High Performance Liquid Chromatography Analysis and Description of Purine Content of Diets Suitable for Dogs with Leishmania Infection during Allopurinol Treatment—A Pilot Trial
Abstract
Simple Summary
Abstract
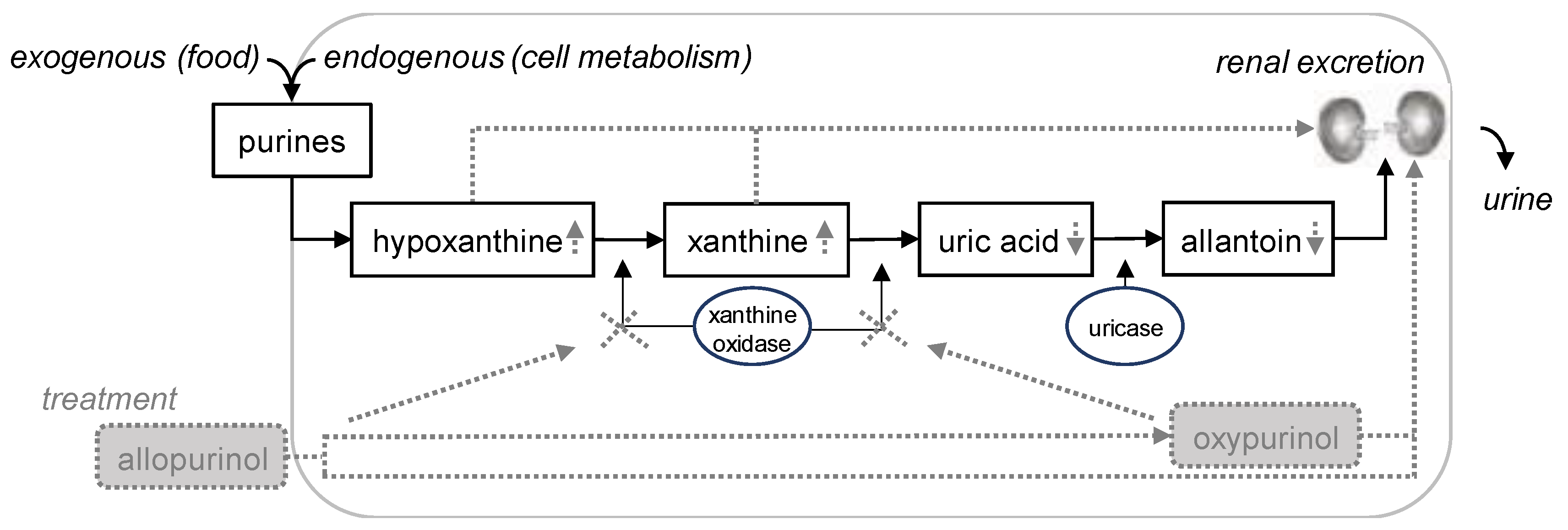
1. Introduction
2. Materials and Methods
2.1. Dog Diets
2.2. Purine Base Measurement and Calculations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreno, J.; Alvar, J. Canine leishmaniasis: Epidemiological risk and the experimental model. Trends Parasitol. 2002, 18, 399–405. [Google Scholar] [CrossRef]
- Morosetti, G.; Toson, M.; Trevisiol, K.; Idrizi, I.; Natale, A.; Lucchese, L.; Michelutti, A.; Ceschi, P.; Lorenzi, G.; Piffer, C.; et al. Canine leishmaniosis in the Italian northeastern Alps: A survey to assess serological prevalence in dogs and distribution of phlebotomine sand flies in the Autonomous Province of Bolzano—South Tyrol, Italy. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100432. [Google Scholar] [CrossRef] [PubMed]
- Espejo, L.A.; Costard, S.; Zagmutt, F.J. Modelling canine leishmaniasis spread to non-endemic areas of Europe. Epidemiol. Infect. 2015, 143, 1936–1949. [Google Scholar] [CrossRef] [PubMed]
- Vrhovec, M.G.; Pantchev, N.; Failing, K.; Bauer, C.; Travers-Martin, N.; Zahner, H. Retrospective analysis of canine vector-borne diseases (CVBD) in Germany with emphasis on the endemicity and risk factors of leishmaniosis. Parasitol. Res. 2017, 116, 131–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naucke, T.J.; Amelung, S.; Lorentz, S. First report of transmission of canine leishmaniosis through bite wounds from a naturally infected dog in Germany. Parasit. Vectors 2016, 9, 256. [Google Scholar] [CrossRef]
- Naucke, T.J.; Lorentz, S. First report of venereal and vertical transmission of canine leishmaniosis from naturally infected dogs in Germany. Parasit. Vectors 2012, 5, 67. [Google Scholar] [CrossRef]
- Geisweid, K.; Mueller, R.; Sauter-Louis, C.; Hartmann, K. Prognostic analytes in dogs with Leishmania infantum infection living in a non-endemic area. Vet. Rec. 2012, 171, 399. [Google Scholar] [CrossRef]
- Fisa, R.; Gállego, M.; Castillejo, S.; Aisa, M.J.; Serra, T.; Riera, C.; Carrió, J.; Gállego, J.; Portús, M. Epidemiology of canine leishmaniosis in Catalonia (Spain) the example of the Priorat focus. Vet. Parasitol. 1999, 83, 87–97. [Google Scholar] [CrossRef]
- Manna, L.; Reale, S.; Viola, E.; Vitale, F.; Foglia Manzillo, V.; Pavone, L.M.; Caracappa, S.; Gravino, A.E. Leishmania DNA load and cytokine expression levels in asymptomatic naturally infected dogs. Vet. Parasitol. 2006, 142, 271–280. [Google Scholar] [CrossRef]
- Otranto, D.; Paradies, P.; de Caprariis, D.; Stanneck, D.; Testini, G.; Grimm, F.; Deplazes, P.; Capelli, G. Toward diagnosing Leishmania infantum infection in asymptomatic dogs in an area where leishmaniasis is endemic. Clin. Vaccine Immunol. 2009, 16, 337–343. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G.; The LeishVet, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit. Vectors 2011, 4, 86. [Google Scholar] [CrossRef] [PubMed]
- Marr, J.J.; Berens, R.L.; Nelson, D.J. Purine metabolism in Leishmania donovani and Leishmania braziliensis. Biochim. Biophys. Acta 1978, 544, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Rosemeyer, H. The chemodiversity of purine as a constituent of natural products. Chem. Biodivers. 2004, 1, 361–401. [Google Scholar] [CrossRef] [PubMed]
- Bartges, J.W.; Osborne, C.A.; Lulich, J.P.; Kruger, J.M.; Sanderson, S.L.; Koehler, L.A.; Ulrich, L.K. Canine urate urolithiasis. Etiopathogenesis, diagnosis, and management. Vet. Clin. North. Am. Small Anim. Pract. 1999, 29, 161–191. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.G.; Huang, C.; et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Marr, J.J. Antileishmanial effect of allopurinol. Antimicrob. Agents Chemother. 1974, 5, 469–472. [Google Scholar] [CrossRef]
- Nelson, D.J.; LaFon, S.W.; Tuttle, J.V.; Miller, W.H.; Miller, R.L.; Krenitsky, T.A.; Elion, G.B.; Berens, R.L.; Marr, J.J. Allopurinol ribonucleoside as an antileishmanial agent. J. Biol. Chem. 1979, 254, 11544–11549. [Google Scholar] [CrossRef]
- Momeni, A.Z.; Reiszadae, M.R.; Aminjavaheri, M. Treatment of cutaneous leishmaniasis with a combination of allopurinol and low-dose meglumine antimoniate. Int. J. Dermatol. 2002, 41, 441–443. [Google Scholar] [CrossRef]
- Koutinas, A.F.; Saridomichelakis, M.N.; Mylonakis, M.E.; Leontides, L.; Polizopoulou, Z.; Billinis, C.; Argyriadis, D.; Diakou, N.; Papadopoulos, O. A randomised, blinded, placebo-controlled clinical trial with allopurinol in canine leishmaniosis. Vet. Parasitol. 2001, 98, 247–261. [Google Scholar] [CrossRef]
- Giesecke, D.; Gaebler, S.; Tiemeyer, W. Purine availability and metabolism in dogs fed single-cell protein or RNA. J. Nutr. 1982, 112, 1822–1826. [Google Scholar] [CrossRef]
- Jesus, L.; Arenas, C.; Domínguez-Ruiz, M.; Silvestrini, P.; Englar, R.E.; Roura, X.; Leal, R.O. Xanthinuria secondary to allopurinol treatment in dogs with leishmaniosis: Current perspectives of the Iberian veterinary community. Comp. Immunol. Microbiol. Infect. Dis. 2022, 83, 101783. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Pastor, J.; Roura, X.; Tabar, M.D.; Espada, Y.; Font, A.; Balasch, J.; Planellas, M. Adverse urinary effects of allopurinol in dogs with leishmaniasis. J. Small Anim. Pract. 2016, 57, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Ling, G.V.; Ruby, A.L.; Harrold, D.R.; Johnson, D.L. Xanthine-containing urinary calculi in dogs given allopurinol. J. Am. Vet. Med. Assoc. 1991, 198, 1935–1940. [Google Scholar] [PubMed]
- Sturgess, K. Dietary management of canine urolithiasis. Practice 2009, 31, 306–312. [Google Scholar] [CrossRef]
- Godoy, A.; de Jesus, C.; Gonçalves, R.S.; Azeredo, F.J.; Rocha, A.; Marques, M.P.; Lanchote, V.L.; Larangeira, D.F.; Barrouin-Melo, S.M. Detection of allopurinol and oxypurinol in canine urine by HPLC/MS-MS: Focus on veterinary clinical pharmacokinetics. J. Pharm. Biomed. Anal. 2020, 185, 113204. [Google Scholar] [CrossRef]
- Souci, S.; Fachmann, W.; Kraut, H. Die Zusammensetzung der Lebensmittel. Nährwert-Tabellen. [The Composition of Foods. Nutrition Tables.], 7th ed.; MedPharm Scientific Publishers: Stuttgart, Germany, 2008. [Google Scholar]
- Cofrades, S.; López-López, I.; Solas, M.T.; Bravo, L.; Jiménez-Colmenero, F. Influence of different types and proportions of added edible seaweeds on characteristics of low-salt gel/emulsion meat systems. Meat Sci. 2008, 79, 767–776. [Google Scholar] [CrossRef]
- Kaneko, K.; Aoyagi, Y.; Fukuuchi, T.; Inazawa, K.; Yamaoka, N. Total purine and purine base content of common foodstuffs for facilitating nutritional therapy for gout and hyperuricemia. Biol. Pharm. Bull. 2014, 37, 709–721. [Google Scholar] [CrossRef]
- Sabolová, M.; Kulma, M.; Petříčková, D.; Kletečková, K.; Kouřimská, L. Changes in purine and uric acid content in edible insects during culinary processing. Food Chem. 2023, 403, 134349. [Google Scholar] [CrossRef]
- Montag, A.; Kölling, I.; Jänicke, S.; Benkmann, R.; Lou, S.N. Zur Kenntnis des Purinbasengehaltes in Lebensmitteln. Aktuelle Ernahrungsmedizin 1989, 14, 243–247. [Google Scholar]
- Bartges, J.W.; Osborne, C.A.; Felice, L.J.; Unger, L.K.; Chen, M. Influence of allopurinol and two diets on 24-hour urinary excretions of uric acid, xanthine, and ammonia by healthy dogs. Am. J. Vet. Res. 1995, 56, 595–599. [Google Scholar]
- Giesecke, D.; Stangassinger, M. [Effects of purine-rich nutrition on the renal and extrarenal excretion of purine catabolites in Dalmatian dogs]. Z. Ernährungswissenschaft 1990, 29, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Queau, Y. Nutritional Management of Urolithiasis. Vet. Clin. North. Am. Small Anim. Pract. 2019, 49, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Roura, X.; Cortadellas, O.; Day, M.J.; Benali, S.L.; Zatelli, A. Canine leishmaniosis and kidney disease: Q&A for an overall management in clinical practice. J. Small Anim. Pract. 2021, 62, 3. [Google Scholar] [CrossRef] [PubMed]
- Westropp, J.L.; Larsen, J.A.; Johnson, E.G.; Bannasch, D.; Fascetti, A.J.; Biourge, V.; Queau, Y. Evaluation of dogs with genetic hyperuricosuria and urate urolithiasis consuming a purine restricted diet: A pilot study. BMC Vet. Res. 2017, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Mireaux, M.; Villaverde, C.; Hervera, M.; Roura, X.; Caussé, E.; Feugier, A.; Biourge, V.; Mougeot, I. Leishmaniose canine et urolithiase à cristaux de xanthine: Intérêt d’un régime alimentaire réduit en purines, étude préliminaire sur 13 chiens. Rev. Vet. Clin. 2014, 49, 23–29. [Google Scholar] [CrossRef]
- Gosch, B.; Montag, A. Zum Nucleopuringehalt des Kaffees. Dtsch. Leb. Rundsch. 1995, 91, 208–214. [Google Scholar]
- Montag, A. Ziele der Nucleopurinanalytik in Lebensmitteln. GIT Fachz. Lab. 1994, 38, 890–892. [Google Scholar]
- National Research Council (USA). Nutrient Requirements of Cats and Dogs; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Dobenecker, B. Vorbeugendes Gewichtsmanagement—Ein Leitfaden für die Praxis. Kleintier Konkret 2013, 16, 9–14. [Google Scholar] [CrossRef][Green Version]
- Thes, M.; Koeber, N.; Fritz, J.; Wendel, F.; Dillitzer, N.; Dobenecker, B.; Kienzle, E. Metabolizable energy intake of client-owned adult dogs. J. Anim. Physiol. Anim. Nutr. 2016, 100, 813–819. [Google Scholar] [CrossRef]
- Kienzle, E.; Opitz, B.; Earle, K.; Smith, P.; Maskell, I.; Iben, C. The development of an improved method of predicting the energy content in prepared dog and cat food. J. Anim. Physiol. Anim. Nutr. 1998, 79, 69–79. [Google Scholar] [CrossRef]
- Giesecke, D.; Gaebler, S.; Stangassinger, M. Quantification and kinetics of purine catabolism in Dalmatian dogs at low and high purine intakes. Comp. Biochem. Physiol. B 1989, 92, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.Y.; Vanselow, B.A.; Walkden-Brown, S.W. One dog’s meat is another dog’s poison–nutrition in the Dalmatian dog. Recent. Adv. Anim. Nutr. Aust. 2003, 14, 123–131. [Google Scholar]
- Freeman, L.M.; Michel, K.E.; Brown, D.J.; Kaplan, P.M.; Stamoulis, M.E.; Rosenthal, S.L.; Keene, B.W.; Rush, J.E. Idiopathic dilated cardiomyopathy in Dalmatians: Nine cases (1990–1995). J. Am. Vet. Med. Assoc. 1996, 209, 1592–1596. [Google Scholar] [PubMed]
- Dobenecker, B. [Phosphate intake with complete food and diets for chronic kidney disease available on the German market]. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere 2021, 49, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Finco, D.R.; Brown, S.A.; Crowell, W.A.; Duncan, R.J.; Barsanti, J.A.; Bennett, S.E. Effects of dietary phosphorus and protein in dogs with chronic renal failure. Am. J. Vet. Res. 1992, 53, 2264–2271. [Google Scholar]
- Clifford, A.; Riumallo, J.; Young, V.; Scrimshaw, N. Effect of oral purines on serum and urinary uric acid of normal, hyperuricemic and gouty humans. J. Nutr. 1976, 106, 428–434. [Google Scholar] [CrossRef]
- Choi, H.K.; Atkinson, K.; Karlson, E.W.; Willett, W.; Curhan, G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N. Engl. J. Med. 2004, 350, 1093–1103. [Google Scholar] [CrossRef]
- Starzonek, J.; von Lindeiner, L.; Vervuert, I. [Assessment of vegan complete diets for dogs and cats available in Germany]. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere 2021, 49, 262–271. [Google Scholar] [CrossRef]
- Carter, N.S.; Yates, P.A.; Gessford, S.K.; Galagan, S.R.; Landfear, S.M.; Ullman, B. Adaptive responses to purine starvation in Leishmania donovani. Mol. Microbiol. 2010, 78, 92–107. [Google Scholar] [CrossRef]
- Martin, J.L.; Yates, P.A.; Soysa, R.; Alfaro, J.F.; Yang, F.; Burnum-Johnson, K.E.; Petyuk, V.A.; Weitz, K.K.; Camp, D.G., 2nd; Smith, R.D.; et al. Metabolic reprogramming during purine stress in the protozoan pathogen Leishmania donovani. PLoS Pathog. 2014, 10, e1003938. [Google Scholar] [CrossRef]
- Stevenson, A.E.; Hynds, W.K.; Markwell, P.J. Effect of dietary moisture and sodium content on urine composition and calcium oxalate relative supersaturation in healthy miniature schnauzers and labrador retrievers. Res. Vet. Sci. 2003, 74, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Bijster, S.; Nickel, R.F.; Beynen, A.C. Comparison of the efficacy of two anti-uric acid diets in dalmatian dogs. Acta Vet. Hung. 2001, 49, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Giesecke, D.; Gallenmüller, P.; Tiemeyer, W.; Gropp, J. Effect of purine-rich nutrients on weight gain, catabolites in blood plasma and the uric acid transport of erythrocytes—A model study in dogs. Z. Ernahrungswiss 1990, 29, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Langfeldt, E.; Holmsen, J. The excretion of purine derivatives in dogs. Biochem. J. 1925, 19, 717–723. [Google Scholar] [CrossRef]
| Feed Material | DM (g) | Total Purines (mg Uric Acid) | ||
|---|---|---|---|---|
| Category | Example | /100 g WW | /100 g WW | /100 g DM |
| High purine content | ||||
| Yeast | Baker yeast | 27.0 | 680 | 2519 |
| Liver (pork) | 28.0 | 515 | 1839 | |
| Organs | Kidney (beef) | 23.9 | 269 | 1126 |
| Heart (beef) | 24.5 | 256 | 1045 | |
| Sardines | 24.0 | 345 | 1438 | |
| Fish | Salmon | 34.5 | 170 | 493 |
| Tuna | 38.5 | 179 | 465 | |
| Seaweed | Nori (dry) | 89 * | 696 ** | 782 |
| Medium purine content | ||||
| Chicken (muscle) | 30.6 | 115 | 376 | |
| Meat | Beef (brisket) | 33.6 | 90 | 268 |
| Pork (belly) | 39.7 | 100 | 252 | |
| Legumes | French beans | 10.5 | 37 | 352 |
| Pea (pod and seed) | 24.8 | 84 | 339 | |
| Soybean (dry seed) | 91.6 | 190 | 207 | |
| Processed soybeans (tofu) | 15.4 | 68 | 442 | |
| Edible insects | Mealworm (Tenebrio molitor) | 32.9 *** | 112 | 339 *** |
| Cricket (Acheta domesticus) | 30.2 *** | 82 | 270 *** | |
| Low purine content | ||||
| Vegetable | Carrot | 11.8 | 17 | 144 |
| Potato | 22.2 | 16 | 72 | |
| Grain | Wheat (whole grain) | 87.3 | 51 | 58 |
| Dairy | Yogurt, 3.5% fat | 13.0 | 8 | 62 |
| Cottage cheese | 21.5 | 9 | 44 | |
| Egg | Chicken | 25.3 | 0 ** | 0 |
| Dog Diet * | Texture | DM 1 (%) | CP (% WW) | CP (% DM) | Manufacturer * Declaration | |
|---|---|---|---|---|---|---|
| Indication | Main Protein Sources | |||||
| Urinary dog diets | ||||||
| U1 | dry | 91.5 | 18 | 20 | Prevention of cystine and urate uroliths | Dried egg, wheat gluten feed, corn gluten feed |
| U2 | dry | 92.5 | 10 | 11 | Reduction of urate and cystine urolith formation, Reduction of copper storage in the liver in adult animals | Dried whole egg |
| U3 | dry | 91.0 | 23 | 25 | Reduction of formation of oxalic, urate and cystine stones | Potato protein, flaked peas, dried whole egg |
| U4 | dry | 94.0 | 24 | 26 | Prevention of xanthine uroliths specifically for dogs with Leishmania infection treated with allopurinol | Dehydrated egg, soy protein, hydrolysed animal protein, plasma proteins, caseinate |
| Kidney dog diets | ||||||
| K1 | dry | 91.5 | 14 | 15 | Support of renal function in case of chronic renal insufficiency, reduction of ingredient and nutrient intolerances | Soy protein isolate |
| K2 | dry | 92.5 | 14 | 15 | Support renal function in chronic or acute renal insufficiency in adult dogs | Dried whole egg, pea protein |
| Vegan dog diet | ||||||
| V | dry | 91.3 | 24 | 26 | No specifically advertised indication | Lentils, peas |
| Low protein dog diets | ||||||
| Lp1 | canned | 22.7 | 6 | 26 | No specifically advertised indication | Wild game muscle meat, chia seeds |
| Lp2 | canned | 21.2 | 6 | 28 | No specifically advertised indication | Calf heart, calf lung, chia seeds |
| Lp3 | canned | 22.4 | 6 | 27 | No specifically advertised indication | Chicken muscle meat, chicken stomach, chicken skin, chia seeds |
| Homemade low purine dog diet | ||||||
| Hm | cooked | 24.5 | 8 | 33 | Prevention of xanthine uroliths specifically for dogs with Leishmania infection treated with allopurinol | Curd, egg, beef muscle meat |
| Regular dog diet | ||||||
| R | dry | 91.5 | 23 | 25 | No specifically advertised indication | Dried poultry protein, hydrolysed animal protein, dried pig protein, wheat feed flour, wheat gluten, corn, rice, beet chips |
| HPLC Analyses | Calculated Values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dog Diet * | Purine Bases | Purine N | Uric Acid Equivalent | Energy | Purine N | Daily Intake | ||||||
| mg/100 g WW | mg/100 g | mg/100 g | kcal/100 g | mg/1000 kcal | g | mg | ||||||
| A | G | HX | X | WW | DM | WW | DM | WW | WW | Purine N | ||
| Urinary dog diets | ||||||||||||
| U1 | 9.8 | 14.1 | <0.01 | <0.01 | 9.3 | 10.2 | 27.9 | 30.5 | 383 | 24 | 235.0 | 21.9 |
| U2 | 11.5 | 17.2 | <0.1 | <0.1 | 11.2 | 12.1 | 33.6 | 36.3 | 399 | 28 | 225.0 | 25.2 |
| U3 | 29.9 | 35.5 | 2.2 | <0.1 | 26.5 | 29.1 | 79.5 | 87.4 | 380 | 70 | 236.0 | 62.5 |
| U4 | 34.4 | 41.5 | 10.8 | 4.1 | 35.7 | 38.0 | 107.0 | 113.8 | 382 | 93 | 235.0 | 83.9 |
| Kidney dog diets | ||||||||||||
| K1 | 16.8 | 29.2 | <0.1 | <0.1 | 17.8 | 19.5 | 53.4 | 58.4 | 398 | 45 | 226.0 | 40.2 |
| K2 | 15.3 | 23.7 | <0.1 | <0.1 | 15.1 | 16.3 | 45.3 | 49.0 | 406 | 37 | 221.0 | 33.4 |
| Vegan dog diet | ||||||||||||
| V | 54.5 | 52.4 | <0.1 | <0.1 | 42.1 | 46.1 | 126.3 | 138.3 | 354 | 119 | 254.0 | 106.9 |
| Low protein dog diets | ||||||||||||
| LP1 | 4.5 | 6.8 | 18.9 | <0.1 | 12.2 | 53.7 | 36.5 | 160.8 | 118 | 103 | 761.0 | 92.7 |
| LP2 | 12.1 | 12.7 | 16.8 | 7.2 | 19.3 | 90.9 | 57.8 | 272.6 | 111 | 173 | 809.0 | 155.8 |
| LP3 | 12.3 | 13.9 | 20.8 | <0.1 | 18.9 | 84.2 | 56.6 | 252.7 | 97 | 194 | 926.0 | 174.7 |
| Homemade low purine dog diet | ||||||||||||
| Hm | 3.2 | 4.3 | 5.0 | <0.1 | 5.0 | 20.2 | 14.9 | 60.7 | 114 | 43 | 575.0 | 28.5 |
| Regular dog diet | ||||||||||||
| R | 23.8 | 35.4 | 27.4 | 3.3 | 35.5 | 38.8 | 106.5 | 116.4 | 386 | 92 | 233.0 | 82.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaempfle, M.; Bergmann, M.; Koelle, P.; Hartmann, K. High Performance Liquid Chromatography Analysis and Description of Purine Content of Diets Suitable for Dogs with Leishmania Infection during Allopurinol Treatment—A Pilot Trial. Animals 2023, 13, 3060. https://doi.org/10.3390/ani13193060
Kaempfle M, Bergmann M, Koelle P, Hartmann K. High Performance Liquid Chromatography Analysis and Description of Purine Content of Diets Suitable for Dogs with Leishmania Infection during Allopurinol Treatment—A Pilot Trial. Animals. 2023; 13(19):3060. https://doi.org/10.3390/ani13193060
Chicago/Turabian StyleKaempfle, Melanie, Michèle Bergmann, Petra Koelle, and Katrin Hartmann. 2023. "High Performance Liquid Chromatography Analysis and Description of Purine Content of Diets Suitable for Dogs with Leishmania Infection during Allopurinol Treatment—A Pilot Trial" Animals 13, no. 19: 3060. https://doi.org/10.3390/ani13193060
APA StyleKaempfle, M., Bergmann, M., Koelle, P., & Hartmann, K. (2023). High Performance Liquid Chromatography Analysis and Description of Purine Content of Diets Suitable for Dogs with Leishmania Infection during Allopurinol Treatment—A Pilot Trial. Animals, 13(19), 3060. https://doi.org/10.3390/ani13193060






