Effects of Genetic Origin of Honeybees and Climate on Prevalence and Infestation Levels of Varroa
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
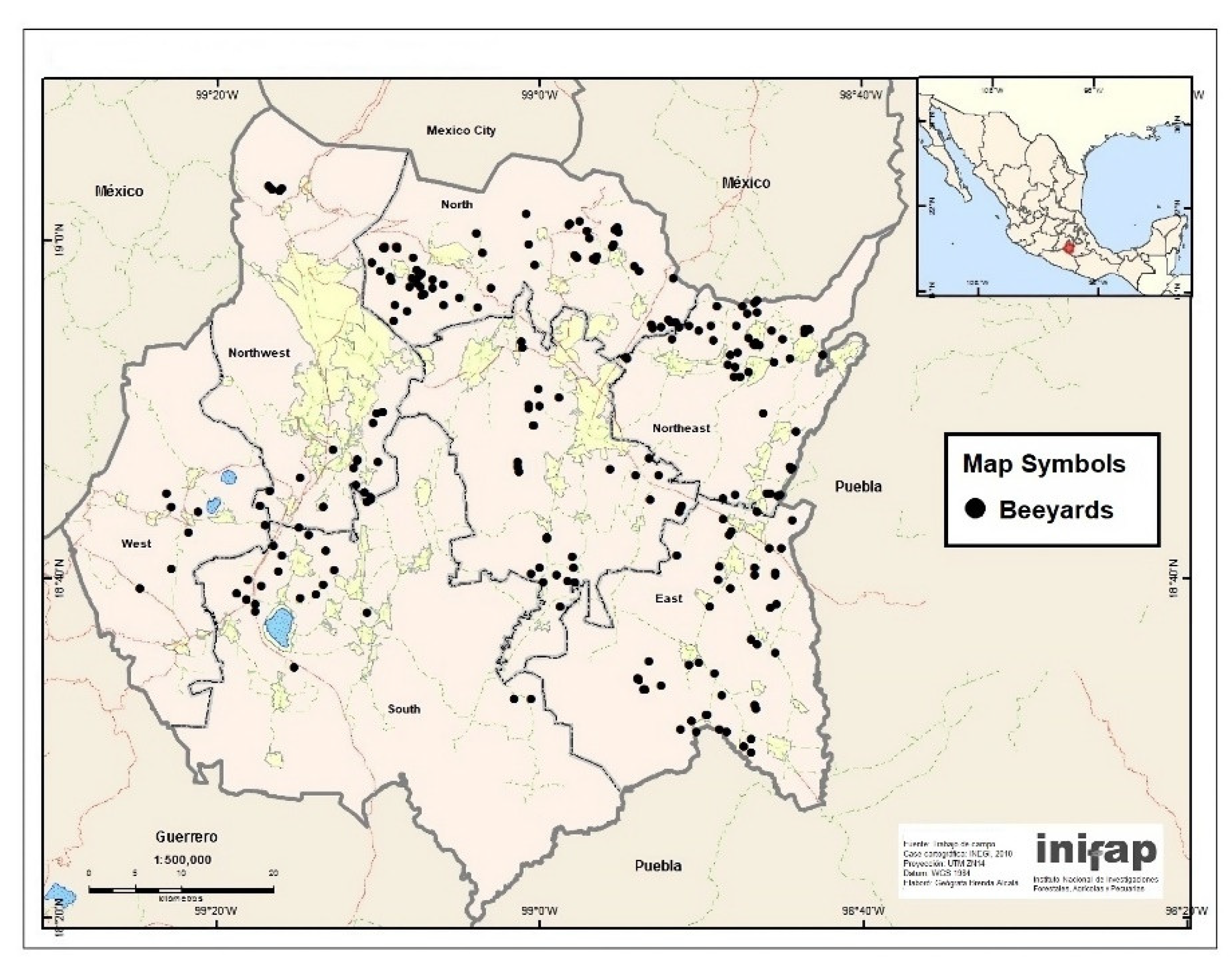
2.1. Selection of Colonies
2.2. Estimation of Prevalence and Infestation Levels of Varroa Destructor
2.3. Colony Morphotype
2.4. Colony Haplotype
2.5. Climate Type
2.6. Effect of the Morphotype, Haplotype and Climate Type on the Prevalence of Varroa Destructor
2.7. Effect of the Morphotype, Haplotype and Climate Type on the Infestation Levels of Varroa Destructor
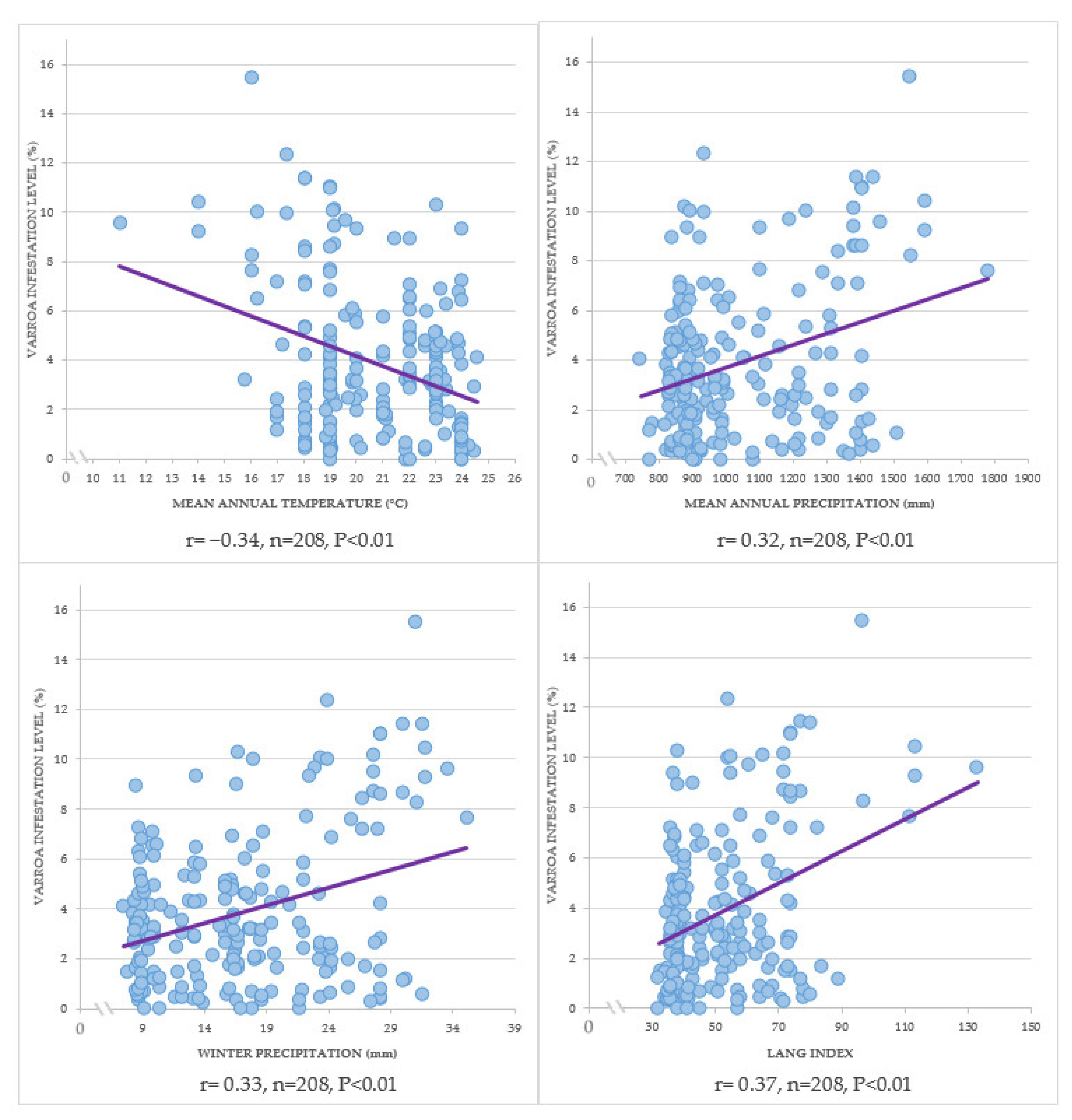
2.8. Effect of Climatic Elements on the Infestation Levels of Varroa Destructor
3. Results
3.1. Estimation of Prevalence and Infestation Levels of Varroa Destructor
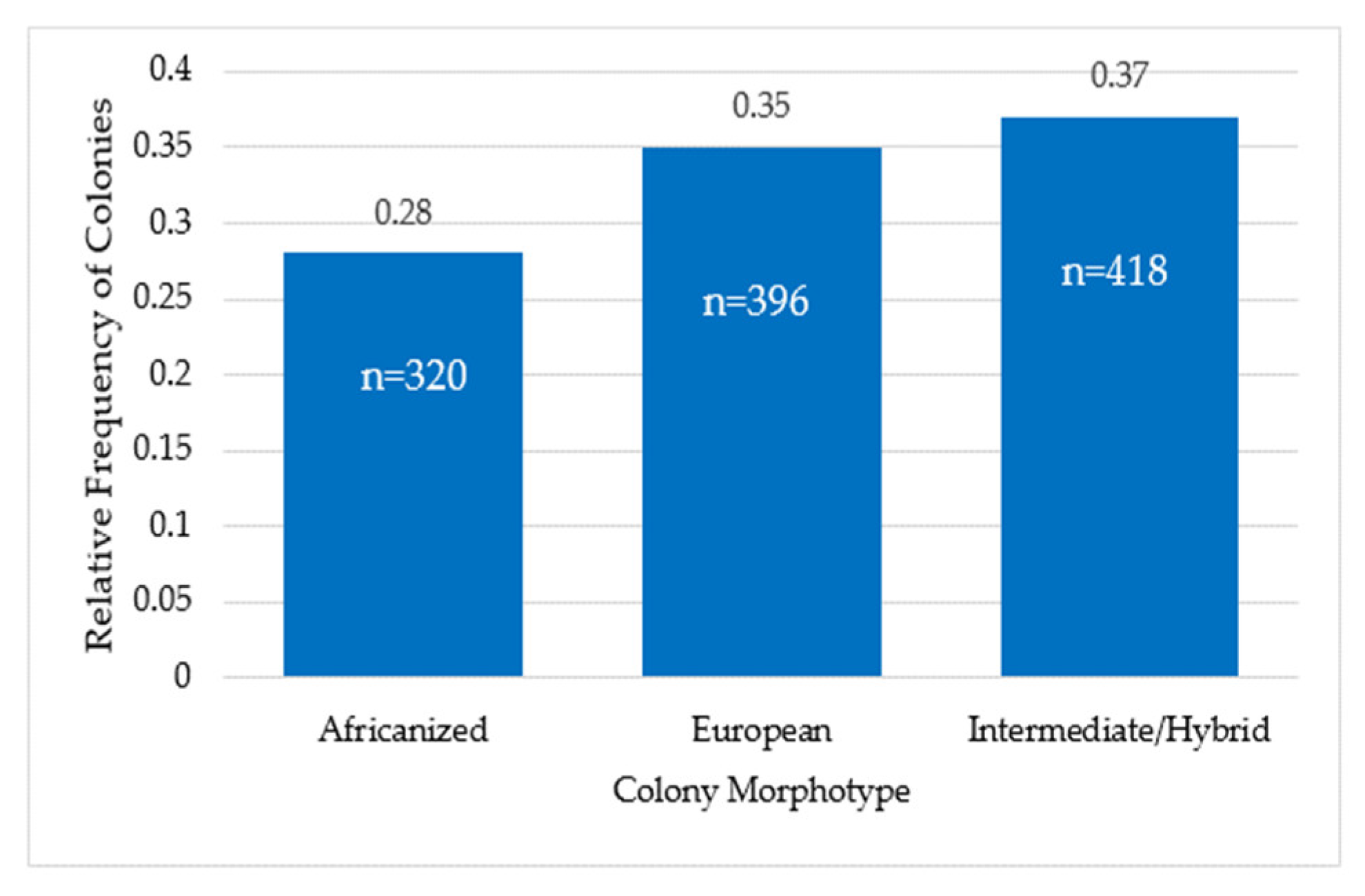
3.2. Colony Morphotype
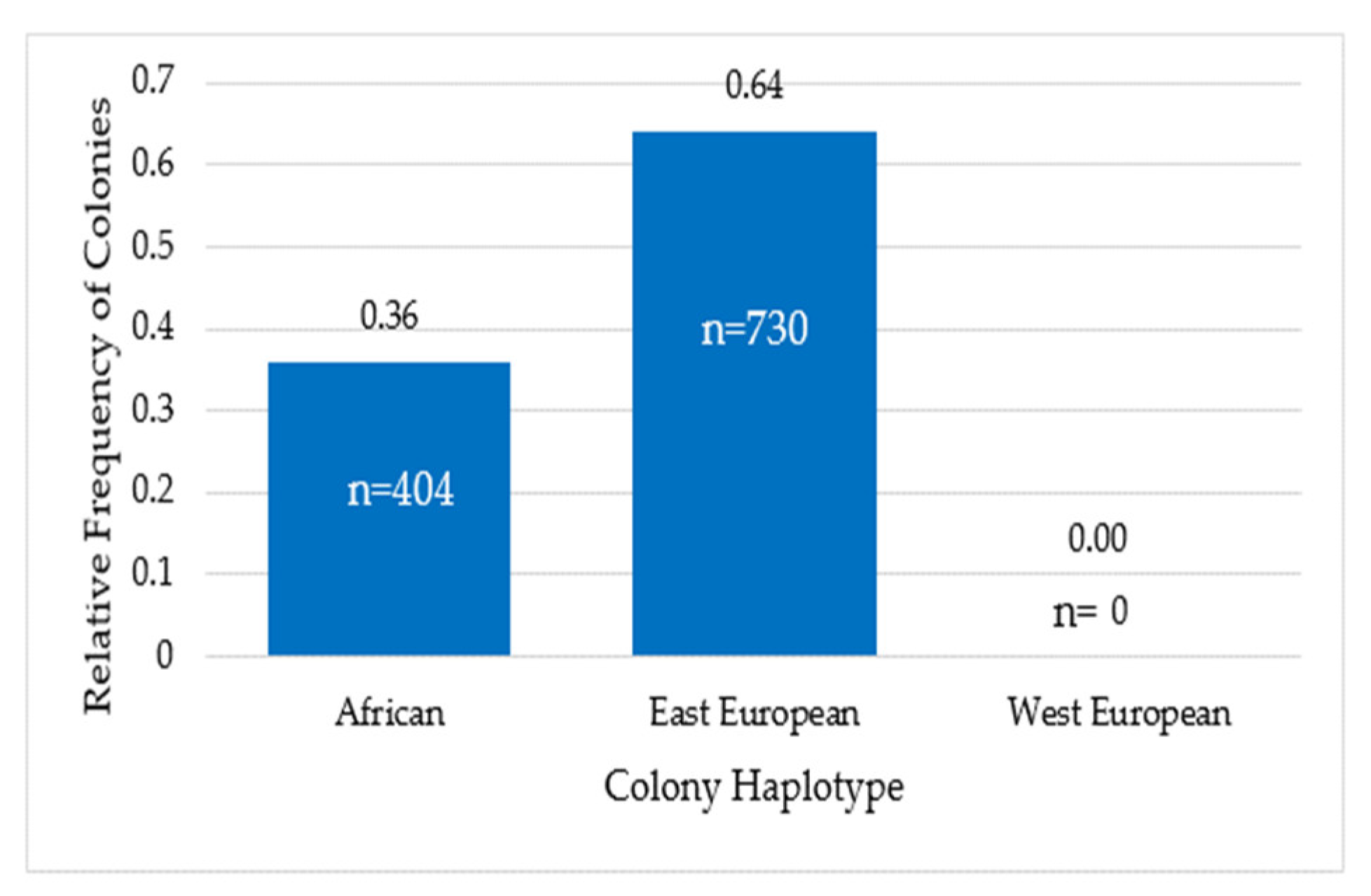
3.3. Colony Haplotype
3.4. Climate Type
3.5. Effect of the Morphotype, Haplotype and Climate Type on the Prevalence of Varroa Destructor
3.6. Effect of the Morphotype, Haplotype and Climate Type on the Infestation Levels of Varroa Destructor
3.7. Effect of Climatic Elements on the Infestation Levels of Varroa Destructor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- De Jong, D.; De Jong, P.H. Longevity of africanized honey bees (Hymenoptera: Apidae) infested by Varroa jacobsoni (Parasitiformes: Varroidae). J. Econ. Entomol. 1983, 76, 766–768. [Google Scholar] [CrossRef]
- De Jong, D.; De Jong, P.H.; Gonçalves, L.S. Weight loss and other damage to developing worker honeybees from infestation with Varroa jacobsoni. J. Apic. Res. 1982, 21, 165–167. [Google Scholar] [CrossRef]
- Anshakova, O.V.; Bobkova, V.V.; Grobov, O.F.; Korjova, L.M.; Langhe, A.B.; Mikitiuk, V.V. Contributions to biological study of Varroa jacobsoni and its influence on honey bees. In Proceedings of the XXVI International Congress of Apiculture, Adelaide, Australia, 13 October 1977; pp. 439–441. [Google Scholar]
- Sipos, T.; Donkó, T.; Jócsák, I.; Keszthelyi, S. Study of Morphological Features in Pre-Imaginal Honey Bee Impaired by Varroa destructor by Means of Computer Tomography. Insects 2021, 12, 717. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Walker, P.; Gunn, A. The effect of the ectoparasitic mite, Varroa destructor on adult worker honeybee (Apis mellifera) emergence weights, water, protein, carbohydrate, and lipid levels. Entomol. Exp. Appl. 2001, 101, 207–217. [Google Scholar] [CrossRef]
- Bernardi, S.; Venturino, E. Viral epidemiology of the adult Apis mellifera infested by the Varroa destructor mite. Heliyon 2016, 2, e00101. [Google Scholar] [CrossRef]
- Kang, Y.; Blanco, K.; Davis, T.; Wang, Y.; De Grandi-Hoffman, G. Disease dynamics of honeybees with Varroa destructor as parasite and virus vector. Math. Biosci. 2016, 275, 71–92. [Google Scholar] [CrossRef]
- Di Prisco, G.; Pennacchio, F.; Caprio, E.; Boncristiani, H.F.; Evans, J.D.; Chen, Y. Varroa destructor is an effective vector of Israeli acute paralysis virus in the honeybee, Apis mellifera. J. Gen. Virol. 2011, 92, 151–155. [Google Scholar] [CrossRef]
- Chen, Y.; Pettis, J.S.; Evans, J.D.; Kramer, M.; Feldlaufer, M.F. Transmission of Kashmir bee virus by the ectoparasitic mite Varroa destructor. Apidologie 2004, 35, 441–448. [Google Scholar] [CrossRef]
- Bowen-Walker, P.L.; Martin, S.J.; Gunn, A. The transmission of deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. J. Invertebr. Pathol. 1999, 73, 101–106. [Google Scholar] [CrossRef]
- Shen, M.; Yang, X.; Cox-Foster, D.; Cui, L. The role of varroa mites in infections of Kashmir bee virus (KBV) and deformed wing virus (DWV) in honey bees. Virology 2005, 342, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Shimanuki, H.; Calderone, N.W.; Knox, D.A. Parasitic mite syndrome: The symptoms. Am. Bee J. 1994, 134, 827–828. [Google Scholar]
- Bailey, L.; Ball, B.V. Honey Bee Pathology, 2nd ed.; Elsevier: Harpenden, UK, 1991; pp. 78–105. [Google Scholar]
- Ritter, W. Varroa disease of the honey bee Apis mellifera. Bee World 1981, 62, 141–153. [Google Scholar] [CrossRef]
- Zaobidna, E.A.; Żółtowska, K.; Lopieńska-Biernat, E. Varroa destructor induces changes in the expression of immunity-related genes during the development of Apis mellifera worker and drone broods. Acta Parasitol. 2017, 62, 779–789. [Google Scholar] [CrossRef]
- Khongphinitbunjong, K.; de Guzman, L.I.; Tarver, M.R.; Rinderer, T.E.; Chen, Y.; Chantawannakul, P. Differential viral levels and immune gene expression in three stocks of Apis mellifera induced by different numbers of Varroa destructor. J. Insect Physiol. 2014, 72, 28–34. [Google Scholar] [CrossRef]
- Gregory, P.G.; Evans, J.D.; Rinderer, T.; de Guzman, L. Conditional immune-gene suppression of honeybees parasitized by Varroa mites. J. Insect Sci. 2005, 5, 7. [Google Scholar] [CrossRef]
- Yang, X.; Cox-Foster, D.L. Impact of an ectoparasite on the immunity and pathology of an invertebrate: Evidence for host immunosuppression and viral amplification. Proc. Natl. Acad. Sci. USA 2005, 102, 7470–7475. [Google Scholar] [CrossRef]
- Reyes-Quintana, M.; Espinosa-Montaño, L.; Prieto-Merlos, D.; Koleoglua, G.; Petukhovaa, T.; Correa-Benítez, A.; Guzmán-Novoa, E. Impact of Varroa destructor and deformed wing virus on emergence, cellular immunity, wing integrity and survivorship of Africanized honey bees in Mexico. J. Invertebr. Pathol. 2019, 164, 43–48. [Google Scholar] [CrossRef]
- Emsen, B.; Guzman-Novoa, E.; Kelly, P.G. Honey production of honey bee (Hymenoptera: Apidae) colonies with high and low Varroa destructor (Acari: Varroidae) infestation rates in eastern Canada. Can. Entomol. 2013, 146, 236–240. [Google Scholar] [CrossRef]
- Medina-Flores, C.A.; Guzmán-Novoa, E.; Aréchiga-Flores, C.F.; Aguilera-Soto, J.I.; Gutiérrez-Piña, F.J. Efecto del nivel de infestación de Varroa destructor sobre la producción de miel de colonias de Apis mellifera en el altiplano semiárido de México. Rev. Mex. Cienc. Pecu. 2011, 2, 313–317. [Google Scholar]
- Arechavaleta, V.M.E.; Guzmán, N.E. Producción de miel de colonias de abejas (Apis mellifera L.) tratadas y no tratadas con fluvalinato contra Varroa jacobsoni Oudemans en Valle de Bravo, Estado de México. Vet. Méx. 2000, 31, 381–384. [Google Scholar]
- Arechavaleta, V.M.E.; Vélez, I.A.; Espinosa, G.J.A.; Amaro, G.R.; Alcalá, E.B.; Vázquez, P.S.; Ramírez, R.F.J. Presencia del Síndrome del Colapso de las Colonias de Abejas (CCD) en México. In Proceedings of the L Reunión Nacional de Investigación Pecuaria, Yucatán, México, 9 October 2014. [Google Scholar]
- Genersch, E.; von der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Büchler, R.; Berg, S.; Ritter, W.; Mühlen, W.; Gisder, S.; et al. The German bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef]
- Currie, R.W.; Pernal, S.F.; Guzmán-Novoa, E. Honey bee colony losses in Canada. J. Apic. Res. 2010, 49, 104–106. [Google Scholar] [CrossRef]
- Dahle, B. The role of Varroa destructor for honey bee colony losses in Norway. J. Apic. Res. 2010, 49, 124–125. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Eccles, L.; Calvete, Y.; Mcgowan, J.; Kelly, P.G.; Correa-Benítez, A. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef]
- Peterson, M.; Gray, A.; Teale, A. Colony losses in Scotland in 2004–2006 from a sample survey. J. Apic. Res. 2010, 48, 145–146. [Google Scholar] [CrossRef]
- Martin, S.J.; Medina, L.M. Africanized honeybees have unique tolerance to Varroa mites. Trends Parasitol. 2004, 20, 112–114. [Google Scholar] [CrossRef]
- Vandame, R.; Colin, M.E.; Monrad, S.; Otero-Colina, G. Levels of compatibility in a new host-parasite association: Apis mellifera/Varroa jacobsoni. Can. J. Zool. 2000, 78, 2037–2044. [Google Scholar] [CrossRef]
- Vieira, G.J.C.; Gonçalves, L.S.; De Jong, D. Africanized honey bees (Apis mellifera L.) are more efficient at removing worker brood artificially infested with the parasitic mite Varroa jacobsoni Oudemans than are Italian bees or Italian/Africanized hybrids. Genet. Mol. Biol. 2000, 23, 89–92. [Google Scholar]
- Rosenkranz, P. Honey bee (Apis mellifera L.) tolerance to Varroa jacobsoni Oud. in South America. Apidologie 1999, 30, 159–172. [Google Scholar] [CrossRef]
- Moretto, G.; João de Mello, L. Varroa jacobsoni infestation of adult Africanized and Italian honey bees (Apis mellifera) in mixed colonies in Brasil. Genet. Mol. Biol. 1999, 22, 321–323. [Google Scholar] [CrossRef]
- Guzmán-Novoa, E.; Sánchez, A.; Page Jr, R.E.; García, T. Susceptibility of European and Africanized honeybees (Apis mellifera L) and their hybrids to Varroa jacobsoni. Apidologie 1996, 27, 93–103. [Google Scholar] [CrossRef]
- Moretto, G.; Pillati, A.; De Jong, D.; Goncalves, L.; Cassini, F. Reduction of Varroa infestations in the state of Santa Catarina, in southern Brazil. Am. Bee J. 1995, 135, 498–500. [Google Scholar]
- Moretto, G.; Gonçalves, L.S.; De Jong, D.; Bichuette, M.Z. The effects of climate and bee race on Varroa jacobsoni Oud infestations in Brazil. Apidologie 1991, 22, 197–203. [Google Scholar] [CrossRef]
- Camazine, S. Differential reproduction of the mite, Varroa jacobsoni (Mesostigmata: Varroidae), on Africanized and European honey bees (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 1986, 79, 801–803. [Google Scholar] [CrossRef]
- Mendoza, Y.; Tomasco, I.H.; Antúnez, K.; Castelli, L.; Branchiccela, B.; Santos, E.; Invernizzi, C. Unraveling Honey Bee–Varroa destructor Interaction: Multiple Factors Involved in Differential Resistance between Two Uruguayan Populations. Vet. Sci. 2020, 7, 116. [Google Scholar] [CrossRef]
- Medina-Flores, C.A.; Guzmán-Novoa, E.; Hamiduzzaman, M.M.; Aréchiga-Flores, C.F.; López-Carlos, M.A. Africanized honey bees (Apis mellifera) have low infestation levels of the mite Varroa destructor in different ecological regions in Mexico. Genet. Mol. Res. 2014, 13, 7282–7293. [Google Scholar] [CrossRef]
- Ramos-Cuellar, A.K.; De la Mora, A.; Contreras-Escareño, F.; Morfin, N.; Tapia-González, J.M.; Macías-Macías, J.O.; Petukhova, T.; Correa-Benítez, A.; Guzmán-Novoa, E. Genotype, but not climate, affects the resistance of honey bees (Apis mellifera) to viral infections and to the mite Varroa destructor. Vet. Sci. 2022, 9, 358. [Google Scholar] [CrossRef]
- Arechavaleta, V.M.E.; Vázquez, P.S.; Ramírez, R.F.; Camacho, R.C.; Robles, R.C.; Amaro, G.R. Distribución de los morfotipos europeo, africanizado e híbrido en poblaciones de colonias de abejas de Morelos. In Proceedings of the XLIX Reunión Nacional de Investigación Pecuaria, Veracruz, México, 11 September 2013. [Google Scholar]
- Vélez-Izquierdo, A.; Espinosa-García, J.; Amaro-Gutiérrez, R.; Arechavaleta-Velasco, M.E. Tipología y caracterización de apicultores del estado de Morelos, México. Rev. Mex. Cienc. Pecu. 2016, 7, 507–524. [Google Scholar] [CrossRef]
- Sylvester, H.A.; Rinderer, T.E. Fast Africanized Bee Identification System. Am. Bee J. 1987, 127, 511–516. [Google Scholar]
- Noriega-Valladolid, G.L. Comparación de los Niveles de Africanización de Colonias de Abejas de Tres Líneas Seleccionadas y Colonias de Abejas no Seleccionadas. Bachelor’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2008. [Google Scholar]
- Hunt, G.J. Insect DNA extraction protocol. In Fingerprinting Methods Based on Arbitrarily Primed PCR; Micheli, M.R., Bova, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 21–24. [Google Scholar]
- Nielsen, D.I.; Ebert, P.R.; Page, R.E.; Hunt, G.J.; Guzmán-Novoa, E. Improved polymerase chain reaction based mitochondrial genotype assay for identification of the africanized honey bee (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 2000, 93, 1–6. [Google Scholar] [CrossRef]
- García, A.E. Modificaciones al Sistema de Clasificación Climática de Köppen, 16th ed.; Universidad Nacional Autónoma de México: Mexico City, Mexico, 2017. [Google Scholar]
- INEGI. Instituto Nacional de Estadística y Geografía. Climatología. México. Available online: https://www.inegi.org.mx/temas/climatologia/ (accessed on 18 August 2023).
- Mondragón, L.; Spivak, M.; Vandame, R. A multifactorial study of the resistance of honeybees Apis mellifera to the mite Varroa destructor over one year in Mexico. Apidologie 2005, 36, 345–358. [Google Scholar] [CrossRef]
- Guzman-Novoa, E.; Vandame, R.; Arechavaleta, M.E. Susceptibility of European and Africanized honey bees (Apis mellifera L.) to Varroa jacobsoni Oud. in Mexico. Apidologie 1999, 30, 173–182. [Google Scholar] [CrossRef]
- Invernizzi, C.; Zefferino, I.; Santos, E.; Sánchez, L.; Mendoza, Y. Multilevel assessment of grooming behavior against Varroa destructor in Italian and Africanized honey bees. J. Apic. Res. 2015, 54, 321–327. [Google Scholar] [CrossRef]
- Arechavaleta-Velasco, M.E.; Alcalá-Escamilla, K.; Robles-Ríos, C.; Tsuruda, J.M.; Hunt, G.J. Fine-scale linkage mapping reveals a small set of candidate genes influencing honey bee grooming behavior in response to Varroa mites. PLoS ONE 2012, 7, e47269. [Google Scholar] [CrossRef] [PubMed]
- Tsuruda, J.M.; Harris, J.W.; Bourgeois, L.; Danka, R.G.; Hunt, G.J. High-resolution linkage analyses to identify genes that influence Varroa sensitive hygiene behavior in honey bees. PLoS ONE 2012, 7, e48276. [Google Scholar] [CrossRef] [PubMed]
- Arechavaleta-Velasco, M.E.; Guzman-Novoa, E. Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies. Apidologie 2001, 32, 157–174. [Google Scholar] [CrossRef]
- Harbo, J.R.; Hoopingarner, R.A. Honey bees (Hymenoptera: Apidae) in the United States that express resistance to Varroa jacobsoni (Mesostigmata: Varroidae). J. Econ. Entomol. 1997, 4, 893–989. [Google Scholar] [CrossRef]
- Tapia-González, J.M.; Alcazar-Oceguera, G.; Macías-Macías, J.O.; Contreras-Escareño, F.; Tapia-Rivera, J.C.; Petukhova, T.; Guzmán-Novoa, E. Varroosis en abejas melíferas en diferentes condiciones ambientales y regionales de Jalisco, México. Ecosist. Recur. Agropec. 2019, 6, 243–251. [Google Scholar] [CrossRef]
- Harris, J.W.; Harbo, J.R.; Villa, J.D.; Danka, R.G. Variable population growth of Varroa destructor (Mesostigmata: Varroidae) in colonies of honey bees (Hymenoptera: Apidae) during a 10-year period. Popul. Ecol. 2003, 32, 1305–1312. [Google Scholar] [CrossRef]


| Colony Morphotype | Number of Varroa-Infested Colonies | Number of Varroa-Free Colonies |
|---|---|---|
| Africanized | 261 (0.82) | 59 (0.18) |
| European | 317 (0.80) | 79 (0.20) |
| Intermediate/Hybrid | 332 (0.79) | 86 (0.21) |
| Colony Haplotype | Number of Varroa-Infested Colonies | Number of Varroa-Free Colonies |
|---|---|---|
| African | 338 (0.84) | 66 (0.16) |
| European | 572 (0.78) | 158 (0.22) |
| Climate Type | Number of Varroa-Infested Colonies | Number of Varroa-Free Colonies |
|---|---|---|
| Temperate Sub-humid | 120 (0.93) | 9 (0.07) |
| Semi-warm | 351 (0.79) | 94 (0.21) |
| Warm sub-humid | 439 (0.78) | 121 (0.22) |
| Source | Sum of Squares | DF | Mean Square | F | p |
|---|---|---|---|---|---|
| Model | 1823.228 | 17 | 107.249 | 4.6621 | <0.0001 |
| Morphotype | 1.2185 | 2 | 0.6092 | 0.0097 | 0.9904 |
| Haplotype | 13.0152 | 1 | 13.0152 | 0.0363 | 0.8490 |
| Climate Type | 754.2876 | 2 | 377.1438 | 15.9460 | <0.0001 |
| Morphotype × Haplotype | 115.9339 | 2 | 57.9669 | 0.1045 | 0.9008 |
| Morphotype × Climate Type | 205.3501 | 4 | 51.3375 | 1.7150 | 0.1446 |
| Haplotype × Climate Type | 28.4102 | 2 | 14.2051 | 0.8476 | 0.4288 |
| Morphotype × Haplotype × Climate Type | 33.7994 | 4 | 8.4498 | 0.5815 | 0.6762 |
| Error | 20,519.930 | 892 | 23.0044 | ||
| Total | 22,343.158 | 909 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Figueroa, C.; Ramírez-Ramírez, F.J.; Alvarado-Avila, L.Y.; Arechavaleta-Velasco, M.E. Effects of Genetic Origin of Honeybees and Climate on Prevalence and Infestation Levels of Varroa. Animals 2023, 13, 3277. https://doi.org/10.3390/ani13203277
García-Figueroa C, Ramírez-Ramírez FJ, Alvarado-Avila LY, Arechavaleta-Velasco ME. Effects of Genetic Origin of Honeybees and Climate on Prevalence and Infestation Levels of Varroa. Animals. 2023; 13(20):3277. https://doi.org/10.3390/ani13203277
Chicago/Turabian StyleGarcía-Figueroa, Claudia, Francisco Javier Ramírez-Ramírez, Laura Yavarik Alvarado-Avila, and Miguel Enrique Arechavaleta-Velasco. 2023. "Effects of Genetic Origin of Honeybees and Climate on Prevalence and Infestation Levels of Varroa" Animals 13, no. 20: 3277. https://doi.org/10.3390/ani13203277
APA StyleGarcía-Figueroa, C., Ramírez-Ramírez, F. J., Alvarado-Avila, L. Y., & Arechavaleta-Velasco, M. E. (2023). Effects of Genetic Origin of Honeybees and Climate on Prevalence and Infestation Levels of Varroa. Animals, 13(20), 3277. https://doi.org/10.3390/ani13203277





