Mild Intermittent Cold Stimulation Affects Cardiac Substance Metabolism via the Neuroendocrine Pathway in Broilers
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
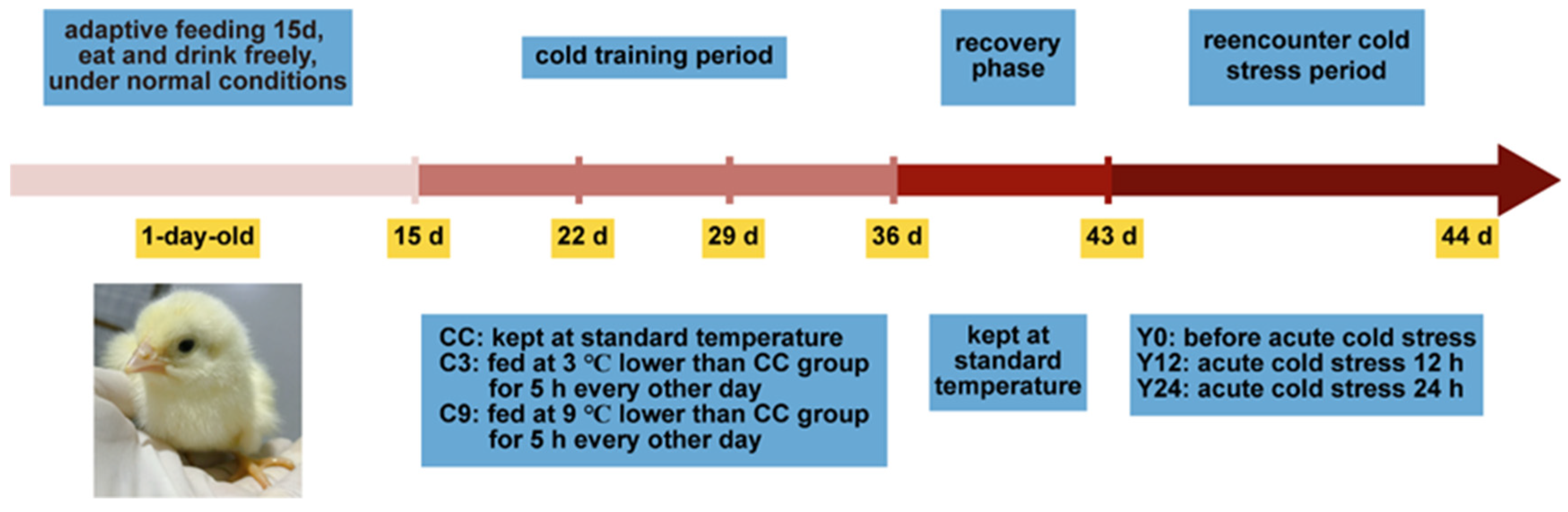
2.1. Animal Experimental Procedure
2.2. Experimental Design
2.3. Sample Collection and Processing
2.4. Total RNA Extraction and Complementary DNA (cDNA) Library Construction
2.5. Transcriptome Sequencing and Bioinformatics Analysis
2.6. Detection of Blood Biochemical Indexes
2.7. Quantitative Real-Time PCR (RT-qPCR)
2.8. Western Blot
2.9. Statistical Analysis
3. Results
3.1. Analysis of Hypothalamic Transcriptome Data of Intermittent Cold Training of Broilers
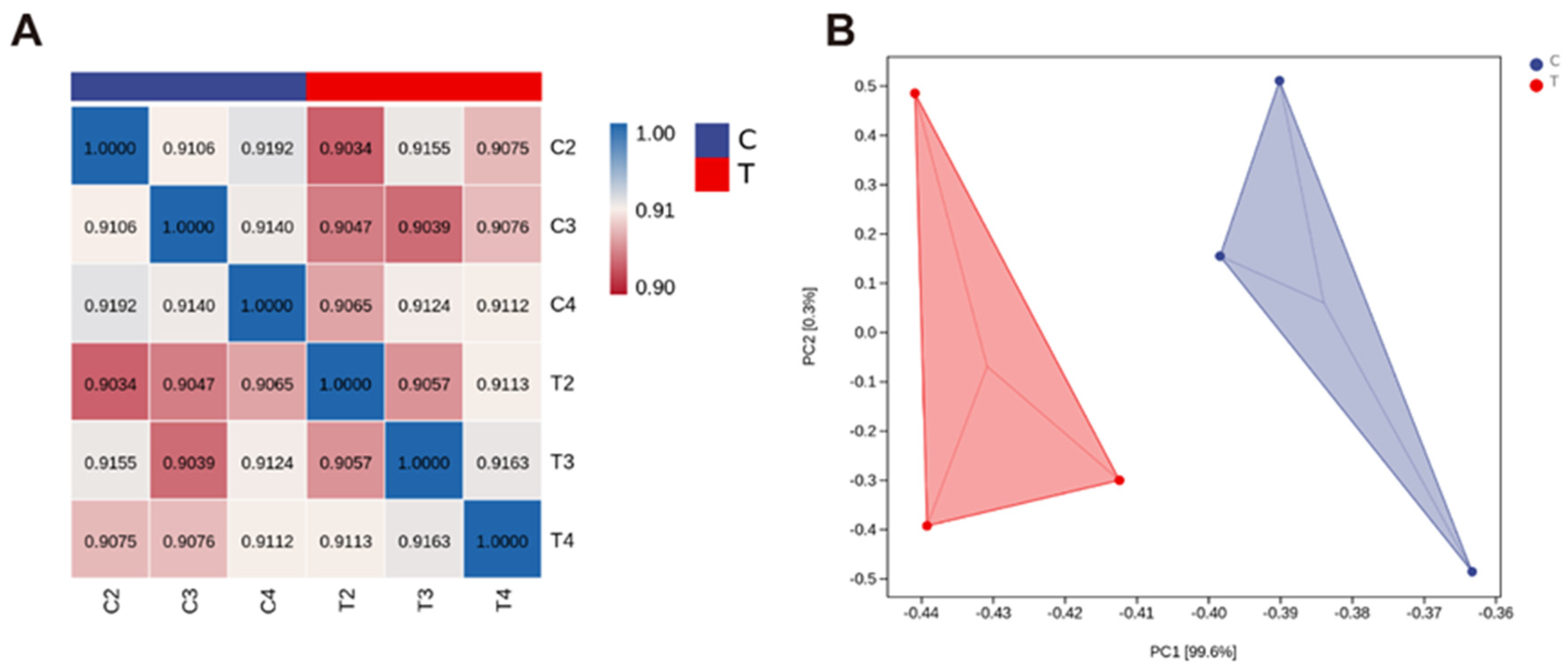
3.1.1. Transcriptome Sequencing Data Comparison
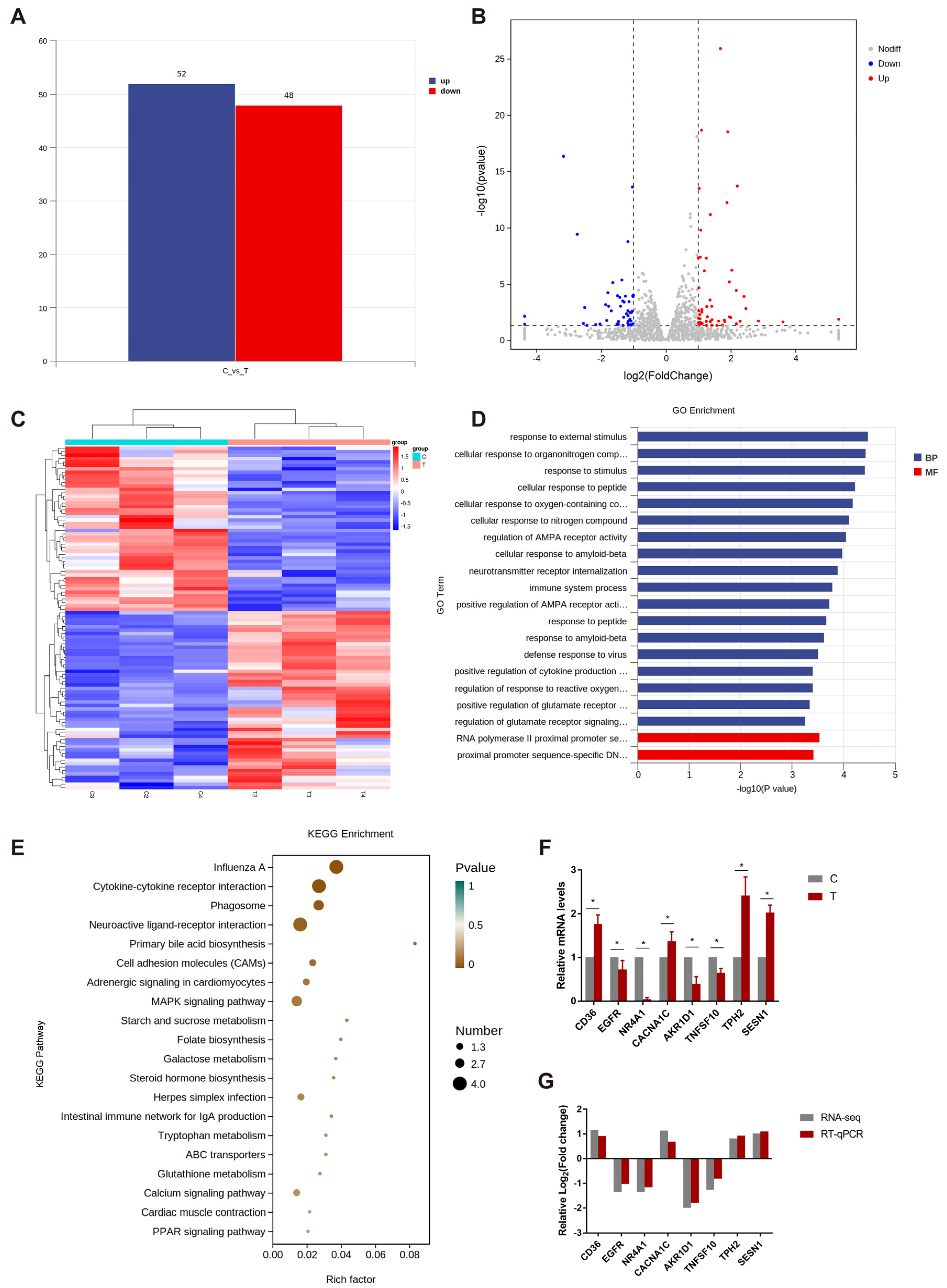
3.1.2. Analysis and Verification of Differential Expression Genes in Hypothalamus
3.2. Effects of Intermittent Cold Training on Blood Biochemical Indices of Broilers
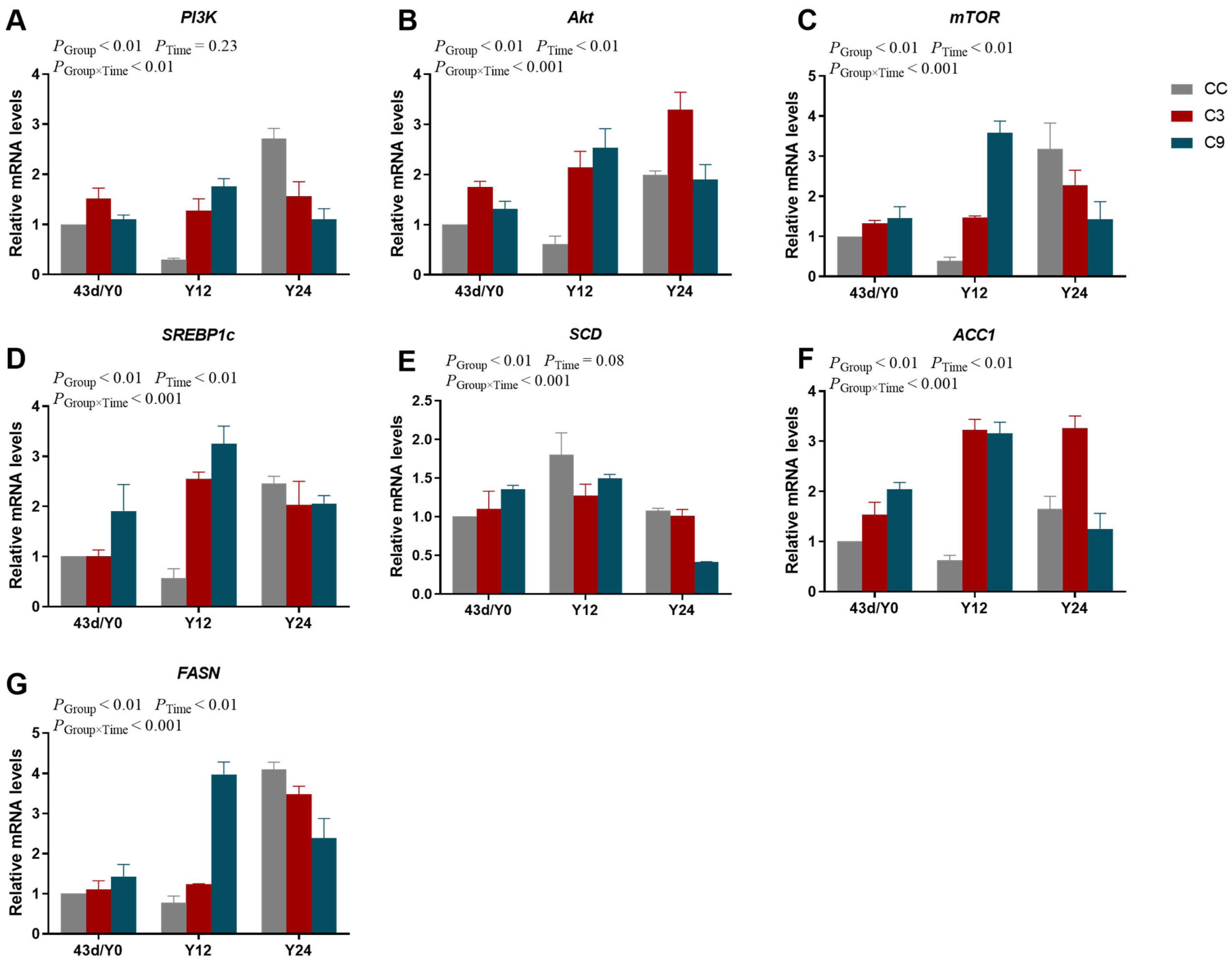
3.3. Effects of Intermittent Cold Training on PI3K/Akt/mTOR Signaling Pathway and Its Downstream Key Genes in the Cardiac Muscle of Broilers
3.4. Effects of Intermittent cold Training on FoxO1 Signaling Pathway and Its Downstream Key Genes in the Cardiac Muscle of Broilers
3.5. Effects of Acute Cold Stress on HSPs and Blood Biochemical Indexes in the Cardiac Muscle of Broilers
3.6. Effects of Acute Cold Stress on PI3K/Akt/mTOR Signaling Pathway and Its Downstream Key Gene mRNA Expression Levels in the Cardiac Muscle of Broilers
3.7. Effects of Acute Cold Stress on FoxO1 Signaling Pathway and Its Downstream Key Gene mRNA Expression Levels in the Cardiac Muscle of Broilers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selye, H. A syndrome produced by diverse nocuous agents. J. Neuropsychiatry Clin. Neurosci. 1936, 10, 230–231. [Google Scholar] [CrossRef]
- Fraser, D. Biology of animal stress: Implications for animal well-being. J. Appl. Anim. Welf. Sci. JAAWS 1999, 2, 157–159. [Google Scholar] [CrossRef]
- Manukhina, E.B.; Downey, H.F.; Mallet, R.T. Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia. Exp. Biol. Med. 2006, 231, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Richer, R.; Zenkner, J.; Küderle, A.; Rohleder, N.; Eskofier, B.M. Vagus activation by Cold Face Test reduces acute psychosocial stress responses. Sci. Rep. 2022, 12, 19270. [Google Scholar] [CrossRef]
- Xue, G.; Yin, J.; Zhao, N.; Liu, Y.; Fu, Y.; Zhang, R.; Bao, J.; Li, J. Intermittent mild cold stimulation improves the immunity and cold resistance of spleens in broilers. Poult. Sci. 2021, 100, 101492. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, S.; Xin, H.; Li, J.; Li, X.; Zhang, R.; Li, J.; Bao, J. Proper cold stimulation starting at an earlier age can enhance immunity and improve adaptability to cold stress in broilers. Poult. Sci. 2020, 99, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Rodan, L.H.; Spillmann, R.C.; Kurata, H.T.; Lamothe, S.M.; Maghera, J.; Jamra, R.A.; Alkelai, A.; Antonarakis, S.E.; Atallah, I.; Bar-Yosef, O.; et al. Phenotypic expansion of CACNA1C-associated disorders to include isolated neurological manifestations. Genet. Med. 2021, 23, 1922–1932. [Google Scholar] [CrossRef]
- Smedler, E.; Louhivuori, L.; Romanov, R.A.; Masini, D.; Ellström, I.D.; Wang, C.; Caramia, M.; West, Z.; Zhang, S.; Rebellato, P.; et al. Disrupted Cacna1c gene expression perturbs spontaneous Ca2+ activity causing abnormal brain development and increased anxiety. Proc. Natl. Acad. Sci. USA 2022, 119, e2108768119. [Google Scholar] [CrossRef]
- Fukuhara, K.; Kvetnansky, R.; Cizza, G.; Pacak, K.; Ohara, H.; Goldstein, D.S.; Kopin, I.J. Interrelations between sympathoadrenal system and hypothalamo-pituitary-adrenocortical/thyroid systemsin rats exposed to cold stress. J. Neuroendocr. 1996, 8, 533–541. [Google Scholar] [CrossRef]
- Forrest, R.H.; Hickford, J.G.; Hogan, A.; Frampton, C. Polymorphism at the ovine beta3-adrenergic receptor locus: Associations with birth weight, growth rate, carcass composition and cold survival. Anim. Genet. 2003, 34, 19–25. [Google Scholar] [CrossRef]
- Asakura, M.; Nagashima, H.; Fujii, S.; Sasuga, Y.; Misonoh, A.; Hasegawa, H.; Osada, K. Influences of chronic stress on central nervous systems. Nihon Shinkei Seishin Yakurigaku Zasshi 2000, 20, 97–105. [Google Scholar]
- Hu, M.; Chen, Y.; Deng, F.; Chang, B.; Luo, J.; Dong, L.; Lu, X.; Zhang, Y.; Chen, Z.; Zhou, J. D-Mannose Regulates Hepatocyte Lipid Metabolism via PI3K/Akt/mTOR Signaling Pathway and Ameliorates Hepatic Steatosis in Alcoholic Liver Disease. Front. Immunol. 2022, 13, 877650. [Google Scholar] [CrossRef]
- Chu, H.; Du, C.; Yang, Y.; Feng, X.; Zhu, L.; Chen, J.; Yang, F. MC-LR Aggravates Liver Lipid Metabolism Disorders in Obese Mice Fed a High-Fat Diet via PI3K/AKT/mTOR/SREBP1 Signaling Pathway. Toxins 2022, 14, 833. [Google Scholar] [CrossRef]
- Gurumayum, S.; Bharadwaj, S.; Sheikh, Y.; Barge, S.R.; Saikia, K.; Swargiary, D.; Ahmed, S.A.; Thakur, D.; Borah, J.C. Taxifolin-3-O-glucoside from Osbeckia nepalensis Hook. mediates antihyperglycemic activity in CC1 hepatocytes and in diabetic Wistar rats via regulating AMPK/G6Pase/PEPCK signaling axis. J. Ethnopharmacol. 2023, 303, 115936. [Google Scholar] [CrossRef]
- Li, D.-D.; Jawale, C.V.; Zhou, C.; Lin, L.; Trevejo-Nunez, G.J.; Rahman, S.A.; Mullet, S.J.; Das, J.; Wendell, S.G.; Delgoffe, G.M.; et al. Fungal sensing enhances neutrophil metabolic fitness by regulating antifungal Glut1 activity. Cell Host Microbe 2022, 30, 530–544.e6. [Google Scholar] [CrossRef] [PubMed]
- Seiler, K.; Humbert, M.; Minder, P.; Mashimo, I.; Schläfli, A.M.; Krauer, D.; Federzoni, E.A.; Vu, B.; Moresco, J.J.; Yates, J.R.; et al. Hexokinase 3 enhances myeloid cell survival via non-glycolytic functions. Cell Death Dis. 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Perby, L.K.; Richter, S.; Weber, K.; Hieber, A.J.; Hess, N.; Crocoll, C.; Mogensen, H.K.; Pribil, M.; Burow, M.; Nielsen, T.H.; et al. Cytosolic phosphofructokinases are important for sugar homeostasis in leaves of Arabidopsis thaliana. Ann. Bot. 2021, 129, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.D.; Jin, A.; Grois, G.A.; Zhang, Y. A role of cytoplasmic p53 in the regulation of metabolism shown by bat-mimicking p53 NLS mutant mice. Cell Rep. 2023, 42, 111920. [Google Scholar] [CrossRef]
- Peng, M.-F.; Tian, S.; Song, Y.-G.; Li, C.-X.; Miao, M.-S.; Ren, Z.; Li, M. Effects of total flavonoids from Eucommia ulmoides Oliv. leaves on polycystic ovary syndrome with insulin resistance model rats induced by letrozole combined with a high-fat diet. J. Ethnopharmacol. 2021, 273, 113947. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Fasano, R.; Paolisso, G. Adiponectin and Cognitive Decline. Int. J. Mol. Sci. 2020, 21, 2010. [Google Scholar] [CrossRef] [PubMed]
- Torrente, C.; Manzanilla, E.G.; Bosch, L.; Villaverde, C.; Pastor, J.; de Gopegui, R.R.; Tvarijonaviciute, A. Adiponectin as a sepsis biomarker in dogs: Diagnostic and prognostic value. Veter-Clin. Pathol. 2020, 49, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Bi, M.Y.; Yao, H.D.; Fu, J.; Li, S.; Xu, S.W. Effect of cold stress on expression of AMPKalpha-PPARalpha pathway and inflammation genes. Avian Dis. 2014, 58, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Feng, Y.; Li, J.; Gu, X. Role of MAPKs in HSP70′s Protection against Heat Stress-Induced Injury in Rat Small Intestine. BioMed Res. Int. 2018, 2018, 1571406. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, R.; Su, Y.; Bi, Y.; Li, X.; Zhang, X.; Li, J.; Bao, J. Effects of Acute Cold Stress After Long-Term Cold Stimulation on Antioxidant Status, Heat Shock Proteins, Inflammation and Immune Cytokines in Broiler Heart. Front. Physiol. 2018, 9, 1589. [Google Scholar] [CrossRef]
- Sugito, S.; Etriwati, E.; Akmal, M.; Rahmi, E.; Delima, M.; Muchlisin, Z.A.; Hasan, D.I. Immunohistochemical Expression of AQP2 and HSP70 in Broiler Kidney Tissue Treated with Salix tetrasperma Roxb. Extract under Heat Exposure. Sci. World J. 2021, 2021, 8711286. [Google Scholar] [CrossRef]
- Loyau, T.; Collin, A.; Yenisey, Ç.; Crochet, S.; Siegel, P.B.; Akşit, M.; Yalçin, S. Exposure of embryos to cyclically cold incubation temperatures durably affects energy metabolism and antioxidant pathways in broiler chickens. Poult. Sci. 2014, 93, 2078–2086. [Google Scholar] [CrossRef]
- Szablewski, L. Glucose transporters in healthy heart and in cardiac disease. Int. J. Cardiol. 2017, 230, 70–75. [Google Scholar] [CrossRef]
- Wei, H.; Li, T.; Zhang, Y.; Liu, X.; Gong, R.; Bao, J.; Li, J. Cold stimulation causes oxidative stress, inflammatory response and apoptosis in broiler heart via regulating Nrf2/HO-1 and NF-κB pathway. J. Therm. Biol. 2023, 116, 103658. [Google Scholar] [CrossRef]
- Manz, C.; Kobitski, A.Y.; Samanta, A.; Nienhaus, K.; Jäschke, A.; Nienhaus, G.U. Exploring the energy landscape of a SAM-I riboswitch. J. Biol. Phys. 2021, 47, 371–386. [Google Scholar] [CrossRef]
- Yao, J.; Hui, J.-W.; Chen, Y.-J.; Luo, D.-Y.; Yan, J.-S.; Zhang, Y.-F.; Lan, Y.-X.; Yan, X.-R.; Wang, Z.-H.; Fan, H.; et al. Lycium barbarum glycopeptide targets PER2 to inhibit lipogenesis in glioblastoma by downregulating SREBP1c. Cancer Gene Ther. 2023, 30, 1084–1093. [Google Scholar]
- Raghow, R.; Yellaturu, C.; Deng, X.; Park, E.A.; Elam, M.B. SREBPs: The crossroads of physiological and pathological lipid homeostasis. Trends Endocrinol. Metab. 2008, 19, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Jiang, M.; Chu, Y.; Wang, W.; Chen, D.; Li, X.; Zhang, Z.; Zhang, D.; Fan, D.; Nie, Y.; et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J. Hepatol. 2018, 68, 773–782. [Google Scholar] [CrossRef]
- Li, X.Z.; Yan, C.G.; Gao, Q.S.; Yan, Y.; Choi, S.H.; Smith, S.B. Adipogenic/lipogenic gene expression and fatty acid composition in chuck, loin, and round muscles in response to grain feeding of Yanbian Yellow cattle. J. Anim. Sci. 2018, 96, 2698–2709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, J.; He, Y.; Wang, N.; Tian, L. Attenuation of lipid accumulation in Bel-7402 cells through ADPN/AMPKα signaling stimulated by Fructus rosae laxae extract. J. Food Biochem. 2020, 44, e13497. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Greene, E.; Ishola, P.; Huff, G.; Donoghue, A.; Bottje, W.; Dridi, S. Chronic Mild Cold Conditioning Modulates the Expression of Hypothalamic Neuropeptide and Intermediary Metabolic-Related Genes and Improves Growth Performances in Young Chicks. PLoS ONE 2015, 10, e0142319. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Wu, H.T.; Wang, Y.C.; Chang, C.J.; Shan, Y.S.; Wu, S.R.; Chiu, Y.C.; Hsu, C.L.; Juan, H.F.; Lan, K.Y.; et al. Secretory autophagy promotes RAB37-mediated insulin secretion under glucose stimulation both in vitro and in vivo. Autophagy 2023, 19, 1239–1257. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Li, Z. Adipokines in glucose and lipid metabolism. Adipocyte 2023, 12, 2202976. [Google Scholar] [CrossRef]
- Chen, J.; Lu, Y.; Tian, M.; Huang, Q. Molecular mechanisms of FOXO1 in adipocyte differentiation. J. Mol. Endocrinol. 2019, 62, R239–R253. [Google Scholar] [CrossRef]
- Li, J.; Hu, Z.-Q.; Yu, S.-Y.; Mao, L.; Zhou, Z.-J.; Wang, P.-C.; Gong, Y.; Su, S.; Zhou, J.; Fan, J.; et al. CircRPN2 Inhibits Aerobic Glycolysis and Metastasis in Hepatocellular Carcinoma. Cancer Res. 2022, 82, 1055–1069. [Google Scholar] [CrossRef]
- Kurakazu, I.; Akasaki, Y.; Hayashida, M.; Tsushima, H.; Goto, N.; Sueishi, T.; Toya, M.; Kuwahara, M.; Okazaki, K.; Duffy, T.; et al. FOXO1 transcription factor regulates chondrogenic differentiation through transforming growth factor β1 signaling. J. Biol. Chem. 2019, 294, 17555–17569. [Google Scholar] [CrossRef]
- Yan, H.; Yang, W.; Zhou, F.; Li, X.; Pan, Q.; Shen, Z.; Han, G.; Newell-Fugate, A.; Tian, Y.; Majeti, R.; et al. Estrogen Improves Insulin Sensitivity and Suppresses Gluconeogenesis via the Transcription Factor Foxo1. Diabetes 2019, 68, 291–304. [Google Scholar] [CrossRef]
- Ng, B.G.; Sosicka, P.; Xia, Z.; Freeze, H.H. GLUT1 is a highly efficient L-fucose transporter. J. Biol. Chem. 2023, 299, 102738. [Google Scholar] [CrossRef] [PubMed]
- Hingst, J.R.; Bjerre, R.D.; Wojtaszewski, J.F.; Jensen, J. Rapid radiochemical filter paper assay for determination of hexokinase activity and affinity for glucose-6-phosphate. J. Appl. Physiol. 2019, 127, 661–667. [Google Scholar] [CrossRef]
- Zuo, J.; Tang, J.; Lu, M.; Zhou, Z.; Li, Y.; Tian, H.; Liu, E.; Gao, B.; Liu, T.; Shao, P. Glycolysis Rate-Limiting Enzymes: Novel Potential Regulators of Rheumatoid Arthritis Pathogenesis. Front. Immunol. 2021, 12, 779787. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiong, Y.; Qiao, T.; Li, X.; Jia, L.; Han, Y. Lactate dehydrogenase A: A key player in carcinogenesis and potential target in cancer therapy. Cancer Med. 2018, 7, 6124–6136. [Google Scholar] [CrossRef]
- Urbańska, K.; Orzechowski, A. Unappreciated Role of LDHA and LDHB to Control Apoptosis and Autophagy in Tumor Cells. Int. J. Mol. Sci. 2019, 20, 2085. [Google Scholar] [CrossRef]
- Tinsley, N.; Iqbal, M.; Pumford, N.R.; Lassiter, K.; Ojano-Dirain, C.; Wing, T.; Bottje, W. Investigation of mitochondrial protein expression and oxidation in heart muscle in low and high feed efficient male broilers in a single genetic line. Poult. Sci. 2010, 89, 349–352. [Google Scholar] [CrossRef] [PubMed]





| Days Old | Control Group (CC) | Cold Adaptation Group (C3) | Cold Stress Group (C9) |
|---|---|---|---|
| 1–3 | 35 °C | ||
| 4–14 | reduce by 0.5 °C per day | ||
| 15 | 29 | 26 | 20 |
| 16 | 28.5 | 28.5 | 28.5 |
| 17 | 28 | 25 | 19 |
| 18 | 27.5 | 27.5 | 27.5 |
| 19 | 27 | 24 | 18 |
| 20 | 26.5 | 26.5 | 26.5 |
| 21 | 26 | 23 | 17 |
| 22 | 25.5 | 25.5 | 25.5 |
| 23 | 25 | 22 | 16 |
| 24 | 24.5 | 24.5 | 24.5 |
| 25 | 24 | 21 | 15 |
| 26 | 23.5 | 23.5 | 23.5 |
| 27 | 23 | 23 | 23 |
| 28 | 22.5 | 19.5 | 13.5 |
| 29 | 22 | 22 | 22 |
| 30 | 21.5 | 18.5 | 12.5 |
| 31 | 21 | 18 | 12 |
| 32 | 20.5 | 20.5 | 20.5 |
| 33 | 20 | 17 | 11 |
| 34 | 20 | 20 | 20 |
| 35 | 20 | 17 | 11 |
| 36–43 | 20 | ||
| 44 | 10 | 10 | 10 |
| Samples | Clean Reads | Clean Base (%) | Q30 Proportion (%) | Map Reads | Map Reads (100%) |
|---|---|---|---|---|---|
| C1 | 37,662,272 | 6,050,529,300 | 90.13 | 34,104,512 | 90.55% |
| C2 | 39,519,606 | 6,358,805,100 | 90.05 | 35,815,992 | 90.63% |
| C3 | 40,606,524 | 6,517,980,600 | 90.19 | 36,767,361 | 90.55% |
| C4 | 40,374,598 | 6,476,575,200 | 90.20 | 36,754,473 | 91.03% |
| T1 | 41,284,360 | 6,643,700,100 | 90.86 | 37,607,189 | 91.09% |
| T2 | 43,470,024 | 6,999,076,500 | 90.38 | 39,304,084 | 90.42% |
| T3 | 41,271,848 | 6,644,863,800 | 90.03 | 37,481,197 | 90.82% |
| T4 | 38,195,746 | 6,149,473,800 | 90.57 | 34,858,213 | 91.26% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xing, L.; Zhang, Y.; Liu, X.; Li, T.; Zhang, S.; Wei, H.; Li, J. Mild Intermittent Cold Stimulation Affects Cardiac Substance Metabolism via the Neuroendocrine Pathway in Broilers. Animals 2023, 13, 3577. https://doi.org/10.3390/ani13223577
Liu Y, Xing L, Zhang Y, Liu X, Li T, Zhang S, Wei H, Li J. Mild Intermittent Cold Stimulation Affects Cardiac Substance Metabolism via the Neuroendocrine Pathway in Broilers. Animals. 2023; 13(22):3577. https://doi.org/10.3390/ani13223577
Chicago/Turabian StyleLiu, Yuanyuan, Lu Xing, Yong Zhang, Xiaotao Liu, Tingting Li, Shijie Zhang, Haidong Wei, and Jianhong Li. 2023. "Mild Intermittent Cold Stimulation Affects Cardiac Substance Metabolism via the Neuroendocrine Pathway in Broilers" Animals 13, no. 22: 3577. https://doi.org/10.3390/ani13223577
APA StyleLiu, Y., Xing, L., Zhang, Y., Liu, X., Li, T., Zhang, S., Wei, H., & Li, J. (2023). Mild Intermittent Cold Stimulation Affects Cardiac Substance Metabolism via the Neuroendocrine Pathway in Broilers. Animals, 13(22), 3577. https://doi.org/10.3390/ani13223577





