Multiparametric Comparison of Two TTA-Based Surgical Techniques in Dogs with Cranial Cruciate Ligament Tears
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dog Selection and Groups
2.2. Surgical Procedures
2.3. Postoperative Assessment
2.4. Experimental Design
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knebel, J. Etiology, pathogenesis, diagnostics and therapy of cranial cruciate ligament rupture in dogs. Tierärztliche Prax. Ausg. K Kleintiere/Heimtiere 2014, 42, 36–47. [Google Scholar]
- Giansetto, T.; Picavet, P.; Lefebvre, M.; Balligant, M. Determination of the stifle angle at standing position in dogs. Vet. Sci. 2022, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Lapman, T.J.; Lund, E.M.; Lipowitz, A.J. Cranial cruciate disease: Current status of diagnosis, surgery, and risk for disease. Vet. Comp. Orthop. Traumatol. 2003, 16, 122–126. [Google Scholar]
- Anderst, W.J.; Tashman, S. The association between velocity of the center of closest proximity on subchondral bones and osteoarthritis progression. J. Orthop. Res. 2009, 27, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Litsky, A.S.; Field, J. Effect of medial meniscal release on tibial translation after tibial plateau leveling osteotomy. Vet. Surg. 2006, 35, 486–494. [Google Scholar] [CrossRef]
- Pozzi, A.; Litsky, A.S.; Field, J.; Apelt, D.; Meadows, C.; Johnson, K.A. Pressure distributions on the medial tibial plateau after medial meniscal surgery and tibial plateau levelling osteotomy in dogs. Vet. Comp. Orthop. Traumatol. 2008, 21, 8–14. [Google Scholar] [CrossRef]
- Lampart, M.; Knell, S.; Pozzi, A. A new approach to treatment selection in dogs with crucial ligament rupture: Patient—Specific treatment recommendations. Schweiz. Arch. Tierheilkd. 2020, 162, 345–364. [Google Scholar] [CrossRef]
- Spinella, G.; Arcamone, G.; Valentini, S. Cranial Cruciate Ligament Rupture in Dogs: Review on Biomechanics, Etiopathogenetic Factors and Rehabilitation. Vet. Sci. 2021, 8, 186. [Google Scholar] [CrossRef]
- Montavon, P.M. Tibial tuberosity advancement (TTA) for the treatment of cranial cruciate disease in dogs: Evidence, technique and initial clinical results. In Proceedings of the 12th ESVOT Congress, Munich, Germany, 10–12 September 2004; pp. 254–255. [Google Scholar]
- Retallack, L.M.; Daye, R.M. A modified Maquet-tibial tuberosity advancement technique for treatment of canine cranial cruciate ligament disease: Short term outcome and complications. Vet. Surg. 2018, 47, 44–51. [Google Scholar] [CrossRef]
- Zhalniarovich, Y.; Mieszkowska, M.; Przyborowska-Zhalniarovich, P.; Głodek, J.; Sobolewski, A.; Waluś, G.; Adamiak, Z. A novel tibial tuberosity advancement technique with cranial implant fixation (TTA CF): A pilot study in sheep. Vet. Res. 2018, 14, 231. [Google Scholar] [CrossRef]
- Etchepareborde, S.; Brunel, L.; Bollen, G.; Balligand, M. Preliminary experience of a modified Maquet technique for repair of cranial cruciate ligament rupture in dogs. Vet. Comp. Orthop. Traumatol. 2011, 24, 223–227. [Google Scholar] [PubMed]
- Bernardi-Villavicencio, C.; Jimenez-Socorro, A.N.; Rojo-Salvador, C.; Robles-Sanmartin, J.; Rodriguez-Quiros, J. Short-term outcomes and complications of 65 cases of porous TTA with flange: A prospective clinical study in dogs. Vet. Res. 2020, 16, 279. [Google Scholar] [CrossRef] [PubMed]
- Crovace, A.M.; Staffieri, F.; Monopoli, D.; Artiles, A.; Fracassi, L.; Crovace, A.; Lacitignola, L. Role of Tibial Tuberosity Fracture/Fissure through the Maquet Hole in Stifle Osteoarthritis after Porous Tibial Tuberosity Advancement in Dogs at Mid-Term Follow-Up. Vet. Sci. 2019, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Millis, D.L. Canine Rehabilitation and Physical Therapy, 2nd ed.; Saunders: Philadelphia, PA, USA, 2004; pp. 211–227. [Google Scholar]
- Cuervo, B.; Rubio, M.; Sopena, J.; Dominguez, J.M.; Vilar, J.; Morales, M.; Cugat, A.; Carrillo, J.M. Hip osteoartritis in dogs: A randomized study using mesenquimal stem cells from adipose tissue and plasma rich in growth factors. Int. J. Mol. Sci. 2014, 15, 13437–13460. [Google Scholar] [CrossRef]
- Vilar, J.M.; Cuervo, B.; Rubio, M.; Sopena, J.; Domínguez, J.M.; Santana, A.; Carrillo, J.M. Effect of intraarticular inoculation of mesenchymal stem cells in dogs with hip osteoarthritis by means of objective force platform gait analysis: Concordance with numeric subjective scoring scales. Vet. Res. 2016, 12, 223. [Google Scholar] [CrossRef]
- Espejo-Baena, A.; Espejo-Reina, A.; Espejo-Reina, M.J.; Ruiz-Del Pino, J. The Finochietto sign as patognomonic of ramp lesión of the medial meniscus. Arthrosc. Tech. 2020, 9, e549–e552. [Google Scholar] [CrossRef]
- Trisciuzzi, R.; Fracassi, L.; Afonso Martin, H.; Monopoli, D.; Amat, D.; Santo-Ruiz, L.; De Palma, E.; Crovace, A. 41 cases of treatment of craneal cruciat ligament ruptura with Porous TTA. Three years of follow up. Vet. Sci. 2019, 6, 18. [Google Scholar] [CrossRef]
- Bjorn, M.; Augat, P.; Berninger, M.; Keppler, L.; Simon, G.; Von Rueden, C.; Birkenmaier, C.; Schipp, R.; Becker, J. Influence of different CCD angles on osseointegration and radiological changes after total hip arthroplasty of a triple wedge shape cementeless femoral stem: A prospective cohort study. Int. Orthop. 2023, 47, 1747–1755. [Google Scholar]
- Muir, P. Advances in the Cranial Crutial Ligament, 1st ed.; Wiley Online Library: Hoboken, NJ, USA, 2017; pp. 217–242. [Google Scholar]
- Vezzoni, A.; Demaria, M.; Corbari, A. Non-traumatic cranial cruciate ligament injuries. In Proceedings of the 1st World Veterinary Orthopedics Congress of the ESVOT/VOS, Munich, Germany, 5–8 September 2002. [Google Scholar]
- Johnson, J.A.; Austin, C.; Breur, G.J. Incidence of canine appendicular musculoeskeletal disorders in 16 veterinary teaching hospitals from 1980–1989. Vet. Comp. Orthop. Traumatol. 1994, 7, 56–69. [Google Scholar] [CrossRef]
- Whitehair, J.G.; Vasseur, P.B.; Willits, N.H. Epidemiology of cranial cruciate ligament rupture in dogs. J. Am. Vet. Med. Assoc. 1993, 203, 1016–1019. [Google Scholar]
- Witsberger, T.H.; Villamil, J.A.; Schultz, L.G.; Hahn, A.W.; Cook, J.L. Prevalence of and risk factors for hip dysplasia and cranial cruciate ligament deficiency in dogs. J. Am. Vet. Med. Assoc. 2008, 232, 1818–1824. [Google Scholar] [CrossRef]
- Ferreira, M.P.; Ferrigno, C.R.; de Souza, A.N.; Caquias, D.F.; de Figueiredo, A.V. Short-term comparison of tibial tuberosity advancement and tibial plateau levelling osteotomy in dogs with cranial cruciate ligament disease using kinetic analysis. Vet. Comp. Orthop. Traumatol. 2016, 29, 209–213. [Google Scholar] [PubMed]
- Livet, V.; Baldinger, A.; Viguier, É.; Taroni, M.; Harel, M.; Carozzo, C.; Cachon, T. Comparison of Outcomes Associated with Tibial Plateau Levelling Osteotomy and a Modified Technique for Tibial Tuberosity Advancement for the Treatment of Cranial Cruciate Ligament Disease in Dogs: A Randomized Clinical Study. Vet. Comp. Orthop. Traumatol. 2019, 32, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Aragosa, F.; Caterino, C.; Della Valle, G.; Fatone, G. Tibial Tuberosity Advancement Techniques (TTAT): A Systematic Review. Animals 2022, 12, 2114. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.V.; Weeren, R.; Paek, M. Extended long-term radiographic and functional comparison of tibial plateau leveling osteotomy vs tibial tuberosity advancement for cranial cruciate ligament rupture in the dog. Vet. Surg. 2020, 49, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Matchwick, A.I.M.; Bridges, J.P.; Scrimgeour, A.B.; Worth, A.J. A retrospective evaluation of complications associated with forkless tibial tuberosity advancement performed in primary care practice. Vet. Surg. 2021, 50, 121–132. [Google Scholar] [CrossRef]
- Costa, M.; Craig, D.; Cambridge, T.; Sebestyen, P.; Su, Y.; Fahie, M.A. Major complications of tibial tuberosity advancement in 1613 dogs. Vet Surg. 2017, 46, 494–500. [Google Scholar] [CrossRef]
- Della Valle, G.; Caterino, C.; Aragosa, F.; Micieli, F.; Costanza, D.; Di Palma, C.; Piscitelli, A.; Fatone, G. Outcome after Modified Maquet Procedure in dogs with unilateral cranial cruciate ligament rupture: Evaluation of recovery limb function by use of force plate gait analysis. PLoS ONE 2021, 16, e0256011. [Google Scholar] [CrossRef]
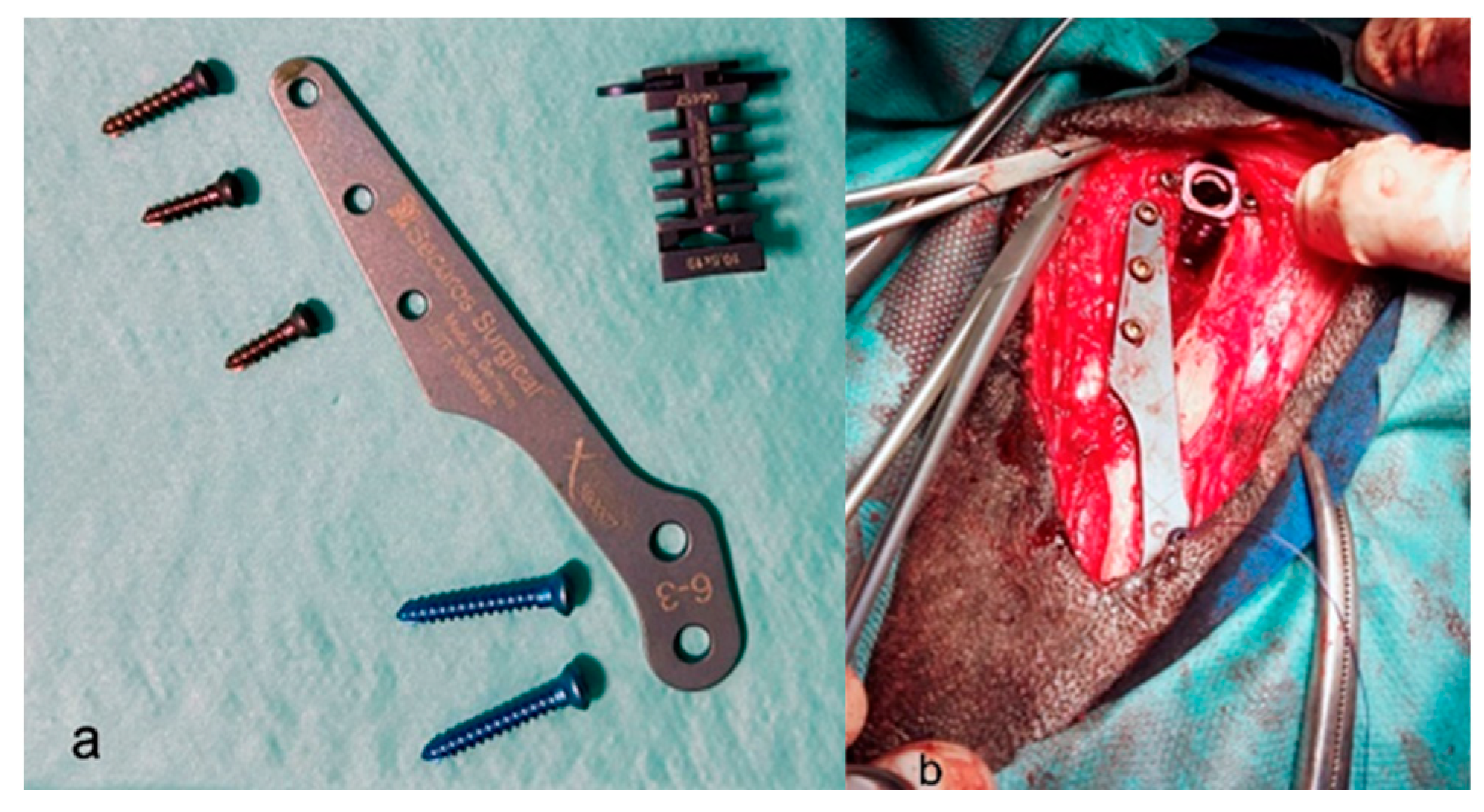
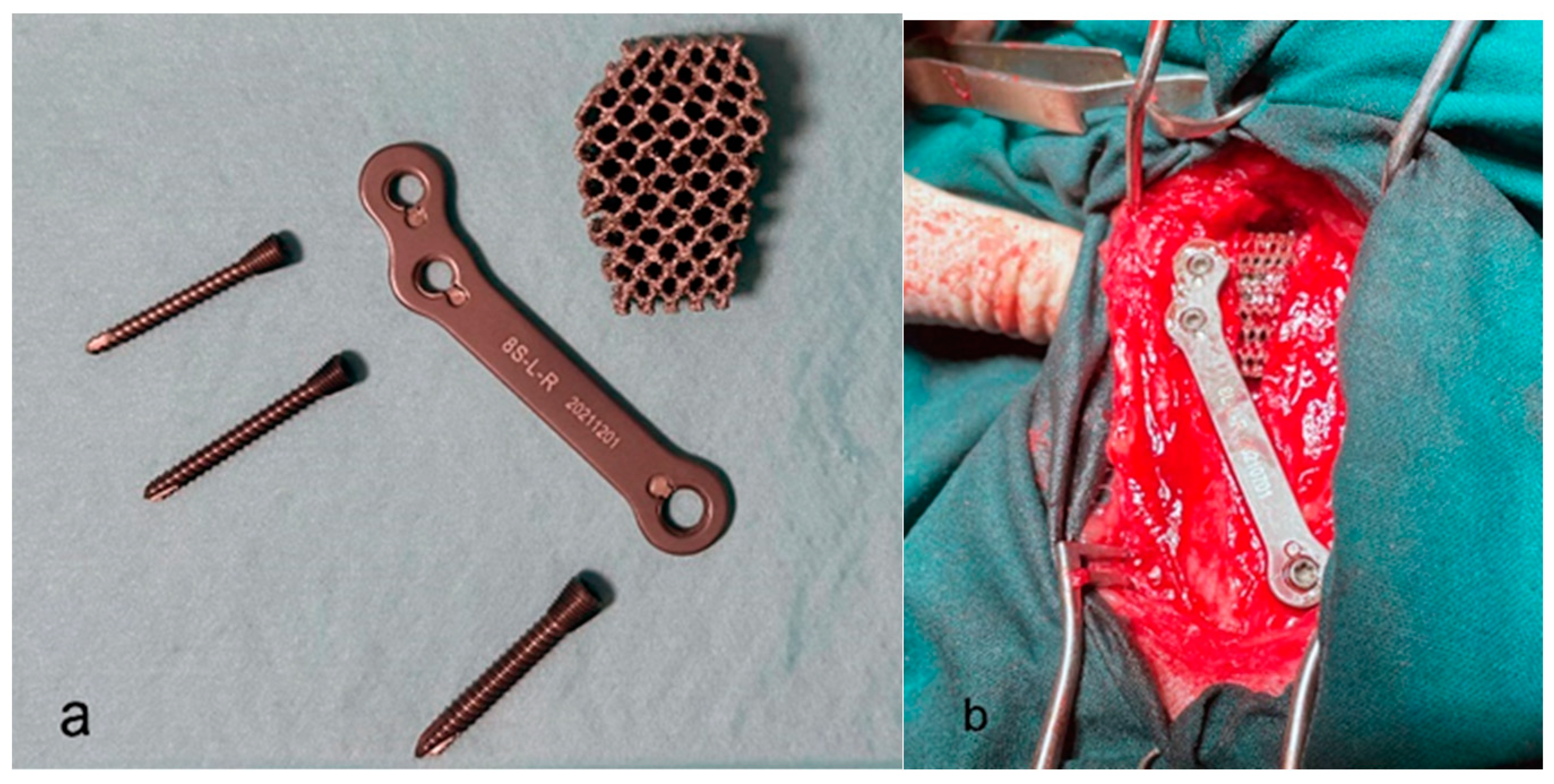
| Variable | Group 1 (n = 15) | Group 2 (n = 15) | p Value |
|---|---|---|---|
| Female | 8 (53.3%) | 7 (46.7%) | 1.0000 |
| Spayed | 3 (20.0%) | 5 (33.3%) | 0.6800 |
| Right stifle | 6 (40.0%) | 8 (53.3%) | 0.7140 |
| Pain | 12 (80.0%) | 11 (73.3%) | 1.0000 |
| Inflammation | 13 (86.7%) | 14 (93.3%) | 1.0000 |
| Joint leak | 13 (86.7%) | 12 (80.0%) | 1.0000 |
| Finochietto | 5 (35.7%) | 6 (37.5%) | 1.0000 |
| Drawer test | 0.8560 | ||
| Absent | 3 (20.0%) | 2 (13.3%) | |
| Present | 8 (53.3%) | 8 (53.3%) | |
| Clear | 4 (26.7%) | 5 (33.3%) |
| Variable | Group 1 | Group 2 | p-Value |
|---|---|---|---|
| Changes in the affected limb while standing | 1.60 ± 0.74 | 1.73 ± 0.70 | 0.5700 |
| Changes in posture while getting up | 1.00 ± 0.65 | 1.20 ± 0.68 | 0.4157 |
| Lameness | 2.13 ± 0.99 | 2.07 ± 0.96 | 0.8538 |
| Lameness after 10 min of walk | 1.67 ± 0.90 | 1.53 ± 0.99 | 0.7103 |
| Resistance to walk | 0.87 ± 0.92 | 0.80 ± 0.94 | 0.8065 |
| Resistance to run and play | 2.00 ± 0.53 | 1.60 ± 0.91 | 0.1363 |
| Resistance to climb stairs | 1.20 ± 0.94 | 1.40 ± 1.06 | 0.6115 |
| Limitation to take small jumps | 1.07 ± 0.70 | 1.07 ± 0.80 | 1.0000 |
| Manual articular mobility of the stifle | 1.33 ± 0.72 | 1.40 ± 0.74 | 0.7797 |
| Limitation of the articular flexion movement | 0.87 ± 0.52 | 0.80 ± 0.56 | 0.7370 |
| Limitation of the articular extension movement | 0.87 ± 0.52 | 0.80 ± 0.56 | 0.7370 |
| Muscular atrophy | 0.60 ± 0.63 | 0.53 ± 0.52 | 0.8690 |
| Variable | Group 1 | Group 2 | p-Value |
|---|---|---|---|
| Changes in the affected limb while standing | 0.33 ± 0.49 | 0.20 ± 0.41 | 0.4326 |
| Changes in posture while getting up | 0.47 ± 0.52 | 0.53 ± 0.64 | 0.8871 |
| Lameness | 0.73 ± 1.03 | 0.80 ± 1.01 | 0.7840 |
| Lameness after 10 min of walk | 0.33 ± 0.82 | 0.33 ± 0.49 | 0.5182 |
| Resistance to walk | 0.40 ± 0.91 | 0.13 ± 0.52 | 0.3087 |
| Resistance to run and play | 0.33 ± 0.62 | 0.33 ± 0.49 | 0.8153 |
| Resistance to climb stairs | 0.27 ± 0.46 | 0.13 ± 0.35 | 0.3856 |
| Limitation to take small jumps | 0.00 ± 0.00 | 0.13 ± 0.35 | 0.1641 |
| Manual articular mobility of the stifle | 0.27 ± 0.59 | 0.13 ± 0.52 | 0.3431 |
| Limitation of the articular flexion movement | 0.13 ± 0.35 | 0.13 ± 0.35 | 1.0000 |
| Limitation of the articular extension movement | 0.07 ± 0.26 | 0.07 ± 0.26 | 1.0000 |
| Muscular atrophy | 0.33 ± 0.62 | 0.13 ± 0.35 | 0.3554 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueirinhas, P.; Gonzalo-Orden, J.M.; Rodriguez, O.; Regueiro-Purriños, M.; Prada, I.; Vilar, J.M.; Rodríguez-Altónaga, J. Multiparametric Comparison of Two TTA-Based Surgical Techniques in Dogs with Cranial Cruciate Ligament Tears. Animals 2023, 13, 3453. https://doi.org/10.3390/ani13223453
Figueirinhas P, Gonzalo-Orden JM, Rodriguez O, Regueiro-Purriños M, Prada I, Vilar JM, Rodríguez-Altónaga J. Multiparametric Comparison of Two TTA-Based Surgical Techniques in Dogs with Cranial Cruciate Ligament Tears. Animals. 2023; 13(22):3453. https://doi.org/10.3390/ani13223453
Chicago/Turabian StyleFigueirinhas, Pedro, José Manuel Gonzalo-Orden, Oliver Rodriguez, Marta Regueiro-Purriños, Ivan Prada, José Manuel Vilar, and José Rodríguez-Altónaga. 2023. "Multiparametric Comparison of Two TTA-Based Surgical Techniques in Dogs with Cranial Cruciate Ligament Tears" Animals 13, no. 22: 3453. https://doi.org/10.3390/ani13223453
APA StyleFigueirinhas, P., Gonzalo-Orden, J. M., Rodriguez, O., Regueiro-Purriños, M., Prada, I., Vilar, J. M., & Rodríguez-Altónaga, J. (2023). Multiparametric Comparison of Two TTA-Based Surgical Techniques in Dogs with Cranial Cruciate Ligament Tears. Animals, 13(22), 3453. https://doi.org/10.3390/ani13223453







