Association Analyses between Single Nucleotide Polymorphisms in ZFAT, FBN1, FAM184B Genes and Litter Size of Xinggao Mutton Sheep
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Preparation and Sample Collection
2.2. Genotyping
2.3. Statistical Analysis
2.4. Protein Structure and Interaction Network Analysis
3. Results
3.1. Genotyping and Population Genetic Analysis of Candidate SNPs in FBN1, FAM184B and ZFAT Genes
3.2. Associations Analysis between loci in FBN1, FAM184B, ZFAT, BMPR1B and Litter Size of Xinggao Mutton Sheep
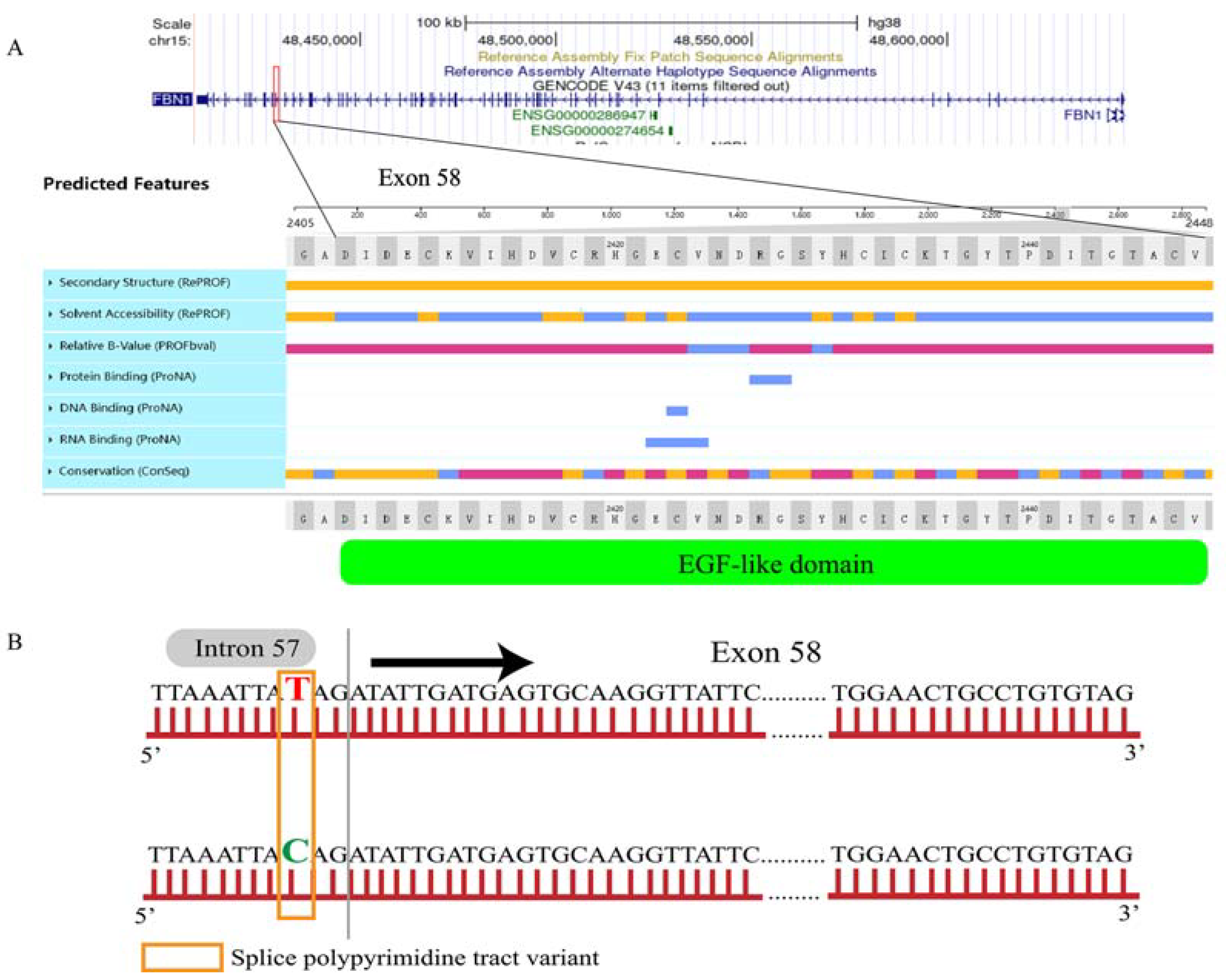
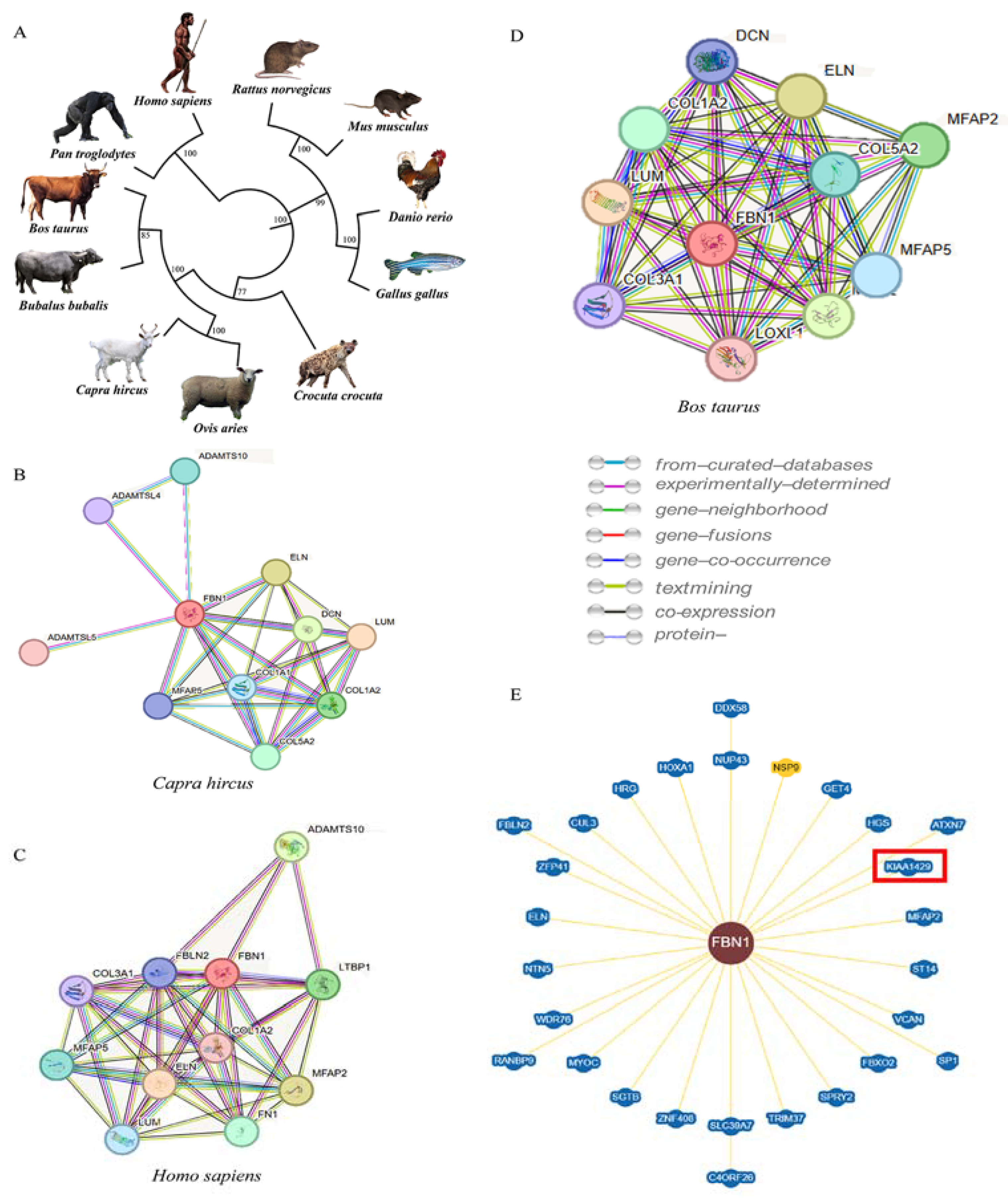
3.3. Protein Structure and Interaction Network Analysis on FBN1
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Li, X.; Yang, J.; Shen, M.; Xie, X.L.; Liu, G.J.; Xu, Y.X.; Lv, F.H.; Yang, H.; Yang, Y.L.; Liu, C.B.; et al. Whole-genome resequencing of wild and domestic sheep identifies genes associated with morphological and agronomic traits. Nat. Commun. 2020, 11, 2815. [Google Scholar] [CrossRef] [PubMed]
- Hatzirodos, N.; Bayne, R.A.; Irving-Rodgers, H.F.; Hummitzsch, K.; Sabatier, L.; Lee, S.; Bonner, W.; Gibson, M.A.; Rainey, W.E.; Carr, B.R.; et al. Linkage of regulators of TGF-β activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 2011, 25, 2256–2265. [Google Scholar] [CrossRef] [PubMed]
- Sakai, L.Y.; Keene, D.R.; Renard, M.; De Backer, J. FBN1: The disease-causing gene for Marfan syndrome and other genetic disorders. Gene 2016, 591, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Azumah, R.; Liu, M.H.; Hummitzsch, K.; Bastian, N.A.; Hartanti, M.D.; Irving-Rodgers, H.F.; Anderson, R.A.; Rodgers, R.J. Candidate genes for polycystic ovary syndrome are regulated by TGF beta in the bovine foetal ovary. Hum. Reprod. 2022, 37, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Prodoehl, M.J.; Irving-Rodgers, H.F.; Bonner, W.M.; Sullivan, T.M.; Micke, G.C.; Gibson, M.A.; Perry, V.E.; Rodgers, R.J. Fibrillins and latent TGF beta binding proteins in bovine ovaries of offspring following high or low protein diets during pregnancy of dams. Mol. Cell Endocrinol. 2009, 307, 133–141. [Google Scholar] [CrossRef]
- Romere, C.; Duerrschmid, C.; Bournat, J.; Constable, P.; Jain, M.; Xia, F.; Saha, P.K.; Del Solar, M.; Zhu, B.; York, B.; et al. Asprosin, a Fasting-Induced Glucogenic Protein Hormone. Cell 2016, 165, 566–579. [Google Scholar] [CrossRef]
- Maylem, E.R.S.; Spicer, L.J.; Batalha, I.; Schutz, L.F. Discovery of a possible role of asprosin in ovarian follicular function. J. Mol. Endocrinol. 2021, 66, 35–44. [Google Scholar] [CrossRef]
- Jiang, Y.; Xie, M.; Chen, W.; Talbot, R.; Maddox, J.F.; Faraut, T.; Wu, C.; Muzny, D.M.; Li, Y.; Zhang, W.; et al. The sheep genome illuminates biology of the rumen and lipid metabolism. Science 2014, 344, 1168–1173. [Google Scholar] [CrossRef]
- Zhang, G.X.; Fan, Q.C.; Wang, J.Y.; Zhang, T.; Xue, Q.; Shi, H.Q. Genome-wide association study on reproductive traits in Jinghai Yellow Chicken. Anim. Reprod. Sci. 2015, 163, 30–34. [Google Scholar] [CrossRef]
- Barbaux, S.; Gascoin-Lachambre, G.; Buffat, C.; Monnier, P.; Mondon, F.; Tonanny, M.B.; Pinard, A.; Auer, J.; Bessières, B.; Barlier, A.; et al. A genome-wide approach reveals novel imprinted genes expressed in the human placenta. Epigenetics 2012, 7, 1079–1090. [Google Scholar] [CrossRef]
- Kobayashi, H. Imprinting genes associated with endometriosis. EXCLI J. 2014, 13, 252–264. [Google Scholar] [PubMed]
- Pan, Z.; Li, S.; Liu, Q.; Wang, Z.; Zhou, Z.; Di, R.; Miao, B.; Hu, W.; Wang, X.; Hu, X.; et al. Whole-genome sequences of 89 Chinese sheep suggest role of RXFP2 in the development of unique horn phenotype as response to semi-feralization. Gigascience 2018, 7, giy019. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tao, L.; He, X.; Di, R.; Wang, X.; Chu, M. Single-nucleotide polymorphisms in FLT3, NLRP5, and TGIF1 are associated with litter size in Small-tailed Han sheep. Arch. Anim. Breed. 2021, 64, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Pan, Z.; Cao, X.; Guo, X.; He, X.; Sun, Q.; Di, R.; Hu, W.; Wang, X.; Zhang, X.; et al. Single Nucleotide Polymorphisms in the HIRA Gene Affect Litter Size in Small Tail Han Sheep. Animals 2018, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, X.; He, X.; Liu, Q.; Di, R.; Hu, W.; Cao, X.; Zhang, X.; Zhang, J.; Chu, M. Effects of FecB Mutation on Estrus, Ovulation, and Endocrine Characteristics in Small Tail Han Sheep. Front. Vet. Sci. 2021, 22, 709737. [Google Scholar] [CrossRef]
- Chambers, J.M.; Freeny, A.E.; Heiberger, R.M. Analysis of variance, Designed experiments. In Statistical Models in S; Chambers, J.M., Hastie, T.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 145–193. [Google Scholar]
- von Mering, C.; Jensen, L.J.; Snel, B.; Hooper, S.D.; Krupp, M.; Foglierini, M.; Jouffre, N.; Huynen, M.A.; Bork, P. STRING: Known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005, 33, D433–D437. [Google Scholar] [CrossRef]
- Winter, A.G.; Wildenhain, J.; Tyers, M. BioGRID REST Service, BiogridPlugin2 and BioGRID WebGraph: New tools for access to interaction data at BioGRID. Bioinformatics 2011, 27, 1043–1044. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shimada, M. The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process. J. Reprod. Dev. 2012, 58, 510–514. [Google Scholar] [CrossRef]
- Diao, H.; Li, X.; Xu, Y.; Xing, X.; Pang, S. Asprosin, a novel glucogenic adipokine implicated in type 2 diabetes mellitus. J. Diabetes Complicat. 2023, 37, 108614. [Google Scholar] [CrossRef]
- Aalders, J.; Léger, L.; Demolder, A.; Muiño Mosquera, L.; Coucke, P.; Menten, B.; De Backer, J.; van Hengel, J. Generation of human induced pluripotent stem cell line UGENTi001-A from a patient with Marfan syndrome carrying a heterozygous c.7754 T > C variant in FBN1 and the isogenic control UGENT001-A-1 using CRISPR/Cas9 editing. Stem Cell Res. 2023, 67, 103036. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Jiang, Q. MFAP5 suppression inhibits migration/invasion, regulates cell cycle and induces apoptosis via promoting ROS production in cervical cancer. Biochem. Biophys. Res. Commun. 2018, 507, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, Z.; Zhao, W.; Fu, Y.; Li, B.; Cheng, J.; Deng, Y.; Li, S.; Li, H. TGF-β1 induces type I collagen deposition in granulosa cells via the AKT/GSK-3β signaling pathway-mediated MMP1 down-regulation. Reprod. Biol. 2022, 22, 100705. [Google Scholar] [CrossRef] [PubMed]
- Hatzirodos, N.; Hummitzsch, K.; Irving-Rodgers, H.F.; Rodgers, R.J. Transcriptome comparisons identify new cell markers for theca interna and granulosa cells from small and large antral ovarian follicles. PLoS ONE 2015, 10, e0119800. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, H.A.; Kwan, P.; Clark, S.A.; Ferdosi, M.H.; Tellam, R.; Gondro, C. Genome-wide association study of body weight in Australian Merino sheep reveals an orthologous region on OAR6 to human and bovine genomic regions affecting height and weight. Genet. Sel. Evol. 2015, 47, 66. [Google Scholar] [CrossRef] [PubMed]
- Bolormaa, S.; Hayes, B.J.; van der Werf, J.H.; Pethick, D.; Goddard, M.E.; Daetwyler, H.D. Detailed phenotyping identifies genes with pleiotropic effects on body composition. BMC Genom. 2016, 17, 224. [Google Scholar] [CrossRef]
- Hua, G.H.; Yang, L.G. A review of research progress of FecB gene in Chinese breeds of sheep. Anim. Reprod. Sci. 2009, 116, 1–9. [Google Scholar] [CrossRef]
- Dietz, H.C.; Cutting, G.R.; Pyeritz, R.E.; Maslen, C.L.; Sakai, L.Y.; Corson, G.M.; Puffenberger, E.G.; Hamosh, A.; Nanthakumar, E.J.; Curristin, S.M.; et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 1991, 352, 337–339. [Google Scholar] [CrossRef]
- Neptune, E.R.; Frischmeyer, P.A.; Arking, D.E.; Myers, L.; Bunton, T.E.; Gayraud, B.; Ramirez, F.; Sakai, L.Y.; Dietz, H.C. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003, 33, 407–411. [Google Scholar] [CrossRef]
- Birchmeier, C.; Birchmeier, W. Molecular aspects of mesenchymal-epithelial interactions. Annu. Rev. Cell Biol. 1993, 9, 511–540. [Google Scholar] [CrossRef]
- Hughesdon, P.E. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet. Gynecol. Surv. 1982, 37, 59–77. [Google Scholar] [CrossRef]
- Maylem, E.R.S.; Spicer, L.J.; Atabay, E.P.; Atabay, E.C.; Batalha, I.; Schutz, L.F. A potential role of fibrillin-1 (FBN1) mRNA and asprosin in follicular development in water buffalo. Theriogenology 2022, 178, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Umehara, T.; Hoshino, Y. Roles of epidermal growth factor (EGF)-like factor in the ovulation process. Reprod. Med. Biol. 2016, 15, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, R.; Kalab, P.; Nagyova, E. Epidermal growth factor-receptor tyrosine kinase activity regulates expansion of porcine oocyte-cumulus cell complexes in vitro. Biol. Reprod. 2003, 68, 797–803. [Google Scholar] [CrossRef]
- Downs, S.M.; Daniel, S.A.; Eppig, J.J. Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: Evidence for a positive stimulus of somatic cell origin. J. Exp. Zool. 1988, 245, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, C.; Perreau, C.; Dupont, J.; Uzbekova, S.; Prigent, C.; Mermillod, P. Several signaling pathways are involved in the control of cattle oocyte maturation. Mol. Reprod. Dev. 2004, 69, 466–474. [Google Scholar] [CrossRef]
- Fan, H.Y.; Liu, Z.; Shimada, M.; Sterneck, E.; Johnson, P.F.; Hedrick, S.M.; Richards, J.S. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 2009, 324, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; Shimada, M.; Liu, Z.; Cahill, N.; Noma, N.; Wu, Y.; Gossen, J.; Richards, J.S. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development 2008, 135, 2127–2137. [Google Scholar] [CrossRef]
- Su, Y.Q.; Wigglesworth, K.; Pendola, F.L.; O’Brien, M.J.; Eppig, J.J. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 2002, 143, 2221–2232. [Google Scholar] [CrossRef]
- Su, Y.Q.; Denegre, J.M.; Wigglesworth, K.; Pendola, F.L.; O’Brien, M.J.; Eppig, J.J. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev. Biol. 2003, 263, 126–138. [Google Scholar] [CrossRef]
- Ochsner, S.A.; Day, A.J.; Rugg, M.S.; Breyer, R.M.; Gomer, R.H.; Richards, J.S. Disrupted function of tumor necrosis factor-alpha-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology 2003, 144, 4376–4384. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kawashima, I.; Yanai, Y.; Nishibori, M.; Richards, J.S.; Shimada, M. Hormone-induced expression of tumor necrosis factor alpha-converting enzyme/A disintegrin and metalloprotease-17 impacts porcine cumulus cell oocyte complex expansion and meiotic maturation via ligand activation of the epidermal growth factor receptor. Endocrinology 2007, 148, 6164–6175. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Chen, C.; Wang, D.; Xu, X.; Qu, C.; Yamaza, T.; Cai, T.; Chen, W.; Sun, L.; Shi, S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012, 10, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Liu, H.; Li, X.; Dai, L.; Gao, Y.; Li, C.; Zhang, L.; Ding, Y.; Yu, X.; Zhang, J. BMP15 prevents cumulus cell apoptosis through CCL2 and FBN1 in porcine ovaries. Cell. Physiol. Biochem. 2013, 32, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ouyang, Z.; Sui, X.; Qi, M.; Li, M.; He, Y.; Cao, Y.; Cao, Q.; Lu, Q.; Zhou, S.; et al. Oocyte competence is maintained by m6A methyltransferase KIAA1429-mediated RNA metabolism during mouse follicular development. Cell Death Differ. 2020, 27, 2468–2483. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xiao, C.; Yang, Z.; Deng, J.; Yang, X. Grade follicles transcriptional profiling analysis in different laying stages in chicken. BMC Genom. 2022, 23, 492. [Google Scholar] [CrossRef] [PubMed]
- Kedem, A.; Ulanenko-Shenkar, K.; Yung, Y.; Youngster, M.; Avraham, S.; Yerushalmi, G.M.; Hourvitz, A. The Involvement of Lumican in Human Ovulatory Processes. Reprod. Sci. 2022, 29, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Hartanti, M.D.; Hummitzsch, K.; Irving-Rodgers, H.F.; Bonner, W.M.; Copping, K.J.; Anderson, R.A.; McMillen, I.C.; Perry, V.E.A.; Rodgers, R.J. Morphometric and gene expression analyses of stromal expansion during development of the bovine fetal ovary. Reprod. Fertil. Dev. 2019, 31, 482–495. [Google Scholar] [CrossRef]
- Turkyilmaz, E.; Guner, H.; Erdem, M.; Erdem, A.; Biri, A.A.; Konac, E.; Alp, E.; Onen, H.I.; Menevse, S. NLF2 gene expression in the endometrium of patients with implantation failure after IVF treatment. Gene 2012, 508, 140–143. [Google Scholar] [CrossRef]
- Yeung, T.L.; Leung, C.S.; Yip, K.P.; Sheng, J.; Vien, L.; Bover, L.C.; Birrer, M.J.; Wong, S.T.C.; Mok, S.C. Anticancer Immunotherapy by MFAP5 Blockade Inhibits Fibrosis and Enhances Chemosensitivity in Ovarian and Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 6417–6428. [Google Scholar] [CrossRef]
- Iwahashi, M.; Muragaki, Y.; Ooshima, A.; Umesaki, N. Increased type III and V collagen expression in human corpora lutea in early pregnancy. Fertil. Steril. 2007, 87, 178–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Stefanovic, B. LARP6 Meets Collagen mRNA: Specific Regulation of Type I Collagen Expression. Int. J. Mol. Sci. 2016, 17, 419. [Google Scholar] [CrossRef] [PubMed]
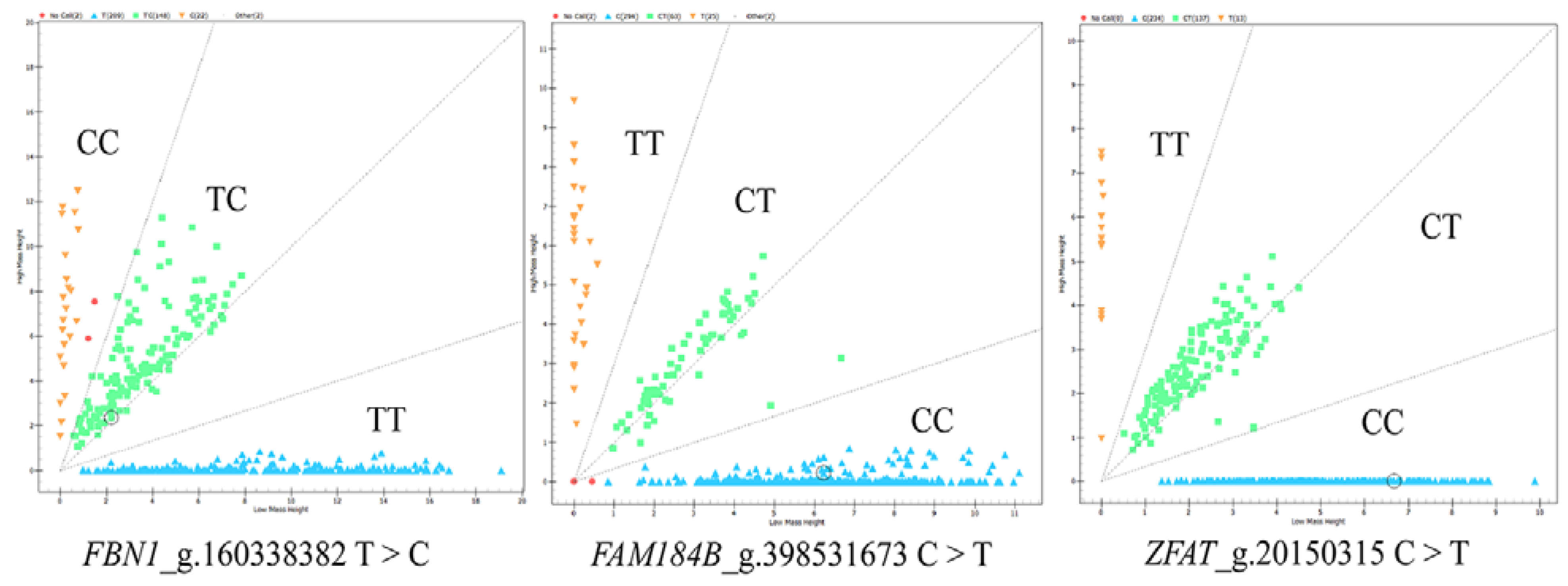
| Breeds | Number | Fertility | Sampling Location |
|---|---|---|---|
| Hu sheep | 96 | Polytocous | Xuzhou, Jiangsu Province, China |
| Cele black sheep | 96 | Polytocous | Cele, Xinjiang Uygur Autonomous Region, China |
| Xinggao mutton sheep | 367 (with litter size) | Polytocous | Xing’an league, Inner Mongolia Autonomous Region, China |
| Sunite sheep | 96 | Monotocous | Bayannaoer, Inner Mongolia Autonomous Region, China |
| Bamei mutton sheep | 96 | Monotocous | Bayannaoer, Inner Mongolia Autonomous Region, China |
| Loci | Primer Sequence (5′-3′) |
|---|---|
| g.160338382 | F-ACGTTGGATGTCCCCATGTCGGCAAACATC |
| R-ACGTTGGATGCCAGTAGTACATATTGACCC | |
| EXT-CCTTGCACTCATCAATATCT | |
| g.398531673 | F-ACGTTGGATGCCTCCTTGAGCTGTGAGTTC |
| R-ACGTTGGATGTCCCAAAGGCAAGAGTGCAG | |
| EXT-GGCAAGGAGGCAGCATCCCT | |
| g.20150315 | F-ACGTTGGATGACCAAGTACCAGGCGCTGGA |
| R-ACGTTGGATGTGGAGCTGACGAACTTCTTG | |
| EXT-ACCCGGCGCTGGAGCTGCACGTC |
| Genes | SNPs | Breeds | Genotype Frequency | Allele Frequency | PIC | He | Ne | Chi-Square Test (p-Value) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FBN1 | Chr7: g.160338382 T > C | CC | TC | TT | C | T | |||||
| Xinggao mutton sheep | 0.06 | 0.40 | 0.55 | 0.26 | 0.74 | 0.31 | 0.38 | 1.61 | 0.44 | ||
| Hu sheep | 0.19 | 0.47 | 0.34 | 0.43 | 0.57 | 0.37 | 0.49 | 1.96 | 0.76 | ||
| Cele sheep | 0.02 | 0.21 | 0.77 | 0.13 | 0.88 | 0.19 | 0.22 | 1.28 | 0.64 | ||
| Sunite sheep | 0.03 | 0.38 | 0.59 | 0.22 | 0.78 | 0.29 | 0.34 | 1.53 | 0.33 | ||
| Bamei mutton sheep | 0.34 | 0.50 | 0.16 | 0.59 | 0.41 | 0.37 | 0.48 | 1.93 | 0.72 | ||
| FAM184B | Chr6: g.398531673 C > T | CC | CT | TT | C | T | |||||
| Xinggao mutton sheep | 0.77 | 0.16 | 0.07 | 0.85 | 0.15 | 0.22 | 0.25 | 1.34 | 0.00 | ||
| Hu sheep | 0.89 | 0.10 | 0.01 | 0.94 | 0.06 | 0.11 | 0.12 | 1.13 | 0.28 | ||
| Cele sheep | 0.54 | 0.32 | 0.14 | 0.70 | 0.30 | 0.33 | 0.42 | 1.72 | 0.02 | ||
| Sunite sheep | 0.81 | 0.14 | 0.05 | 0.88 | 0.12 | 0.19 | 0.21 | 1.27 | 0.00 | ||
| Bamei mutton sheep | 0.99 | 0.01 | 0.00 | 0.99 | 0.01 | 0.01 | 0.01 | 1.01 | NA | ||
| ZFAT | Chr9: g.20150315 C > T | CC | CT | TT | C | T | |||||
| Xinggao mutton sheep | 0.61 | 0.36 | 0.03 | 0.79 | 0.21 | 0.28 | 0.33 | 1.50 | 0.19 | ||
| Hu sheep | 0.96 | 0.04 | 0.00 | 0.98 | 0.02 | 0.04 | 0.04 | 1.04 | 0.83 | ||
| Cele sheep | 1.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 1.00 | NA | ||
| Sunite sheep | 0.98 | 0.02 | 0.00 | 0.99 | 0.01 | 0.02 | 0.02 | 1.02 | NA | ||
| Bamei mutton sheep | 1.00 | 0.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 1.00 | NA | ||
| BMPR1B | Chr6: g.29382188 T > C | TT | TC | CC | T | C | |||||
| Xinggao mutton sheep | 0.14 | 0.48 | 0.38 | 0.38 | 0.62 | 0.36 | 0.47 | 1.88 | 0.67 | ||
| Hu sheep | 0.01 | 0.06 | 0.93 | 0.04 | 0.96 | 0.08 | 0.09 | 1.09 | 0.03 | ||
| Cele sheep | 0.42 | 0.52 | 0.06 | 0.68 | 0.32 | 0.34 | 0.44 | 1.77 | 0.06 | ||
| Sunite sheep | 0.81 | 0.18 | 0.01 | 0.90 | 0.10 | 0.16 | 0.18 | 1.22 | 0.95 | ||
| Baimei mutton sheep | 0.99 | 0.01 | 0.00 | 1.00 | 0.00 | 0.01 | 0.01 | 1.01 | NA | ||
| Genes | SNPs | Genotype | Litter Size (Mean ± SD) | |
|---|---|---|---|---|
| Second Parity (N) | Third Parity (N) | |||
| FBN1 | g.160338382 T > C | CC | 1.85 ± 0.555(13) b | 2.20 ± 0.837(5) b |
| TC | 2.32 ± 0.877(103) ab | 2.83 ± 1.124(35) ab | ||
| TT | 2.70 ± 0.789(133) a | 3.15 ± 0.931(55) a | ||
| FAM184B | g.398531673 C > T | CC | 2.43 ± 0.914(208) | 2.89 ± 1.090(71) |
| CT | 2.53 ± 0.869(45) | 3.05 ± 0.887(20) | ||
| TT | 2.36 ± 0.842(14) | 3.00 ± 1.225(5) | ||
| ZFAT | g.20150315 C > T | CC | 2.40 ± 0.888(173) | 2.87 ± 0.919(67) |
| CT | 2.53 ± 0.918(89) | 3.08 ± 1.324(26) | ||
| TT | 2.71 ± 1.113(7) | 3.25 ± 1.258(4) | ||
| BMPR1B | g.29382188 T > C | TT | 1.95 ± 0.664(37) c | 2.46 ± 0.660(13) b |
| TC | 2.24 ± 0.849(124) b | 2.60 ± 0.849(43) ab | ||
| CC | 2.82 ± 0.825(95) a | 3.29 ± 1.071(31) a | ||
| Parity | SNPs | Df | Sum sq | Mean Sq | F Value | Pr (>F) | |
|---|---|---|---|---|---|---|---|
| Second Parity | g.160338382 | FecB | 4 | 2.16 | 0.540 | 0.713 | 0.584 |
| Third Parity | g.160338382 | FecB | 3 | 1.06 | 0.354 | 0.337 | 0.798 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Chen, Q.; He, X.; Wang, X.; He, X.; Wang, Y.; Pan, Z.; Chu, M.; Di, R. Association Analyses between Single Nucleotide Polymorphisms in ZFAT, FBN1, FAM184B Genes and Litter Size of Xinggao Mutton Sheep. Animals 2023, 13, 3639. https://doi.org/10.3390/ani13233639
Gong Y, Chen Q, He X, Wang X, He X, Wang Y, Pan Z, Chu M, Di R. Association Analyses between Single Nucleotide Polymorphisms in ZFAT, FBN1, FAM184B Genes and Litter Size of Xinggao Mutton Sheep. Animals. 2023; 13(23):3639. https://doi.org/10.3390/ani13233639
Chicago/Turabian StyleGong, Yiming, Qiuju Chen, Xiaolong He, Xiangyu Wang, Xiaoyun He, Yunfei Wang, Zhangyuan Pan, Mingxing Chu, and Ran Di. 2023. "Association Analyses between Single Nucleotide Polymorphisms in ZFAT, FBN1, FAM184B Genes and Litter Size of Xinggao Mutton Sheep" Animals 13, no. 23: 3639. https://doi.org/10.3390/ani13233639
APA StyleGong, Y., Chen, Q., He, X., Wang, X., He, X., Wang, Y., Pan, Z., Chu, M., & Di, R. (2023). Association Analyses between Single Nucleotide Polymorphisms in ZFAT, FBN1, FAM184B Genes and Litter Size of Xinggao Mutton Sheep. Animals, 13(23), 3639. https://doi.org/10.3390/ani13233639






