Lateralised Behavioural Responses in Livestock to Environmental Stressors: Implications for Using Infrared Thermography to Assess Welfare Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Relationships between Sensory Lateralisation and Behavioural Responses
2.1. Visual Lateralisation
2.2. Auditory Lateralisation
2.3. Olfactory Lateralisation
3. Relationship between Sensory Behavioural Lateralisation and Emotions
4. Relationship between Lateralisation and Behavioural Responses
5. Relationship between Posture Lateralisation and Behavioural Responses
6. Factors Affecting Lateralisation Manifestation
7. Relationship between Behavioural Lateralisation and Farm Management Practices
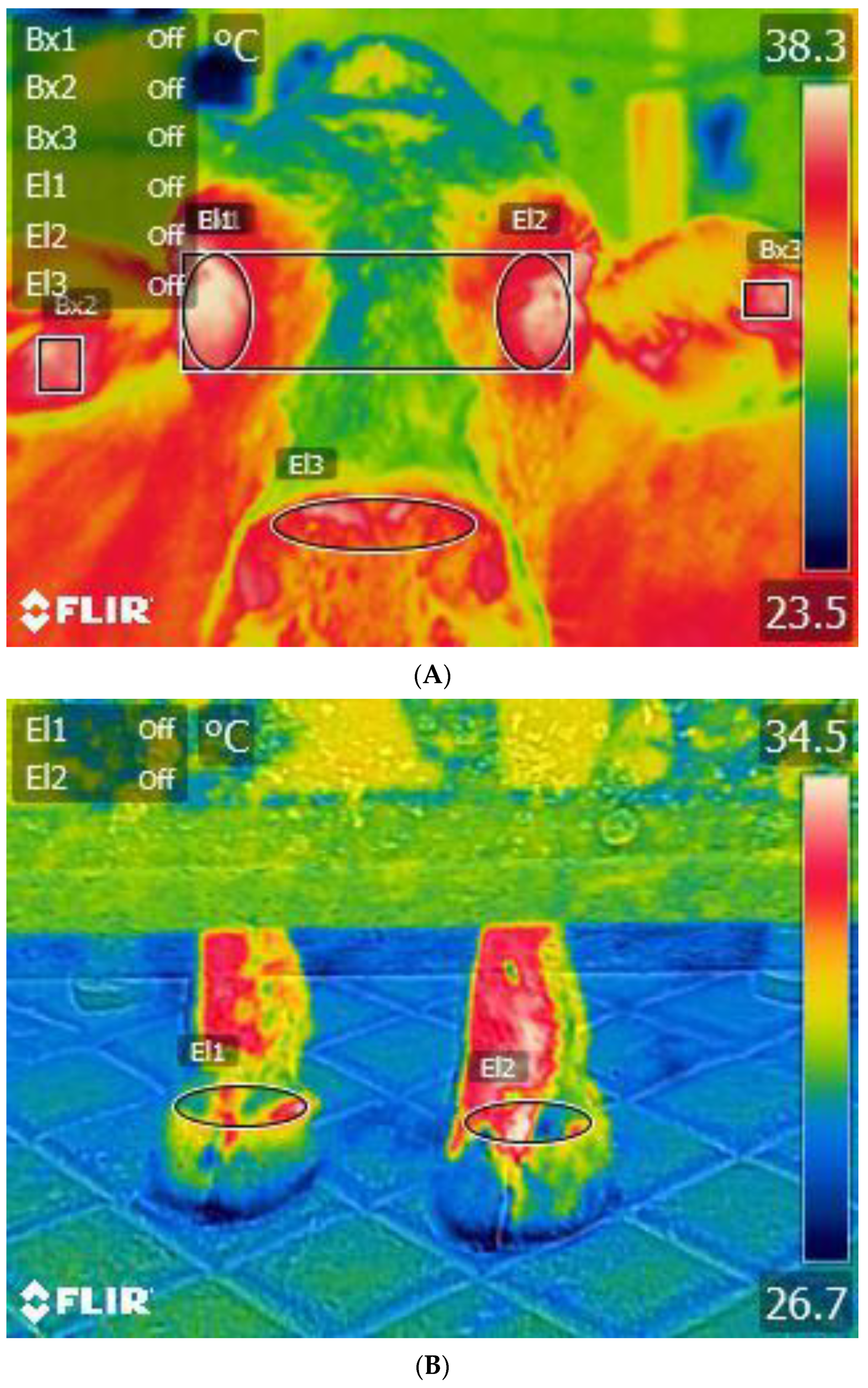
8. Relationship between Behavioural Lateralisation and the Infrared Thermography (IRT) Technique
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Boissy, A.; Manteuffel, G.; Bak Jensen, M.; Oppermann Mor, R.; Spruijt, B.; Keeling, L.J.; Langbein, J. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Duncan, I.J.H. The changing concept of animal sentience. Appl. Anim. Behav. Sci. 2006, 100, 11–19. [Google Scholar] [CrossRef]
- Manteuffel, G.; Langbein, J.; Puppe, B. From operant learning to cognitive enrichment in farm animal housing: Bases and applicability. Anim. Welf. 2009, 18, 87–95. [Google Scholar] [CrossRef]
- Mendl, M.; Paul, E.S. Consciousness, emotion and animal welfare: Insights from cognitive science. Anim. Welf. 2004, 13, S17–S25. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; Mclean, A.N.; Mcgreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human—Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef] [PubMed]
- McGlone, J.J. What is animal welfare? J. Agric. Environ. Ethics 1993, 6, 26–36. [Google Scholar]
- Moberg, G.P. Using risk assessment to define domestic animal welfare. J. Agric. Environ. Ethics 1993, 6, 1–7. [Google Scholar]
- Knierim, U.; Van Dongen, S.; Forkman, B.; Tuyttens, F.A.M.; Špinka, M.; Campo, J.L.; Weissengruber, G.E. Fluctuating asymmetry as an animal welfare indicator—A review of methodology and validity. Physiol. Behav. 2007, 92, 398–421. [Google Scholar] [CrossRef]
- Robins, A.; Phillips, C. Lateralised visual processing in domestic cattle herds responding to novel and familiar stimuli. Laterality 2010, 15, 514–534. [Google Scholar] [CrossRef]
- Phillips, C.J.C.; Oevermans, H.; Syrett, K.L.; Jespersen, A.Y.; Pearce, G.P. Lateralisation of behaviour in dairy cows in response to conspecifics and novel persons. J. Dairy Sci. 2015, 98, 2389–2400. [Google Scholar] [CrossRef]
- Vallortigara, G. Cerebral Lateralisation: A Common theme in the organization of the vertebrate brain. Cortex 2006, 42, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Frasnelli, E.; Vallortigara, G.; Rogers, L.J. Left-right asymmetries of behaviour and nervous system in invertebrates. Neurosci. Biobehav. Rev. 2012, 36, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Rogers, L.J. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralisation. Behav. Brain Sci. 2005, 28, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Hand and paw preferences in relation to the lateralized brain. Philos. Trans. R. Soc. B 2009, 364, 943–954. [Google Scholar] [CrossRef]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, UK, 2013; p. 229. [Google Scholar] [CrossRef]
- Vallortigara, G.; Chiandetti, C.; Rugani, R.; Sovrano, V.A.; Regolin, L. Animal cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2010, 1, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Lateralisation in Its Many Forms, and Its Evolution and Development. In The Evolution of Hemispheric Specialization in Primates; Hopkins, W.D., Ed.; American Society for Primatologists, Academic Press: London, UK, 2007. [Google Scholar] [CrossRef]
- Denenberg, V.H. Behaviour al symmetry and reverse asymmetry in the chick and rat. Behav. Brain Sci. 2005, 28, 597. [Google Scholar] [CrossRef]
- Lyons, D.M.; Afarian, H.; Schatzberg, A.F.; Sawyer-Glover, A.; Moseley, M.E. Experience-dependent asymmetric variation in primate prefrontal morphology. Behav. Brain Res. 2002, 136, 51–59. [Google Scholar] [CrossRef]
- Clarke, G.M. Developmental stability–fitness relationships in animals: Some theoretical considerations. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: Oxford, UK, 2003; pp. 187–195. [Google Scholar]
- Zakharov, V.M. Linking developmental stability and environmental stress: A whole organism approach. In Developmental Instability: Causes and Consequences; Polak, M., Ed.; Oxford University Press: Oxford, UK, 2003; pp. 402–414. [Google Scholar]
- MohanKumar, S.M.J.; Balasubramanian, P.; Dharmaraj, M.; MohanKumar, P.S. Neuroendocrine Regulation of Adaptive Mechanisms in Livestock. In Environmental Stress and Amelioration in Livestock Production; Sejian, V., Naqvi, S., Ezeji, T., Lakritz, J., Lal, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 263–298. [Google Scholar] [CrossRef]
- Phillips, C.J.C.; Llewellyn, S.; Claudia, A. Laterality in bovine behaviour in an extensive partially suckled herd and an intensive dairy herd. J. Dairy Sci. 2003, 86, 3167–3173. [Google Scholar] [CrossRef]
- Stub, C.; Ritskes-Hoitinga, M.; Thon, R.; Hansen, C.K.; Hansen, A.K. Fluctuating asymmetry in mice and rats: Evaluation of the method. Lab. Anim. 2002, 36, 193–199. [Google Scholar] [CrossRef]
- Tuyttens, F.A.; Maertens, L.; Van Poucke, E.; Van Nuffel, A.; Debeuckelaere, S.; Creve, J.; Lens, L. Measuring fluctuating asymmetry in fattening rabbits: A valid indicator of performance and housing quality? J. Anim. Sci. 2005, 83, 2645–2652. [Google Scholar] [CrossRef]
- Mendl, M.; Brooks, J.; Basse, C.; Burman, O.; Paul, E.; Blackwell, E.; Casey, R. Dogs showing separation-related behaviour exhibit a ‘pessimistic’ cognitive bias. Curr. Biol. 2010, 20, R839–R840. [Google Scholar] [CrossRef] [PubMed]
- Puppe, B.; Zebunke, M.; Düpjan, S.; Langbein, J. Kognitiv-emotionale Umweltbewältigung beim Hausschwein—Herausforderung für Tierhaltung und Tierschutz. Züchtungskunde 2012, 84, 307–319. [Google Scholar]
- Deng, C.; Rogers, L.J. Social recognition and approach in the chick: Lateralisation and effect of visual experience. Anim. Behav. 2002, 63, 697–706. [Google Scholar] [CrossRef]
- Vallortigara, G.; Regolin, L.; Pagni, P. Detour behaviour, imprinting and visual lateralisation in the domestic chick. Cogn. Brain Res. 1999, 7, 307–320. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Quaranta, A.; Rogers, L.J. Hemispheric specialization in dogs for processing different acoustic stimuli. PLoS ONE 2008, 3, e3349. [Google Scholar] [CrossRef]
- Teufel, C.; Ghazanfar, A.A.; Fischer, J. On the Relationship Between Lateralized Brain Function and Orienting Asymmetries. Behav. Neurosci. 2010, 124, 437–445. [Google Scholar] [CrossRef]
- Byrne, R.W.; Bates, L.A. Sociality, evolution and cognition. Curr. Biol. 2007, 17, R714–R723. [Google Scholar] [CrossRef]
- Coulon, M.; Deputte, B.L.; Heyman, Y.; Richard, C.; Delatouche, L.; Baudoin, C. Visual discrimination by heifers (Bos taurus) of their own species. J. Comp. Psychol. 2007, 121, 198–204. [Google Scholar] [CrossRef]
- Rosa Salva, O.; Regolin, L.; Mascalzoni, E.; Valortigara, G. Cerebral and behaviour al asymmetries in animal social recognition. Comp. Cogn. Behav. Rev. 2012, 7, 110–138. [Google Scholar] [CrossRef]
- Lee, C.; Café, L.M.; Robinson, S.L.; Doyle, R.E.; Lea, J.M.; Small, A.H.; Colditz, I.G. Anxiety influences attention bias but not flight speed and crush score in beef cattle. Appl. Anim. Behav. Sci. 2018, 205, 210–215. [Google Scholar] [CrossRef]
- Bouwknecht, J.A.; Olivier, B.; Paylor, R.E. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: A review of pharmacological and genetic studies in the mouse. Neurosci. Biobehav. Rev. 2007, 31, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Proctor, H.; Carder, G. Can changes in nasal temperature be used as an indicator of emotional state in cows? Appl. Anim. Behav. Sci. 2016, 184, 1–6. [Google Scholar] [CrossRef]
- Mader, T.L.; Gaughan, J.B.; Johnson, L.J.; Hahn, G.L. Tympanic temperature in confined beef cattle exposed to excessive heat load. Int. J. Biometeorol. 2010, 54, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Lefcourt, A.M.; Adams, W.R. Radiotelemetry measurement of body temperatures of feedlot steers during summer. J. Anim. Sci. 1996, 74, 2633–2640. [Google Scholar] [CrossRef] [PubMed]
- Vickers, L.A.; Burfeind, O.; von Keyserlingk, M.A.G.; Veira, D.M.; Weary, D.M.; Heuwieser, W. Technical note: Comparison of rectal and vaginal temperatures in lactating dairy cows. J. Dairy Sci. 2010, 93, 5246–5251. [Google Scholar] [CrossRef]
- Lees, A.; Lea, J.; Salvin, H.; Café, L.; Colditz, I.; Lee, C. Relationship between rectal temperature and vaginal temperature in grazing Bos taurus heifers. Animals 2018, 8, 156. [Google Scholar] [CrossRef]
- Lees, A.M.; Lees, J.C.; Lisle, A.T.; Sullivan, M.L.; Gaughan, J.B. Effect of heat stress on rumen temperature of three breeds of cattle. Int. J. Biometeorol. 2018, 62, 207–215. [Google Scholar] [CrossRef]
- Jenkins, S.; Brown, R.; Rutterford, N. Comparing thermographic, EEG, and subjective measures of affective experience during simulated product interactions. Int. J. Des. 2009, 3, 53–65. [Google Scholar]
- Johnson, S.R.; Rao, S.; Hussey, S.B.; Morley, P.S.; Traub-Dargatz, J.L. Thermographic eye temperature as an index to body temperature in ponies. J. Equine Vet. Sci. 2011, 31, 63–66. [Google Scholar] [CrossRef]
- Stewart, M.M.; Wilson, M.T.; Schaefer, A.L.; Huddart, F.; Sutherland, M.A. The use of infrared thermography and accelerometers for remote monitoring of dairy cow health and welfare. J. Dairy Sci. 2017, 100, 3893–3901. [Google Scholar] [CrossRef]
- Lowe, G.L.; Sutherland, M.A.; Waas, J.R.; Schaefer, A.L.; Cox, N.R.; Stewart, M. Physiological and behaviour al responses as indicators for early disease detection in dairy calves. J. Dairy Sci. 2019, 102, 5389–5402. [Google Scholar] [CrossRef] [PubMed]
- Perez Marquez, H.J.; Ambrose, D.J.; Schaefer, A.L.; Cook, N.J.; Bench, C.J. Infrared thermography and behaviour al biometrics associated with estrus indicators and ovulation in estrus-synchronized dairy cows housed in tiestalls. J. Dairy Sci. 2019, 102, 4427–4440. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Cogi, N.H.; Heinzl, E.U.L.; Dalla Costa, E.; Canali, E.; Minero, M. Validation of a fear test in sport horses using infrared thermography. J. Vet. Behav. Clin. Appl. Res. 2015, 10, 128–136. [Google Scholar] [CrossRef]
- Hall, C.; Burton, K.; Maycock, E.; Wragg, E. A preliminary study into the use of infrared thermography as a means of assessing the horse’s response to different training methods. J. Vet. Behav. Clin. Appl. Res. 2011, 6, 291–292. [Google Scholar] [CrossRef]
- Squibb, K.; Griffin, K.; Favier, R.; Ijichi, C. Poker Face: Discrepancies in behaviour and affective states in horses during stressful handling procedures. Appl. Anim. Behav. Sci. 2018, 202, 34–38. [Google Scholar] [CrossRef]
- Stewart, M.; Schaefer, A.; Haley, D.; Colyn, J.; Cook, N.; Stafford, K.; Webster, J. Infrared thermography as a non-invasive method for detecting fear-related responses of cattle to handling procedures. Anim. Welf. 2008, 17, 387–393. [Google Scholar] [CrossRef]
- Stewart, M.; Verkerk, G.A.; Stafford, K.J.; Schaefer, A.L.; Webster, J.R. Non-invasive assessment of autonomic activity for evaluation of pain in calves, using surgical castration as a model. J. Dairy Sci. 2010, 93, 3602–3609. [Google Scholar] [CrossRef]
- Stewart, M.; Webster, J.R.; Stafford, K.J.; Schaefer, A.L.; Verkerk, G.A. Technical note: Effects of an epinephrine infusion on eye temperature and heart rate variability in bull calves. J. Dairy Sci. 2010, 93, 5252–5257. [Google Scholar] [CrossRef]
- Proctor, H.S.; Carder, G. Measuring positive emotions in cows: Do visible eye whites tell us anything? Physiol. Behav. 2015, 147, 1–6. [Google Scholar] [CrossRef]
- Valera, M.; Bartolomé, E.; Sánchez, M.J.; Molina, A.; Cook, N.; Schaefer, A. Changes in Eye Temperature and Stress Assessment in Horses During Show Jumping Competitions. J. Equine Vet. Sci. 2012, 32, 827–830. [Google Scholar] [CrossRef]
- Ijichi, C.; Griffin, K.; Squibb, K.; Favier, R. Stranger danger? An investigation into the influence of human-horse bond on stress and behaviour. Appl. Anim. Behav. Sci. 2018, 206, 59–63. [Google Scholar] [CrossRef]
- Fenner, K.; Yoon, S.; White, P.; Starling, M.; McGreevy, P. The effect of noseband tightening on horses’ behaviour, eye temperature, and cardiac responses. PLoS ONE 2016, 11, e0154179. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Gargano, M.; Luzi, F.; Carenzi, C.; Verga, M. Technical note: Applicability of infrared thermography as a non invasive measurements of stress in rabbit. World Rabbit Sci. 2007, 15, 199–206. [Google Scholar] [CrossRef]
- Travain, T.; Colombo, E.S.; Heinzl, E.; Bellucci, D.; Previde, E.P.; Valsecchi, P. Hot dogs: Thermography in the assessment of stress in dogs (Canis familiaris)—A pilot study. J. Vet. Behav. 2015, 10, 17–23. [Google Scholar] [CrossRef]
- Uddin, J.; Phillips, C.J.C.; Goma, A.A.; McNeill, D.M. Relationships between infrared temperature and Laterality. Appl. Anim. Behav. Sci. 2019, 220, 104855. [Google Scholar] [CrossRef]
- Uddin, J.; Phillips, C.J.C.; Auboeuf, M.; McNeill, D.M. Relationships between body temperatures and behaviours in lactating dairy cows. Appl. Anim. Behav. Sci. 2021, 241, 105359. [Google Scholar] [CrossRef]
- Tucker, C.B.; Cox, N.R.; Weary, D.M.; Špinka, M. Laterality of lying behaviour in dairy cattle. Appl. Anim. Behav. Sci. 2009, 120, 125–131. [Google Scholar] [CrossRef]
- Morgante, M.; Vallortigara, G. Animal welfare: Neuro-cognitive approaches. Ital. J. Anim. Sci. 2009, 8, 255–264. [Google Scholar] [CrossRef]
- Rogers, L.J. Relevance of brain and behavioural lateralisation to animal welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]
- Rogers, L.J. Does brain lateralisation have practical implications for improving animal welfare? CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2011, 6, 1–10. [Google Scholar] [CrossRef]
- Mounaix, B.; Boivin, X.; Brule, A.; Schmitt, I. Cattle Behaviour and the Human-Animal Relationship: Variation Factors and Consequences in Breeding; Institut de l’Élevage: Paris, France, 2014; pp. 1–61. [Google Scholar]
- Villalba, J.J.; Provenza, F.D. Polyethylene glycol influences selection of foraging location by sheep consuming quebracho tannin. J. Anim. Sci. 2002, 80, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Launchbaugh, K.L.; Howery, L.D. Understanding landscape use patterns of livestock as a consequence of foraging behaviour. Rangel. Ecol. Manag. 2005, 58, 99–108. [Google Scholar] [CrossRef]
- Wredle, E.; Rushen, J.; De Passile, A.M.; Munksgarrd, L. Training cattle to approach a feed source in response to auditory signals. Can. J. Anim. Sci. 2004, 84, 567–572. [Google Scholar] [CrossRef]
- Kluever, B.M.; Howery, L.D.; Breck, S.W.; Bergman, D.L. Predator and heterospecific stimuli alter behaviour in cattle. Behav. Proc. 2009, 81, 85–91. [Google Scholar] [CrossRef] [PubMed]
- De Boyer Des Roches, A.; Richard-Yris, M.A.; Henry, S.; Ezzaouïa, M.; Hausberger, M. Laterality and emotions: Visual laterality in the domestic horse (Equus caballus) differs with objects’ emotional value. Physiol. Behav. 2008, 94, 487–490. [Google Scholar] [CrossRef]
- Adamczyk, K.; Górecka-Bruzda, A.; Nowicki, J.; Gumułka, M.; Molik, E.; Schwarz, T.; Klocek, C. Perception of environment in farm animals. A review. Ann. Anim. Sci. 2015, 15, 565–589. [Google Scholar] [CrossRef]
- Hanggi, E.B.; Ingersoll, J.F. Lateral vision in horses: A behaviour al investigation. Behav. Proc. 2012, 91, 70–76. [Google Scholar] [CrossRef]
- Vallortigara, G. Comparative neuropsychology of the dual brain: A stroll through animals’ left and right perceptual worlds. Brain Lang. 2000, 73, 189–219. [Google Scholar] [CrossRef]
- Jozet-Alves, C.; Viblanc, V.A.; Romagny, S.; Dacher, M.; Healy, S.D.; Dickel, L. Visual lateralisation is task and age dependent in cuttlefish, Sepia officinalis. Anim. Behav. 2012, 83, 1313–1318. [Google Scholar] [CrossRef]
- Fraser, D.; Matthews, L.R. Preference and motivation testing. In Animal Welfare; Appleby, M.C., Hughes, B.O., Eds.; CAB International: New York, NY, USA, 1997; pp. 159–173. [Google Scholar]
- Farmer, K.; Krueger, K.; Byrne, R.W. Visual laterality in the domestic horse (Equus caballus) interacting with humans. Anim. Cogn. 2010, 13, 229–238. [Google Scholar] [CrossRef]
- Heffner, H.E. Auditory awareness. Appl. Anim. Behav. Sci. 1998, 57, 259–268. [Google Scholar] [CrossRef]
- Heffner, R.S.; Heffner, H.E. Hearing in large mammals: Sound-localization acuity in cattle (Bos taurus) and goats (Capra hircus). J. Comp. Psychol. 1992, 106, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.J.C. Cattle Behaviour; Farming Press: Ipswich, UK, 1993; p. 58. [Google Scholar]
- Andrew, R.J. Arousal and the causation of behaviour. Behaviour 1974, 51, 135–165. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Eye and Ear Preferences. In Lateralized Brain Functions; Rogers, L., Vallortigara, G., Eds.; Neuromethods; Humana Press: New York, NY, USA, 2017; Volume 122. [Google Scholar] [CrossRef]
- Scheumann, M.; Zimmermann, E. Sex-specific asymmetries in communication sound perception are not related to hand preference in an early primate. BMC Biol. 2008, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Waring, G.H. Horse Behaviour, 2nd ed.; Noyes Publications/William Andrew Publishing: Norwich, NY, USA, 2003; pp. 18–299. [Google Scholar]
- Basile, M.; Boivin, S.; Boutin, A.; Blois-Heulin, C.; Hausberger, M.; Lemasson, A. Socially dependent auditory laterality in domestic horses (Equus caballus). Anim. Cogn. 2009, 12, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.; Pearson, L. The Vertebrate Brain; Academic Press: London, UK, 1976; p. 744. [Google Scholar]
- Ebbesson, S.E. On the organisation of the central visual pathways in vertebrates. Brain Behav. Evol. 1970, 3, 178–194. [Google Scholar] [CrossRef]
- Hugdahl, K. Lateralisation of cognitive processes in the brain. Acta Psychol. 2000, 105, 211–235. [Google Scholar] [CrossRef] [PubMed]
- Ocklenburg, S.; Ströckens, F.; Güntürkün, O. Lateralisation of conspecific vocalisation in non-human vertebrates. Laterality 2013, 18, 1–31. [Google Scholar] [CrossRef]
- Arave, C.W. Assessing sensory capacity of animals using operant technology. J. Anim. Sci. 1996, 74, 1996–2009. [Google Scholar] [CrossRef]
- Waynert, D.F.; Stookey, J.M.; Schwartzkopf-Genswein, K.S.; Waltz, C.S. The response of beef cattle to noise during handling. Appl. Anim. Behav. Sci. 1999, 62, 27–42. [Google Scholar] [CrossRef]
- Waiblinger, S.; Boivin, X.; Pedersen, V.; Tosi, M.V.; Janczak, A.M.; Visser, E.K.; Jones, R.B. Assessing the human–animal relationship in farmed species: A critical review. Appl. Anim. Behav. Sci. 2006, 101, 185–242. [Google Scholar] [CrossRef]
- Pajor, E.A.; Rushen, J.; De Passillé, A.M.B. Dairy cattle’s choice of handling treatments in a Y-maze. Appl. Anim. Behavi. Sci. 2003, 80, 93–107. [Google Scholar] [CrossRef]
- Wackermannova, M.; Pinc, L.; Jebavy, L. Olfactory Sensitivity in Mammalian Species. Physiol. Res. 2016, 65, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Boissy, A.; Terlouw, C.; Le Neindre, P. Presence of cues from stressed conspecifics increases reactivity to aversive events in cattle: Evidence for the existence of alarm substances in urine. Physiol. Behav. 1998, 63, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Bouissou, M.F.; Boissy, A.; Le Neindre, P.; Vessier, I. The social behaviour of cattle. In Social Behaviour in Farm Animals; Keeling, L.J., Gonyou, H.W., Eds.; CABI Publishing: New York, NY, USA, 2001; pp. 113–145. [Google Scholar]
- Brancucci, A.; Lucci, G.; Mazzatenta, A.; Tommasi, L. Asymmetries of the human social brain in the visual, auditory and chemical modalities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 895–914. [Google Scholar] [CrossRef] [PubMed]
- Savic, I.; Berglund, H. Right-nostril dominance in discrimination of unfamiliar, but not familiar, odours. Chem. Sens. 2000, 25, 517–523. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Broman, D.A.; Olsson, M.J.; Nordin, S. Lateralisation of olfactory cognitive functions: Effects of rhinal side of stimulation. Chem. Sens. 2001, 26, 1187–1192. [Google Scholar] [CrossRef]
- Royet, J.P.; Plailly, J. Lateralisation of olfactory processes. Chem. Sens. 2004, 29, 731–745. [Google Scholar] [CrossRef]
- Andrew, R.J.; Rogers, L.J. The nature of lateralisation in tetrapods. In Comparative Vertebrate Lateralisation; Rogers, L.J., Andrew, R.J., Eds.; Cambridge University Press: New York, NY, USA, 2002; pp. 94–125. [Google Scholar]
- McGreevy, P.D.; Rogers, L.J. Motor and sensory laterality in thoroughbred horses. Appl. Anim. Behav. Sci. 2005, 92, 337–352. [Google Scholar] [CrossRef]
- Rogers, L.J.; Vallortigara, G. From antenna to antenna: Lateral shift of olfactory memory recall by honeybees. PLoS ONE 2008, 3, e2340. [Google Scholar] [CrossRef]
- Vallortigara, G.; Rogers, L.J.; Bisazza, A. Possible evolutionary origins of cognitive brain lateralisation. Brain Res. Rev. 1999, 30, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Chiandetti, C.; Sovrano, V.A. Brain asymmetry (animal). Wiley Interdiscip. Rev. Cogn. Sci. 2011, 2, 146–157. [Google Scholar] [CrossRef] [PubMed]
- MacNeilage, P.F.; Rogers, L.J.; Vallortigara, G. Neuroscience origins of the left and right brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Forebrain emotional asymmetry: A neuroanatomical basis? Trends Cogn. Sci. 2005, 9, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Sasso, R.; Pepe, A.M.; Vallortigara, G.; Quaranta, A. Dogs turn left to emotional stimuli. Behav. Brain Res. 2010, 208, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Kappel, S.; Mendl, M.T.; Barrett, D.C.; Murrell, J.C.; Whay, H.R. Lateralized behaviour as indicator of affective state in dairy cows. PLoS ONE 2017, 12, e0184933. [Google Scholar] [CrossRef]
- Goma, A.A.; Pearce, G.P.; Uddin, J.; Rimon, E.; Davies, H.; Phillips, C.J.C. A forced lateralisation test for dairy cows and its relation to their behaviour. Appl. Anim. Behav. Sci. 2018, 207, 8–19. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Lateralisation of agonistic and vigilance responses in Przewalski horses (Equus przewalskii). Appl. Anim. Behav. Sci. 2014, 151, 43–50. [Google Scholar] [CrossRef]
- Sankey, C.; Henry, S.; Clouard, C.; Richard-Yris, M.A.; Hausberger, M. Asymmetry of behaviour al responses to a human approach in young naive vs. trained horses. Physiol. Behav. 2011, 104, 464–468. [Google Scholar] [CrossRef]
- Esch, L.; Wöhr, C.; Erhard, M.; Krüger, K. Horses’ (Equus caballus) laterality, stress hormones, and task related behaviour in innovative problem-solving. Animals 2019, 9, 265. [Google Scholar] [CrossRef]
- Felici, M.; Reddon, A.R.; Maglieri, V.; Lanatà, A.; Baragli, P. Heart and brain: Change in cardiac entropy is related to lateralised visual inspection in horses. PLoS ONE 2023, 18, e0289753. [Google Scholar] [CrossRef] [PubMed]
- Farmer, K.; Krüger, K.; Byrne, R.W.; Marr, I. Sensory laterality in affiliative interactions in domestic horses and ponies (Equus caballus). Anim. Cogn. 2018, 21, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Kieson, E.; Goma, A.A.; Medhat, R. Tend and Befriend in Horses: Partner Preferences, Lateralisation and Contextualization of Allogrooming in Two Socially Stable Herds of Quarter Horse Mares. Animals 2023, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Leliveld, L.M.C.; Langbein, J.; Puppe, B. The emergence of emotional lateralisation: Evidence in non-human vertebrates and implications for farm animals. Appl. Anim. Behav. Sci. 2013, 145, 1–14. [Google Scholar] [CrossRef]
- Briefer, E.F. Vocal expression of emotions in mammals: Mechanisms of production and evidence. J. Zool. 2012, 288, 1–20. [Google Scholar] [CrossRef]
- Briefer, E.F. Vocal contagion of emotions in non-human animals. Proc. R. Soc. B 2018, 285, 20172783. [Google Scholar] [CrossRef] [PubMed]
- Nickel, R.; Schummer, A.; Seiferle, E. Lehrbuch der Anatomie der Haustiere [Handbook of Anatomy of Domestic Animals]; Paul Parey Verlag: Berlin, Germany, 1968; Volume 1. [Google Scholar]
- Schmied, C.; Waiblinger, S.; Scharl, T.; Leisch, F.; Boivin, X. Stroking of different body regions by a human: Effects on behaviour and heart rate of dairy cows. Appl. Anim. Behav. Sci. 2008, 109, 25–38. [Google Scholar] [CrossRef]
- De Oliveira, D.; Keeling, L.J. Routine activities and emotion in the life of dairy cows: Integrating body language into an affective state framework. PLoS ONE 2018, 13, e0195674. [Google Scholar] [CrossRef]
- Ocklenburg, S.; Korte, S.M.; Peterburs, J.; Wolf, O.T.; Gu¨ntu¨rku¨n, O. Stress and laterality—The comparative perspective. Physiol. Behav. 2016, 164, 321–329. [Google Scholar] [CrossRef]
- Schoenbaum, G.; Chiba, A.A.; Gallagher, M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J. Neurosci. 1999, 19, 1876–1884. [Google Scholar] [CrossRef]
- Armony, J.L. Current emotion research in behaviour al neuroscience: The role(s) of the amygdala. Emot. Rev. 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Janak, P.H.; Tye, K.M. From circuits to behaviour in the amygdala. Nature 2015, 517, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Young, E.J.; Williams, C.L. Valence dependent asymmetric release of norepinephrine in the basolateral amygdala. Behav. Neurosci. 2010, 124, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Young, E.J.; Williams, C.L. Differential activation of amygdala Arc expression by positive and negatively valenced emotional learning conditions. Front. Behav. Neurosci. 2013, 7, 191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murphy, J.; Arkins, S. Equine learning behaviour. Behav. Proc. 2007, 76, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Cerri, F.; Carluccio, A.; Baciadonna, L. Space availability influence laterality in donkeys (Equus asinus). Behav. Proc. 2011, 88, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Savin, H. The Effects of Lateralisation on Detour-Based Problem Solving in Horses (Equus caballus). Master’s Thesis, University of Plymouth, School of Biomedical and Biological Sciences, Plymouth, UK, 2015. Available online: http://hdl.handle.net/10026.1/3512 (accessed on 1 August 2023).
- Baragli, P.; Vitale, V.; Paoletti, E.; Sighieri, C.; Reddon, A.R. Detour behaviour in horses (Equus caballus). J. Ethol. 2011, 29, 227–234. [Google Scholar] [CrossRef]
- Proops, L.; McComb, K. Cross-modal individual recognition in domestic horses (Equus caballus) extends to familiar humans. Proc. Biol. Sci. 2012, 279, 3131–3138. [Google Scholar] [CrossRef]
- Marr, I.; Farmer, K.; Krüger, K. Evidence for right-sided horses being more optimistic than left-sided horses. Animals 2018, 8, 219. [Google Scholar] [CrossRef]
- Gabor, V.; Gerken, M. Horses use procedural learning rather than conceptual learning to solve matching to sample. Appl. Anim. Behav. Sci. 2010, 126, 119–124. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Thomson, P.C. Differences in motor laterality between breeds of performance horse. Appl. Anim. Behav. Sci. 2006, 99, 183–190. [Google Scholar] [CrossRef]
- Larose, C.; Richard-Yris, M.A.; Hausberger, M.; Rogers, L.J. Laterality of horses associated with emotionality in novel situations. Laterality 2006, 11, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.; Schwarz, S.; Marr, I.; Farmer, K. Laterality in Horse Training: Psychological and Physical Balance and Coordination and Strength Rather Than Straightness. Animals 2022, 12, 1042. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Marr, I.; Farmer, K.; Graf, K.; Stefanski, V.; Krueger, K. Does Carrying a Rider Change Motor and Sensory Laterality in Horses? Animals 2022, 12, 992. [Google Scholar] [CrossRef] [PubMed]
- Byström, A.; Clayton, H.M.; Hernlund, E.; Rhodin, M.; Egenvall, A. Equestrian and biomechanical perspectives on laterality in the horse. Comp. Exerc. Physiol. 2020, 16, 35–45. [Google Scholar] [CrossRef]
- Ganskopp, D. Free-ranging angora goats: Left- or right-handed tendencies while grazing? Appl. Anim. Behav. Sci. 1995, 43, 141–146. [Google Scholar] [CrossRef]
- Eberhart, N.L.; Krawczel, P.D. The Effect of Hock Injury Laterality and Lameness on Lying Behaviour s and Lying Laterality in Holstein Dairy Cows. Animals 2017, 7, 86. [Google Scholar] [CrossRef]
- Arave, C.W.; Walters, J.L. Factors affecting lying behaviour and stall utilization of dairy cattle. Appl. Anim. Ethol. 1980, 6, 369–376. [Google Scholar] [CrossRef]
- Bao, J.; Giller, P.S. Observations on the changes in behavioural activities of dairy cows prior to and after parturition. Ir. Vet. J. 1991, 44, 43–47. [Google Scholar]
- Jones, R.B.; Roper, T.J. Olfaction in the domestic fowl: A critical review. Physiol. Behav. 1997, 62, 1009–1018. [Google Scholar] [CrossRef]
- Sommerville, B.A.; Broom, D.M. Olfactory awareness. Appl. Anim. Behav. Sci. 1998, 57, 269–286. [Google Scholar] [CrossRef]
- Tanaka, T.; Hashimoto, A.; Tanida, H.; Yoshimoto, T. Studies on the visual acuity of sheep using shape—Discrimination learning. J. Ethol. 1995, 13, 69–75. [Google Scholar] [CrossRef]
- Zonderland, J.J.; Cornelissen, L.; Wolthuis-Fillerup, M.; Spoolder, H.A.M. Visual acuity of pigs at different light intensities. Appl. Anim. Behav. Sci. 2008, 111, 28–37. [Google Scholar] [CrossRef]
- Sugnaseelan, S.; Prescott, N.B.; Broom, D.M.; Wathes, C.M.; Phillips, C.J.C. Visual discrimination learning and spatial acuity in sheep. Appl. Anim. Behav. Sci. 2013, 147, 104–111. [Google Scholar] [CrossRef]
- Corballis, M.C. Of mice and men—And lopsided birds. Cortex 2008, 44, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Fenner, K.; Freire, R.; McLean, A.; McGreevy, P. Behaviour al, demographic, and management influences on equine responses to negative reinforcement. J. Vet. Behav. 2019, 29, 11–17. [Google Scholar] [CrossRef]
- Kikkers, B.H.; Ózsvári, L.; Van Eerdenburg, F.J.C.M.; Bajcsy, Á.C.; Szenci, O. The influence of laterality on mastitis incidence in dairy cattle-preliminary study. Acta Vet. Hung. 2006, 54, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, A.M.; Pettersson, G.; Ljungberg, T.; Svennersten-Sjaunja, K. A brief note about cow lying behaviour—Do cows choose left and right lying side equally? Appl. Anim. Behav. Sci. 2008, 114, 32–36. [Google Scholar] [CrossRef]
- Lane, A.; Phillips, C. A note on behavioural laterality in neonatal lambs. Appl. Anim. Behav. Sci. 2004, 86, 161–167. [Google Scholar] [CrossRef]
- Lanier, J.L.; Grandin, T.; Green, R.D.; Avery, D.; McGee, K. The relationship between the reaction to sudden, intermittent movements and sounds, and temperament. J. Anim. Sci. 2000, 78, 1467–1474. [Google Scholar] [CrossRef]
- Broucek, J.; Uhrincat, M.; Mihina, S.; Soch, M.; Mrekajova, A.; Hanus, A. Dairy Cows Produce Less Milk and Modify Their Behaviour during the Transition between Tie-Stall to Free-Stall. Animals 2017, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.L. Animal asymmetry and human heredity. Dextrality, tool use and language in evolution-10 years after Walker (1980). Br. J. Psychol. 1991, 82, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Wagnon, K.A.; Rollins, W.C. Bovine laterality. J. Anim. Sci. 1972, 35, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.J.; Colenbrander, V.F.; Albright, J.L. Effect of particle size of forage and rumen cannulation upon chewing activity and laterality in dairy cows. J. Dairy Sci. 1990, 73, 3158–3164. [Google Scholar] [CrossRef] [PubMed]
- Mattachini, G.; Tamburini, A.; Zucali, M.; Bava, L.; Riva, E.; Provolo, G.; Sandrucci, A. Relationships among lying and standing behaviour, body condition score and milk production in primiparous cows. Ital. J. Anim. Sci. 2020, 19, 772–782. [Google Scholar] [CrossRef]
- Hixson, C.L.; Krawczel, P.D.; Caldwell, J.M.; Miller-Cushon, E.K. Behaviour al changes in group-housed dairy calves infected with Mannheimia haemolytica. J. Dairy Sci. 2018, 101, 10351–10360. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, N.L.; Storer, J.M.; Caldwell, M.; Saxton, A.M.; Krawczel, P.D. Behaviour al and physiologic changes in Holstein steers experimentally infected with Mannheimia haemolytica. Am. J. Vet. Res. 2017, 78, 1056–1064. [Google Scholar] [CrossRef]
- Černý, T.; Večeřa, M.; Falta, D.; Chládek, G. The effect of the season on the behaviour and milk yield of the czech fleckvieh cows. Acta Univ. Agric. Silvic. Mendel. Brun. 2016, 64, 1125–1130. [Google Scholar] [CrossRef]
- Boris, L.M. The food-borne ultimatum: Proposing federal legislation to create humane living conditions for animals raised for food in order to improve human health. J. Law Health 2011, 24, 285. [Google Scholar]
- D’Silva, J. Adverse impact of industrial animal agriculture on the health and welfare of farmed animals. Integr. Zool. 2006, 1, 53–58. [Google Scholar] [CrossRef]
- Gunderson, R. From cattle to capital: Exchange value, animal commodification, and barbarism. Crit. Sociol. 2013, 39, 259–275. [Google Scholar] [CrossRef]
- Fraser, A.F.; Broom, D.M. Farm Animal Behaviour and Welfare, 3rd ed.; CAB International: Wallingford, UK, 1997; p. 437. [Google Scholar]
- Prelle, I.; Phillips, C.J.C.; Paranhos Da Costa, M.J.; Vandenberghe, N.C.; Broom, D.M. Are cows that consistently enter the same side of a two-sided milking parlour more fearful of novel situations or more competitive? Appl. Anim. Behav. Sci. 2004, 87, 193–203. [Google Scholar] [CrossRef]
- Hopster, H.; Van Der Werf, J.T.N.; Blokhuis, H.J. Side preference of dairy cows in the milking parlour and its effects on behaviour and heart rate during milking. Appl. Anim. Behav. Sci. 1998, 55, 213–229. [Google Scholar] [CrossRef]
- Tanner, M.; Grandin, T.; Cattell, M.; Deesing, M. The relationship between facial hair whorls and milking parlor side preferences. J. Anim. Sci. 1994, 72, 207. [Google Scholar]
- Zucs, E.; Acs, I.; Csiba, A.; Ugry, K. A csoportletszam szerepe a fejostehenek tartastechnologiajanak kialakitasaban. 3. Kozlemeny: A fejoallas hasznatala. Allattenyesztes-es-Takarmanyozas 1992, 41, 133–152. [Google Scholar]
- Paranhos Da Costa, M.J.R.; Broom, D.M. Consistency of side choice in the milking parlour by Holstein-Friesian cows and its relationship with their reactivity and milk yield. Appl. Anim. Behav. Sci. 2001, 70, 177–186. [Google Scholar] [CrossRef]
- Hansen, S.W.; Damgaard, B.M. Behavioural and adrenocortical coping strategies and the effect on eosinophil leucocyte level and heterophil/lymphocyte-ratio in beech marten (Martes foina). Appl. Anim. Behav. Sci. 1993, 35, 369–388. [Google Scholar] [CrossRef]
- Miguel-Pacheco, G.G.; Thomas, H.J.; Kaler, J.; Craigon, J.; Huxley, J.N. Effects of lameness treatment for claw horn lesions on lying behaviour in dairy cows. Appl. Anim. Behav. Sci. 2016, 179, 11–16. [Google Scholar] [CrossRef]
- Večeřa, M.; Falta, D.; Filipčík, R.; Chládek, G.; Lategan, F. The effect of low and high cowshed temperatures on the behaviour and milk performance of Czech fleckvieh cows. Ann. Anim. Sci. 2016, 16, 1153–1161. [Google Scholar] [CrossRef]
- Rizhova, L.Y.; Kokorina, E.P. Behavioural asymmetry is involved in regulation of autonomic processes: Left side presentation of food improves reproduction and lactation in cows. Behav. Brain Res. 2005, 161, 75–81. [Google Scholar] [CrossRef]
- Thorbergson, Z.W.; Nielsen, S.G.; Beaulieu, R.J.; Doyle, R.E. Physiological and Behaviour al Responses of Horses to Wither Scratching and Patting the Neck When Under Saddle. J. Appl. Anim. Welf. Sci. 2016, 19, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.M.; Salvin, H.E.; Colditz, I.; Lee, C. The influence of temperament on body temperature response to handling in angus cattle. Animals 2020, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Webster, J.R.; Schaefer, A.L.; Cook, N.J.; Scott, S.L. Infrared thermography as a non-invasive tool to study animal welfare. Anim. Welf. 2005, 14, 319–325. [Google Scholar] [CrossRef]
- McCafferty, D.J.; Gallon, S.; Nord, A. Challenges of measuring body temperatures of free-ranging birds and mammals. Anim. Biotelem. 2015, 3, 33. [Google Scholar] [CrossRef]
- Montanholi, Y.R.; Odongo, N.E.; Swanson, K.C.; Schenkel, F.S.; McBride, B.W.; Miller, S.P. Application of infrared thermography as an indicator of heat and methane production and its use in the study of skin temperature in response to physiological events in dairy cattle (Bos taurus). J. Therm. Biol. 2008, 33, 468–475. [Google Scholar] [CrossRef]
- DiGiacomo, K.; Marett, L.C.; Wales, W.J.; Hayes, B.J.; Dunshea, F.R.; Leury, B.J. Thermoregulatory differences in lactating dairy cattle classed as efficient or inefficient based on residual feed intake. Anim. Prod. Sci. 2014, 54, 1877–1881. [Google Scholar] [CrossRef]
- Uddin, J.; McNeill, D.M.; Lisle, A.T.; Phillips, C.J.C. A sampling strategy for the determination of infrared temperature of relevant external body surfaces of dairy cows. Int. J. Biometeorol. 2020, 64, 1583–1592. [Google Scholar] [CrossRef]
- Idris, M.; Uddin, J.; Sullivan, M.; McNeill, D.M.; Phillips, C.J.C. Non-invasive physiological indicators of heat stress in cattle. Animals 2021, 11, 71. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goma, A.A.; Uddin, J.; Kieson, E. Lateralised Behavioural Responses in Livestock to Environmental Stressors: Implications for Using Infrared Thermography to Assess Welfare Conditions. Animals 2023, 13, 3663. https://doi.org/10.3390/ani13233663
Goma AA, Uddin J, Kieson E. Lateralised Behavioural Responses in Livestock to Environmental Stressors: Implications for Using Infrared Thermography to Assess Welfare Conditions. Animals. 2023; 13(23):3663. https://doi.org/10.3390/ani13233663
Chicago/Turabian StyleGoma, Amira A., Jashim Uddin, and Emily Kieson. 2023. "Lateralised Behavioural Responses in Livestock to Environmental Stressors: Implications for Using Infrared Thermography to Assess Welfare Conditions" Animals 13, no. 23: 3663. https://doi.org/10.3390/ani13233663
APA StyleGoma, A. A., Uddin, J., & Kieson, E. (2023). Lateralised Behavioural Responses in Livestock to Environmental Stressors: Implications for Using Infrared Thermography to Assess Welfare Conditions. Animals, 13(23), 3663. https://doi.org/10.3390/ani13233663





