aquat. Anim. Health 2016, 28, 97–106. [Google Scholar] [CrossRef]
- Peatman, E.; Li, C.; Peterson, B.C.; Straus, D.L.; Farmer, B.D.; Beck, B.H. Basal polarization of the mucosal compartment in Flavobacterium columnare susceptible and resistant channel catfish (Ictalurus punctatus). Mol. Immunol. 2013, 56, 317–327. [Google Scholar] [CrossRef]
- Dong, H. Emerging, Re-Emerging and New Diseases of Tilapia. In FAO/China Intensive Training Course on Tilapia Lake Virus (TLV); FAO: Guangzhou, China, 2018. [Google Scholar]
- Huang, S.-L.; Chen, W.-C.; Shei, M.-C.; Liao, I.; Chen, S.-N. Studies on epizootiology and pathogenicity of Staphylococcus epidermidis in Tilapia (Oreochromis spp.) cultured in Taiwan. Zool. Stud. 1999, 38, 178–188. [Google Scholar]
- Nicholson, P.; Mon-on, N.; Jaemwimol, P.; Tattiyapong, P.; Surachetpong, W. Coinfection of tilapia lake virus and Aeromonas hydrophila synergistically increased mortality and worsened the disease severity in tilapia (Oreochromis spp.). Aquaculture 2020, 520, 734–746. [Google Scholar] [CrossRef]
- Bejerano, Y.; Sarig, S.; Horne, M.; Roberts, R. Mass mortalities in silver carp Hypophthalmichthys molitrix (Valenciennes) associated with bacterial infection following handling. J. Fish Dis. 1979, 2, 49–56. [Google Scholar] [CrossRef]
- Ramkumar, R.; Ravi, M.; Jayaseelan, C.; Rahuman, A.A.; Anandhi, M.; Rajthilak, C.; Perumal, P. Description of Providencia vermicola isolated from diseased Indian major carp, Labeo rohita (Hamilton, 1822). Aquaculture 2014, 420, 193–197. [Google Scholar] [CrossRef]
- Conroy, G. Most common tilapia diseases in Latin America and the Caribbean. In Ninth International Aquaculture Forum; World Aquaculture Society: Guadalajara, Mexico, 2014. [Google Scholar]
- Roon, S.R.; Alexander, J.D.; Jacobson, K.C.; Bartholomew, J.L. Effect of Nanophyetus salmincola and bacterial co-infection on mortality of juvenile chinook salmon. J. Aquat. Anim. Health 2015, 27, 209–216. [Google Scholar] [CrossRef]
- Zhang, C.; Li, D.-l.; Chi, C.; Ling, F.; Wang, G.-X. Dactylogyrus intermedius parasitism enhances Flavobacterium columnare invasion and alters immune-related gene expression in Carassius auratus. Dis. Aquat. Org. 2015, 116, 11–21. [Google Scholar] [CrossRef]
- Kotob, M.H.; Menanteau-Ledouble, S.; Kumar, G.; Abdelzaher, M.; El-Matbouli, M. The impact of co-infections on fish: A review. Vet. Res. 2017, 47, 98. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.; Dawood, M.A.; Menanteau-Ledouble, S.; El-Matbouli, M. The nature and consequences of co-infections in tilapia: A review. J. Fish Dis. 2020, 43, 651–664. [Google Scholar] [CrossRef]
- Speare, D.J. Aquatic Animal Health Code. Can. Vet. J. 2006, 47, 1217. [Google Scholar]
- Bou, G.; Fernández-Olmos, A.; García, C.; Sáez-Nieto, J.A.; Valdezate, S. Métodos de identificación bacteriana en el laboratorio de microbiología. Enfermedades Infecc. Y Microbiol. Clínica 2011, 29, 601–608. [Google Scholar] [CrossRef]
- Kleinbaum, D.G.; Klein, M.; Kleinbaum, D.G.; Klein, M. Maximum likelihood techniques: An overview. In Logistic Regression: A Self-Learning Text; Springer: New York, NY, USA, 2010; pp. 103–127. [Google Scholar]
- Huerta-Cepas, J.; Serra, F.; Bork, P. ETE 3: Reconstruction, analysis, and visualization of phylogenomic data. Mol. Biol. Evol. 2016, 33, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- NOM-062-Z00-1999; Official Mexican Standard-062-Z00-1999, Especificaciones Técnicas para la Producción, Cuidado y Uso de Animales de Laboratorio. Secretaria de Agricultura y Desarrollo Rural: Mexico City, Mexico, 1999.
- Lafferty, K.D.; Harvell, C.D.; Conrad, J.M.; Friedman, C.S.; Kent, M.L.; Kuris, A.M.; Powell, E.N.; Rondeau, D.; Saksida, S.M. Infectious diseases affect marine fisheries and aquaculture economics. Annu. Rev. Mar. Sci. 2015, 7, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Huicab-Pech, Z.; Landeros-Sánchez, C.; Castañeda-Chávez, M.; Lango-Reynoso, F.; López-Collado, C.; Platas Rosado, D. Current state of bacteria pathogenicity and their relationship with host and environment in tilapia Oreochromis niloticus. J. Aquac. Res. Dev. 2016, 7, 1–10. [Google Scholar]
- Huang, S.L.; Liao, I.C.; Chen, S.N. Induction of apoptosis in tilapia, Oreochromis aureus Steindachner, and in TO-2 cells by Staphylococcus epidermidis. J. Fish Dis. 2000, 23, 363–368. [Google Scholar] [CrossRef]
- Huss, H.H. Fresh Fish-Quality and Quality Changes: A Training Manual Prepared for the FAO/DANIDA Training Programme on Fish Technology and Quality Control; Food & Agriculture Organization: Rome, Italy, 1988. [Google Scholar]
- Çanak, Ö.; Timur, G. An initial survey on the occurrence of staphylococcal infections in Turkish marine aquaculture (2013–1014). J. Appl. Ichthyol. 2020, 36, 932–941. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Dong, Z.-X.; Luo, Z.-W.; Jiao, Y.-J.; Guo, J.; Deng, X.-Y.; Wang, F.; Chen, J.-Y.; Lin, L.-B. MicroRNA profile of immune response in gills of zebrafish (Danio rerio) upon Staphylococcus aureus infection. Fish Shellfish Immunol. 2019, 87, 307–314. [Google Scholar] [CrossRef]
- Kanchan, C.; Imjai, P.; Kanchan, N.; Chaiyara, A.; Panchai, K. Occurrence of parasitic and bacterial diseases in Thai freshwater fish. J. Agric. Crops 2020, 8, 210–214. [Google Scholar] [CrossRef]
- Ge, L.; Liu, Q.; Wang, Y.; Li, Y.; Yan, X.; Kang, L. Research on the pathogenicity of Staphylococcus sp. from fish to Carassius auratus and Danio rerio. Heilongjiang Agric. Sci. 2014, 8, 69–70. [Google Scholar]
- Souza, C.D.F.; Baldissera, M.D.; Verdi, C.M.; Santos, R.C.; Da Rocha, M.I.U.; da Veiga, M.L.; da Silva, A.S.; Baldisserotto, B. Oxidative stress and antioxidant responses in Nile tilapia Oreochromis niloticus experimentally infected by Providencia rettgeri. Microb. Pathog. 2019, 131, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Preena, P.G.; Dharmaratnam, A.; Raj, N.S.; Raja, S.A.; Nair, R.R.; Swaminathan, T.R. Antibiotic resistant Enterobacteriaceae from diseased freshwater goldfish. Arch. Microbiol. 2021, 203, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wei, Y.; Zhang, S.; Cheng, J.; Cheng, X.; Qian, C.; Wang, Y.; Zhang, Y.; Yin, Z.; Chen, H. Comparative Genomic Analysis Reveals Genetic Mechanisms of the Variety of Pathogenicity, Antibiotic Resistance, and Environmental Adaptation of Providencia Genus. Front. Microbiol. 2020, 11, 572–642. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.; Vijayakumar, P.; Dhas, T.S.; Velu, K.; Inbakandan, D.; Thamaraiselvi, C.; Greff, B.; Chandrasekaran, M.; Almutairi, S.M.; Alharbi, F.S.; et al. Synthesis of biogenic silver nanoparticles using butter fruit pulp extract and evaluation of their antibacterial activity against Providencia vermicola in Rohu. J. King Saud Univ.-Sci. 2022, 34, 101–114. [Google Scholar] [CrossRef]
- Ramesh, D.; Souissi, S. Antibiotic resistance and virulence traits of bacterial pathogens from infected freshwater fish, Labeo rohita. Microb. Pathog. 2018, 116, 113–119. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Descovi, S.N.; Verdi, C.M.; Santos, R.C.; da Silva, A.S.; Da Rocha, M.I.U.; da Veiga, M.L.; Baldisserotto, B. Purinergic signaling displays a pro-inflammatory profile in lymphoid immune organs of Oreochromis niloticus experimentally infected by Providencia rettgeri: The role of pathophysiology. Aquaculture 2019, 510, 176–181. [Google Scholar] [CrossRef]
- Popp, W.; Faisal, M.; Refai, M. High mortality in the Nile tilapia Oreochromis niloticus caused by Providencia rettgeri. Anim. Res. Dev. 1988, 29, 95. [Google Scholar]
- Oh, W.T.; Jun, J.W.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.G.; Park, S.C. Staphylococcus xylosus infection in rainbow trout (Oncorhynchus mykiss) as a primary pathogenic cause of eye protrusion and mortality. Microorganisms 2019, 7, 330. [Google Scholar] [CrossRef]
- Martinez-Porchas, M.; Martinez-Cordova, L.R. World aquaculture: Environmental impacts and troubleshooting alternatives. Sci. World J. 2012, 2012, 389623. [Google Scholar] [CrossRef]
- Rajme-Manzur, D.; Gollas-Galván, T.; Vargas-Albores, F.; Martínez-Porchas, M.; Hernández-Oñate, M.Á.; Hernández-López, J. Granulomatous bacterial diseases in fish: An overview of the host’s immune response. Comp. Biochem. Physiol. 2021, 261, 111058. [Google Scholar] [CrossRef]



| Signs/Injuries | Presentation Frequency |
|---|---|
| Reduced food intake | +++ |
| Erratic swimming | +++ |
| Distended abdomen | +++ |
| Ulcers in the head | +++ |
| Eye injuries | -+ |
| Ascites | ++ |
| Friable liver | ++ |
| Splenomegaly | + |
| Hemorrhage in internal organs | ++ |
| Mortality | ++ |
| Antimicrobial | Bacterial Genus | |||
|---|---|---|---|---|
| Staphylococcus | Staphylococcus | Providencia | Providencia | |
| Oxolinic acid | R | R | S | S |
| Nalidixic acid | R | R | S | S |
| Ofloxacin | R | R | S | S |
| Ciprofloxacin | R | S | S | S |
| Enrofloxacin | S | S | S | S |
| Erythromycin | R | R | R | R |
| Fosfomycin | S | S | S | R |
| Chloramphenicol | S | S | R | S |
| Florfenicol | S | S | S | S |
| Oxytetracycline | R | R | R | R |
| Cotrimoxazole | S | S | S | S |
| Penicillin G | S | S | R | R |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajme-Manzur, D.; Hernández-López, J.; Martínez-Porchas, M.; Vargas-Albores, F.; Garibay-Valdez, E.; Coronado-Molina, D.E.; Hernández-Oñate, M.Á.; Vázquez-Ramírez, F.; Velázquez-Valencia, L.A.; Santacruz, A. Staphylococcus haemolyticus and Providencia vermicola Infections Occurring in Farmed Tilapia: Two Potentially Emerging Pathogens. Animals 2023, 13, 3715. https://doi.org/10.3390/ani13233715
Rajme-Manzur D, Hernández-López J, Martínez-Porchas M, Vargas-Albores F, Garibay-Valdez E, Coronado-Molina DE, Hernández-Oñate MÁ, Vázquez-Ramírez F, Velázquez-Valencia LA, Santacruz A. Staphylococcus haemolyticus and Providencia vermicola Infections Occurring in Farmed Tilapia: Two Potentially Emerging Pathogens. Animals. 2023; 13(23):3715. https://doi.org/10.3390/ani13233715
Chicago/Turabian StyleRajme-Manzur, David, Jorge Hernández-López, Marcel Martínez-Porchas, Francisco Vargas-Albores, Estefanía Garibay-Valdez, Daniel Eduardo Coronado-Molina, Miguel Ángel Hernández-Oñate, Francisco Vázquez-Ramírez, Luis Alfonso Velázquez-Valencia, and Azucena Santacruz. 2023. "Staphylococcus haemolyticus and Providencia vermicola Infections Occurring in Farmed Tilapia: Two Potentially Emerging Pathogens" Animals 13, no. 23: 3715. https://doi.org/10.3390/ani13233715
APA StyleRajme-Manzur, D., Hernández-López, J., Martínez-Porchas, M., Vargas-Albores, F., Garibay-Valdez, E., Coronado-Molina, D. E., Hernández-Oñate, M. Á., Vázquez-Ramírez, F., Velázquez-Valencia, L. A., & Santacruz, A. (2023). Staphylococcus haemolyticus and Providencia vermicola Infections Occurring in Farmed Tilapia: Two Potentially Emerging Pathogens. Animals, 13(23), 3715. https://doi.org/10.3390/ani13233715





