Cryopreservation Cooling Rate Impacts Post-Thaw Sperm Motility and Survival in Litoria booroolongensis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Spermic Urine Collection and Evaluation
2.3. Sample Cryopreservation
2.4. Post-Thaw Sample Handling and Assessment
2.5. Effect of CPA Dilution Ratio on Post-Thaw Sperm Recovery
2.6. Statistics
3. Results
3.1. Raw Spermic Urine Quality Assessment
3.2. Effects of CPA and Cooling Rate on Sperm Quality
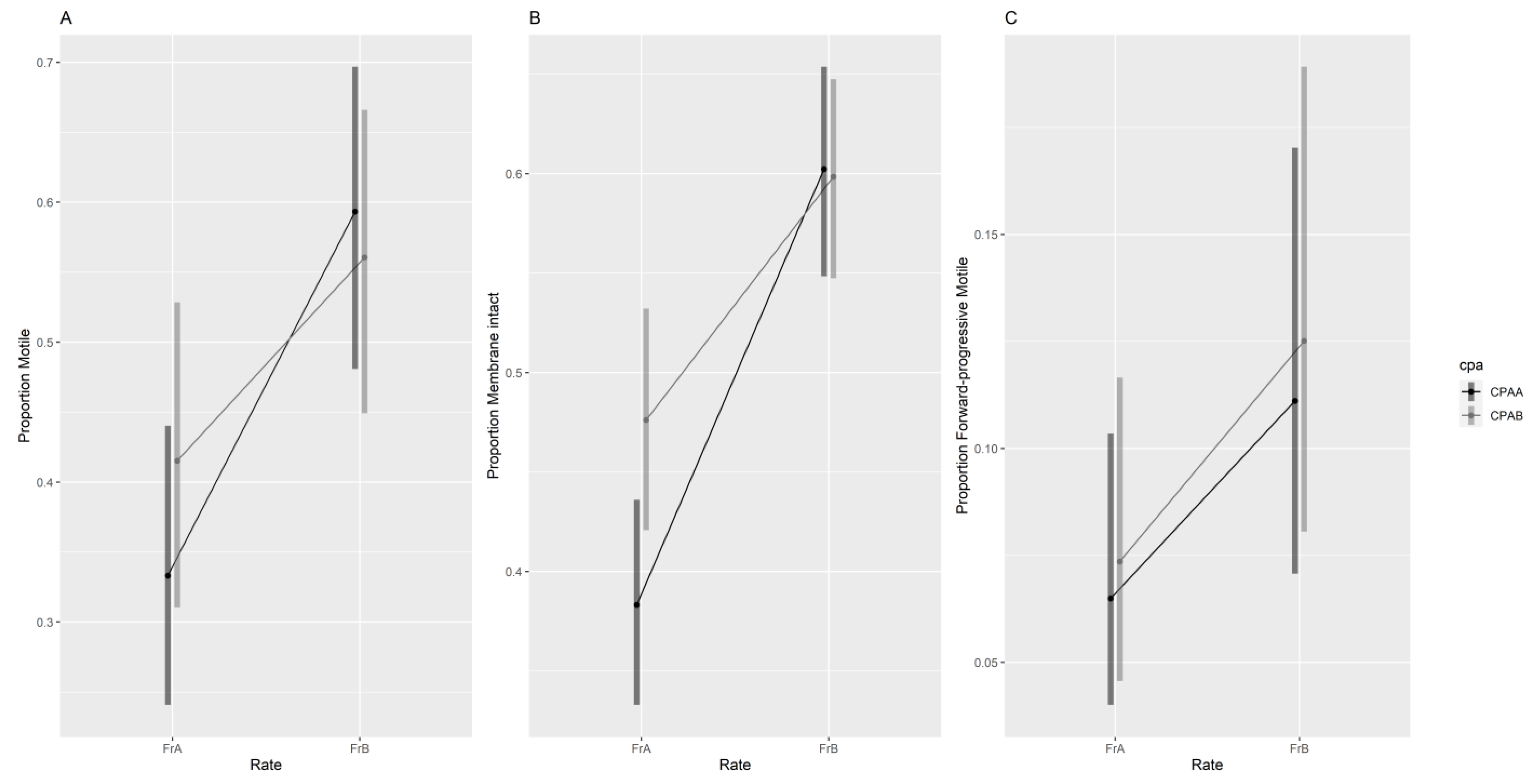
3.2.1. Manual Assessment of Sperm Quality
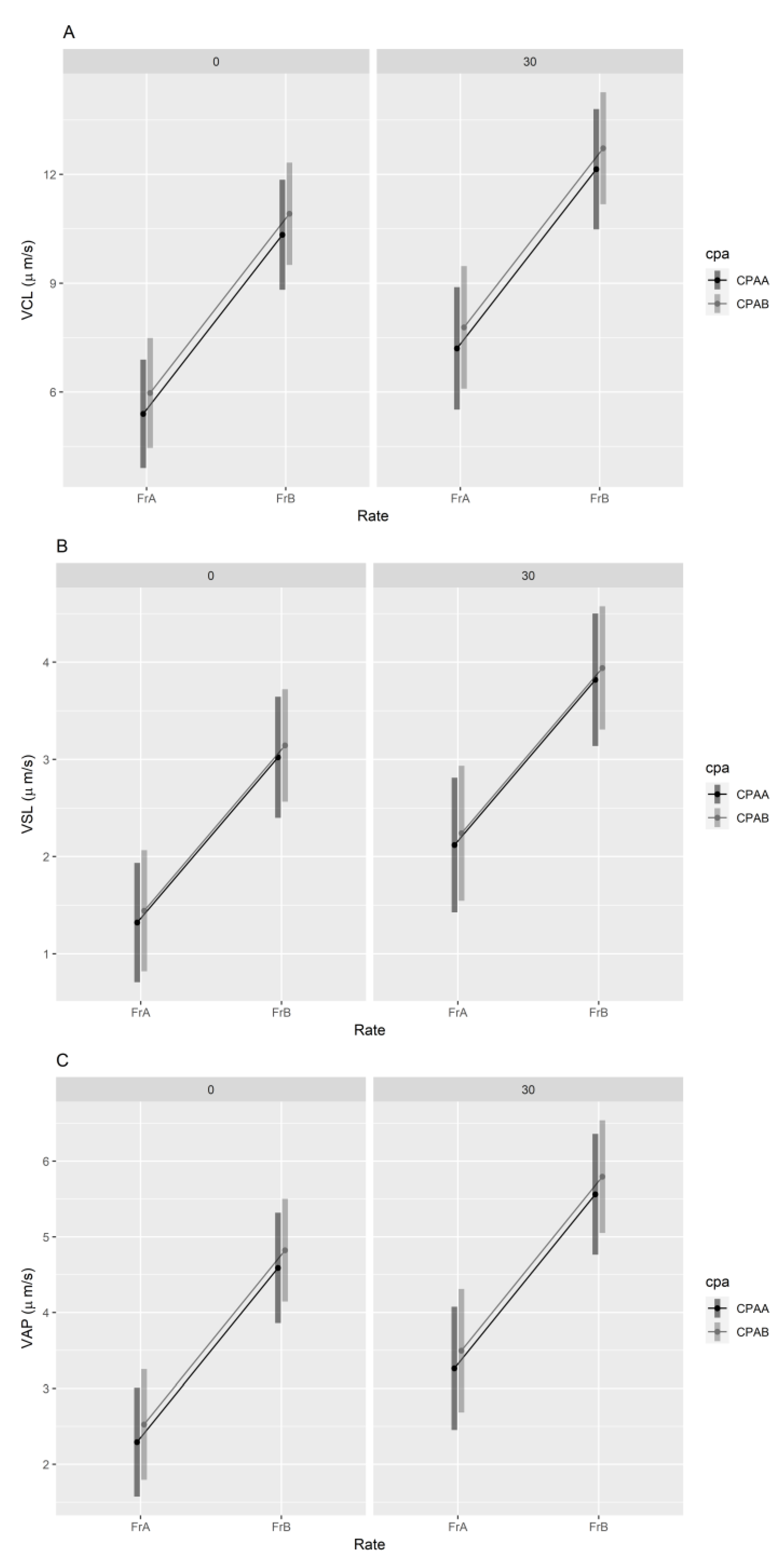
3.2.2. Sperm Velocity
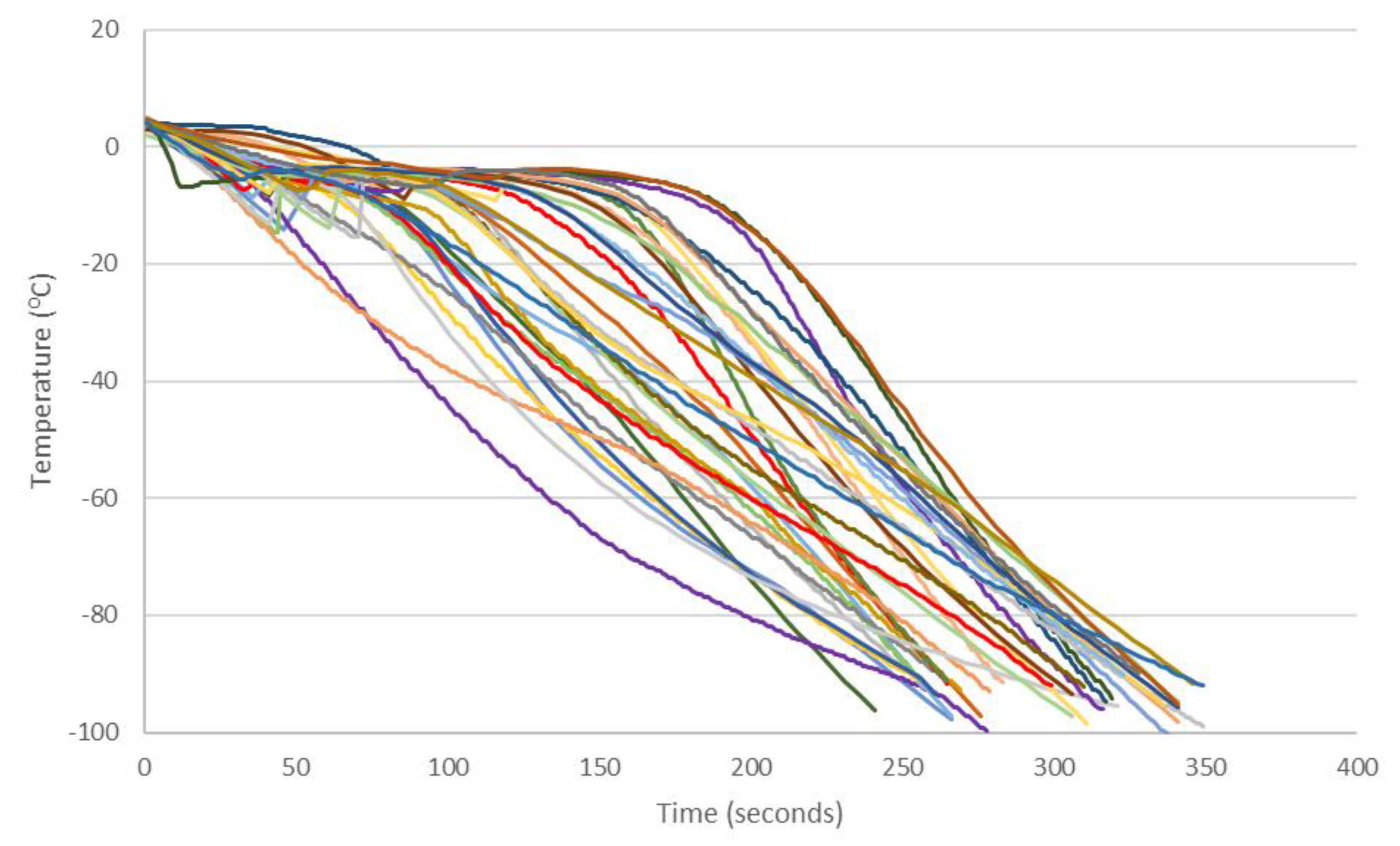
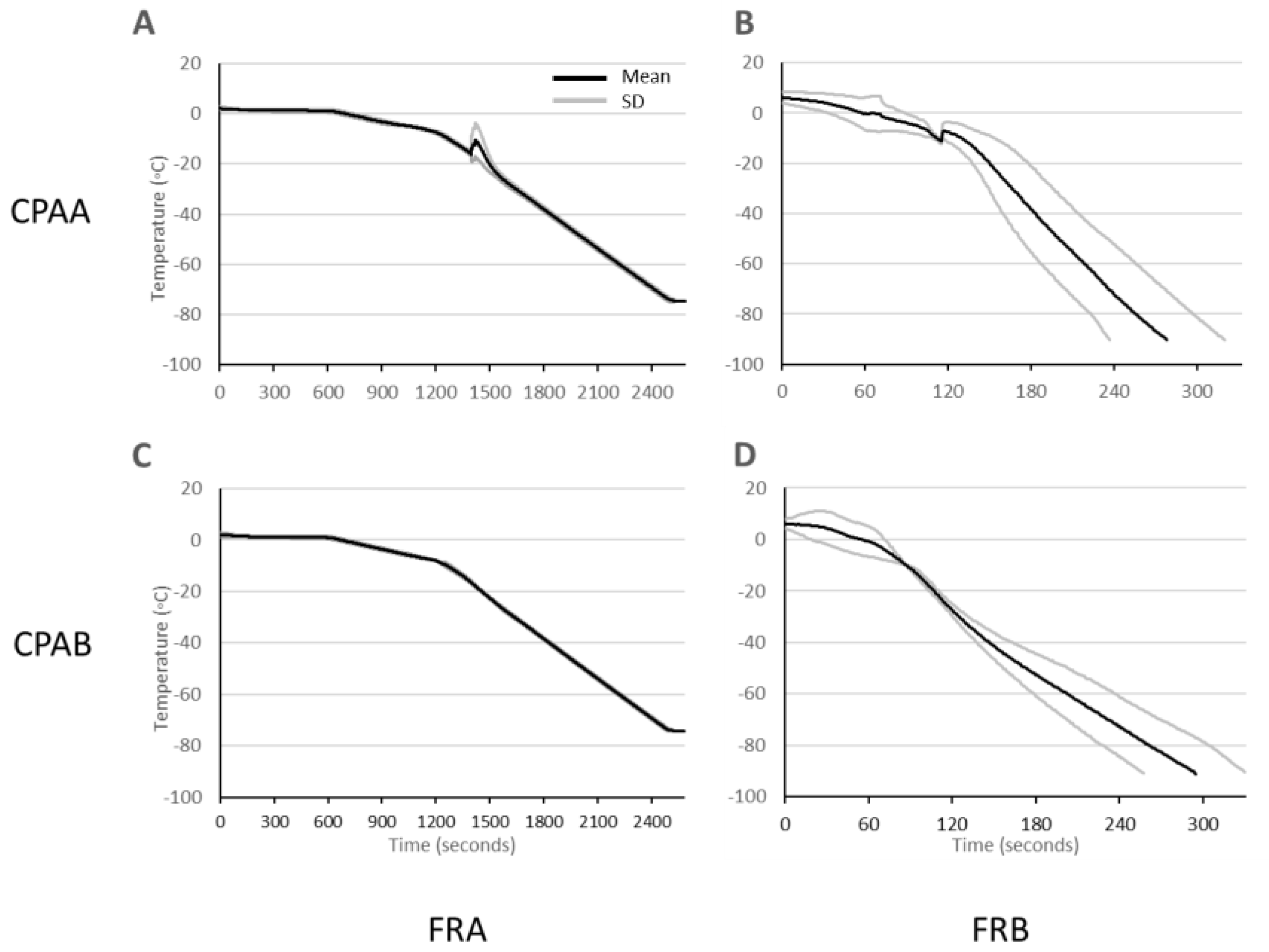
3.3. Cooling Rates
3.4. Effects of Sperm Dilution Rate on Post-Thaw Sperm Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- International Union for Conservation of Nature Species Survival Commission, Amphibian Specialist Group. Amphibian Conservation Action Plan; IUCN: Gland, Switzerland; Cambridge, UK, 2007; p. 64. [Google Scholar]
- International Union for Conservation of Nature Species Survival Commission, Amphibian Specialist Group. The Amphibian Conservation Action Plan (ACAP): A Status Review and Roadmap for Global Amphibian Conservation; The World Conservation Union (IUCN): Gland, Switzerland, 2022; Volume Preprint. [Google Scholar]
- Howell, L.G.; Mawson, P.R.; Frankham, R.; Rodger, J.C.; Upton, R.M.O.; Witt, R.R.; Calatayud, N.E.; Clulow, S.; Clulow, J. Integrating biobanking could produce significant cost benefits and minimise inbreeding for Australian amphibian captive breeding programs. Reprod. Fertil. Dev. 2021, 33, 573–587. [Google Scholar] [CrossRef]
- Della Togna, G.; Howell, L.G.; Clulow, J.; Langhorne, C.J.; Marcec-Greaves, R.; Calatayud, N.E. Evaluating amphibian biobanking and reproduction for captive breeding programs according to the Amphibian Conservation Action Plan objectives. Theriogenology 2020, 150, 412–431. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; Calatayud, N.E.; Trudeau, V.L. Amphibian reproductive technologies: Approaches and welfare considerations. Conserv. Physiol. 2021, 9, coab011. [Google Scholar] [CrossRef] [PubMed]
- Anastas, Z.M.; Byrne, P.G.; O’Brien, J.K.; Hobbs, R.J.; Upton, R.; Silla, A.J. The Increasing Role of Short-Term Sperm Storage and Cryopreservation in Conserving Threatened Amphibian Species. Animals 2023, 13, 2094. [Google Scholar] [CrossRef]
- Clulow, J.; Upton, R.; Trudeau, V.L.; Clulow, S. Amphibian Assisted Reproductive Technologies: Moving from Technology to Application. In Reproductive Sciences in Animal Conservation; Comizzoli, P., Brown, J.L., Holt, W.V., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 413–463. [Google Scholar]
- Strand, J.; Thomsen, H.; Jensen, J.B.; Marcussen, C.; Nicolajsen, T.B.; Skriver, M.B.; Søgaard, I.M.; Ezaz, T.; Purup, S.; Callesen, H.; et al. Biobanking in amphibian and reptilian conservation and management: Opportunities and challenges. Conserv. Genet. Resour. 2020, 12, 709–725. [Google Scholar] [CrossRef]
- Clulow, J.; Upton, R.M.O.; Trudeau, V.L.; Clulow, S. Cryopreservation of amphibian genomes: Targeting the Holy Grail, cryopreservation of maternal haploid and embryonic diploid genomes. In Reproductive Technologies and Biobanking as Tools for the Conservation of Amphibians; Silla, A., Kouba, A., Heatwole, H., Eds.; CSIRO: Sydney, Australia, 2022. [Google Scholar]
- Harding, G.; Griffiths, R.A.; Pavajeau, L. Developments in amphibian captive breeding and reintroduction programs. Conserv. Biol. 2016, 30, 340–349. [Google Scholar] [CrossRef]
- McFadden, M.; Gilbert, D.; Bradfield, K.; Evans, M.; Marantelli, G.; Byrne, P. The role of ex situ amphibian conservation in Australia. In Status of Conservation and Decline of Amphibians: Australia, New Zealand, and Pacific Islands; Heatwole, H., Rowley, J., Eds.; CSIRO Publishing: Clayton South, VC, USA, 2018; Volume 11, pp. 125–140. [Google Scholar]
- Hughes, L.; Steffen, W.; Mullins, G.; Dean, A.; Weisbrot, E.; Rice, M. Summer of Crisis; Climate Council: Sidney, Australia, 2020; Available online: https://apo.org.au/node/277911 (accessed on 6 May 2020).
- Committee, T.S.S. Conservation Advice Litoria booroolongensis Booroolong Frog. 2021. Available online: https://www.environment.gov.au/biodiversity/threatened/species/pubs/1844-conservation-advice.pdf (accessed on 12 July 2023).
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. Available online: https://www.iucnredlist.org (accessed on 11 May 2023).
- Mansour, N.; Lahnsteiner, F.; Patzner, R.A. Optimization of the cryopreservation of African clawed frog (Xenopus laevis) sperm. Theriogenology 2009, 72, 1221–1228. [Google Scholar] [CrossRef]
- Mansour, N.; Lahnsteiner, F.; Patzner, R.A. Motility and cryopreservation of spermatozoa of European common frog, Rana temporaria. Theriogenology 2010, 74, 724–732. [Google Scholar] [CrossRef]
- Marcec-Graves, R.M. Development of Assisted Reproductive Technologies for Endangered North American Salamanders. Ph.D. Thesis, Mississippi State University, Starkville, CO, USA, 2016. [Google Scholar]
- Browne, R.K.; Clulow, J.; Mahony, M. The short-term storage and cryopreservation of spermatozoa from hylid and myobatrachid frogs. Cryo Lett. 2002, 23, 129–136. [Google Scholar]
- Upton, R.; Clulow, S.; Colyvas, K.; Mahony, M.; Clulow, J. Paradigm shift in frog sperm cryopreservation: Reduced role for non-penetrating cryoprotectants. Reproduction 2023, 165, 583–592. [Google Scholar] [CrossRef]
- Upton, R.; Clulow, S.; Calatayud, N.E.; Colyvas, K.; Seeto, R.G.Y.; Wong, L.A.M.; Mahony, M.J.; Clulow, J. Generation of reproductively mature offspring from the endangered green and golden bell frog Litoria aurea using cryopreserved spermatozoa. Reprod. Fertil. Dev. 2021, 33, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Upton, R.; Clulow, S.; Mahony, M.J.; Clulow, J. Generation of a sexually mature individual of the Eastern dwarf tree frog, Litoria fallax, from cryopreserved testicular macerates: Proof of capacity of cryopreserved sperm derived offspring to complete development. Conserv. Physiol. 2018, 6, coy043. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; Langhorne, C.J. Protocols for hormonally induced spermiation, and the cold storage, activation, and assessment of amphibian sperm. In Reproductive Technologies and Biobanking for the Conservation of Amphibians; CSIRO Publishing: London, UK, 2022; p. 106. [Google Scholar]
- Upton, R.; Natalie, E.C.; Simon, C.; Darcie, B.; Alana, L.B.; Kim, C.; Michael, M.; John, C. Refrigerated storage and cryopreservation of hormone induced sperm in a threatened frog. bioRxiv 2023. [Google Scholar] [CrossRef]
- Kaurova, S.A.; Uteshev, V.K.; Gapeyev, A.B.; Shishova, N.V.; Gakhova, E.N.; Browne, R.K.; Kramarova, L.I. Cryopreservation of spermatozoa obtained postmortem from the European common frog Rana temporaria. Reprod. Fertil. Dev. 2021, 33, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Shishova, N.R.; Uteshev, V.K.; Kaurova, S.A.; Browne, R.K.; Gakhova, E.N. Cryopreservation of hormonally induced sperm for the conservation of threatened amphibians with Rana temporaria as a model research species. Theriogenology 2011, 75, 220–232. [Google Scholar] [CrossRef]
- Langhorne, C.J. Developing Assisted Reproductive Technologies for Endangered North American Amphibians. Ph.D. Thesis, Mississippi State University, Starkville, CO, USA, 2016. [Google Scholar]
- Burger, I.J.; Lampert, S.S.; Kouba, C.K.; Morin, D.J.; Kouba, A.J. Development of an amphibian sperm biobanking protocol for genetic management and population sustainability. Conserv. Physiol. 2022, 10, coac032. [Google Scholar] [CrossRef]
- Burger, I.; Julien, A.R.; Kouba, A.J.; Barber, D.; Counsell, K.R.; Pacheco, C.; Krebs, J.; Kouba, C.K. Linking in-situ and ex-situ populations of threatened amphibians through genome banking. Conserv. Sci. Pract. 2021, 3, e525. [Google Scholar] [CrossRef]
- Ogielska, M.; Bartmahska, J. Spermatogenesis and Male Reproductive System in Amphibia—Anura. In Reproduction of Amphibians, 1st ed; Ogielska, M., Ed.; Science Publishers: New Hampshire, MA, USA, 2009; Volume 4, pp. 34–99. [Google Scholar]
- Zhou, W.; De Iuliis, G.N.; Dun, M.D.; Nixon, B. Characteristics of the Epididymal Luminal Environment Responsible for Sperm Maturation and Storage. Front. Endocrinol. 2018, 9, 59. [Google Scholar] [CrossRef]
- Bedford, J.M. Enigmas of mammalian gamete form and function. Biol. Rev. 2004, 79, 429–460. [Google Scholar] [CrossRef]
- Silla, A.J.; McFadden, M.S.; Byrne, P.G. Hormone-induced sperm-release in the critically endangered Booroolong frog (Litoria booroolongensis): Effects of gonadotropin-releasing hormone and human chorionic gonadotropin. Conserv. Physiol. 2019, 7, coy080. [Google Scholar] [CrossRef]
- Silla, A.J.; Keogh, L.M.; Byrne, P.G. Sperm motility activation in the critically endangered booroolong frog: The effect of medium osmolality and phosphodiesterase inhibitors. Reprod. Fertil. Dev. 2017, 29, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Keogh, L.M.; Byrne, P.G.; Silla, A.J. The effect of antioxidants on sperm motility activation in the Booroolong frog. Anim. Reprod. Sci. 2017, 183, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Silla, A.J.; Keogh, L.M.; Byrne, P.G. Antibiotics and oxygen availability affect the short-term storage of spermatozoa from the critically endangered booroolong frog, Litoria booroolongensis. Reprod. Fertil. Dev. 2015, 27, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.K.; Clulow, J.; Mahony, M.; Clark, A. Successful Recovery of Motility and Fertility of Cryopreserved Cane Toad (Bufo marinus) Sperm. Cryobiology 1998, 37, 339–345. [Google Scholar] [CrossRef]
- Roth, T.L.; Bush, L.M.; Wildt, D.E.; Weiss, R.B. Scimitar-Horned Oryx (Oryx dammah) Spermatozoa Are Functionally Competent in a Heterologous Bovine In Vitro Fertilization System after Cryopreservation on Dry Ice, in a Dry Shipper, or over Liquid Nitrogen Vapor1. Biol. Reprod. 1999, 60, 493–498. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.5 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 8 June 2023).
- Team, R.C. R: A language and Environment for Statstical Computing. R Foundation for Statistical Computing, Vienna, Austria 2022. Available online: http://www.r-project.org/ (accessed on 8 June 2023).
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.4.8. 2022. Available online: https://CRAN.Rproject.org/package=emmeans (accessed on 8 June 2023).
- Wickham, H. ggplot:Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Auguie, B. gridExtra: Miscellaneous Functions for "Grid" Graphics. R Package Version 2.3. 2017. Available online: https://CRAN.R-project.org/package=gridExtra (accessed on 8 June 2023).
- Mortimer, S.T.; Mortimer, D. Kinematics of human spermatozoa incubated under capacitating conditions. J. Androl. 1990, 11, 195–203. [Google Scholar] [CrossRef]
- Browne, R.K.; Clulow, J.; Mahony, M. The effect of saccharides on the post-thaw recovery of cane toad (Bufo marinus) spermatozoa. Cryo Lett. 2002, 23, 121–128. [Google Scholar]
- Browne, R.K.; Mahony, M.; Clulow, J. A comparison of sucrose, saline, and saline with egg-yolk diluents on the cryopreservation of cane toad (Bufo marinus) sperm. Cryobiology 2002, 44, 251–257. [Google Scholar] [CrossRef]
- Poo, S.; Hinkson, K.M. Applying cryopreservation to anuran conservation biology. Conserv. Sci. Pract. 2019, 1, e91. [Google Scholar] [CrossRef]
- Guy, E.L.; Gillis, A.B.; Kouba, A.J.; Barber, D.; Poole, V.; Marcec-Greaves, R.M.; Kouba, C.K. Sperm collection and cryopreservation for threatened newt species. Cryobiology 2020, 94, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Kouba, A.J.; Vance, C.K.; Frommeyer, M.A.; Roth, T.L. Structural and functional aspects of Bufo americanus spermatozoa: Effects of inactivation and reactivation. J. Exp. Zool. Part A Comp. Exp. Biol. 2003, 295A, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.Y.; Jamieson, B.G.M. The ultrastructure of the spermatozoa of bufonid and hylid frogs (Anura, Amphibia): Implications for phylogeny and fertilization biology. Zool. Scr. 1993, 22, 309–323. [Google Scholar] [CrossRef]
- Chen, D.M.; Moore, M.G.; Willis, E.L.; Kouba, A.J.; Kouba, C.K. The impact of time and environmental factors on the mitochondrial vesicle and subsequent motility of amphibian sperm. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 268, 111191. [Google Scholar] [CrossRef]
- Liu, J.; Hermo, L.; Ding, D.; Wei, C.; Mann, J.M.; Yan, X.; Melnick, A.F.; Wu, Y.; Withrow, A.; Cibelli, J.; et al. SYPL1 defines a vesicular pathway essential for sperm cytoplasmic droplet formation and male fertility. Nat. Commun. 2023, 14, 5113. [Google Scholar] [CrossRef]
- Burger, I.J.; Chen, L.-D.; Lampert, S.S.; Kouba, C.K.; Barber, D.; Smith, D.; Cobos, C.; Kouba, A.J. Applying sperm collection and cryopreservation protocols developed in a model amphibian to three threatened anuran species targeted for biobanking management. Biol. Conserv. 2023, 277, 109850. [Google Scholar] [CrossRef]
- Mazur, P. Kinetics of water loss from cells at subzero temperatures and the likelihood of intracellular freezing. J. Gen. Physiol. 1963, 47, 347–369. [Google Scholar] [CrossRef]
- Naranjo, R.E.; Naydenova, E.; Proaño-Bolaños, C.; Vizuete, K.; Debut, A.; Arias, M.T.; Coloma, L.A. Development of assisted reproductive technologies for the conservation of Atelopus sp. (spumarius complex). Cryobiology 2022, 105, 20–31. [Google Scholar] [CrossRef]
- Browne, R.K.; Kaurova, S.A.; Uteshev, V.K.; Shishova, N.V.; McGinnity, D.; Figiel, C.R.; Mansour, N.; Agnew, D.; Wu, M.; Gakhova, E.N.; et al. Sperm motility of externally fertilizing fish and amphibians. Theriogenology 2015, 83, 1–13.e18. [Google Scholar] [CrossRef]
- Levitan, D.R. Sperm limitation, gamete competition and sexual selection in external fertilisers. In Sperm Competition and Sexual Selection; Birkhead, T.R., Moller, A.P., Eds.; Academic Press: London, UK, 1998; pp. 175–218. [Google Scholar]
- Silla, A.J.; Byrne, P.G. The Role of Reproductive Technologies in Amphibian Conservation Breeding Programs. Annu. Rev. Anim. Biosci. 2019, 7, 499–519. [Google Scholar] [CrossRef]
- Clulow, J.; Pomering, M.; Herbert, D.; Upton, R.; Calatayud, N.; Clulow, S.; Mahony, M.J.; Trudeau, V.L. Differential success in obtaining gametes between male and female Australian temperate frogs by hormonal induction: A review. Gen. Comp. Endocrinol. 2018, 265, 141–148. [Google Scholar] [CrossRef]
- Arregui, L.; Kouba, A.J.; Germano, J.M.; Barrios, L.; Moore, M.; Kouba, C.K. Fertilization potential of cold-stored Fowler’s toad (Anaxyrus fowleri) spermatozoa: Temporal changes in sperm motility based on temperature and osmolality. Reprod. Fertil. Dev. 2022, 34, 461–469. [Google Scholar] [CrossRef]
- Browne, R.; Clulow, J.; Mahony, M. Short-term storage of cane toad (Bufo marinus) gametes. Reproduction 2001, 121, 167–173. [Google Scholar] [CrossRef]
- Della Togna, G.; Gratwicke, B.; Evans, M.; Augustine, L.; Chia, H.; Bronikowski, E.; Murphy, J.B.; Comizzoli, P. Influence of extracellular environment on the motility and structural properties of spermatozoa collected from hormonally stimulated Panamanian Golden Frog (Atelopus zeteki). Theriogenology 2018, 108, 153–160. [Google Scholar] [CrossRef]
- Christensen, J.R.; Pauli, B.D.; Richardson, J.S.; Bishop, C.A.; Elliott, J. Effects of pH and dilution on African clawed frog (Xenopus laevis) sperm motility. Can. J. Zool. 2004, 82, 555–563. [Google Scholar] [CrossRef]
- Watanabe, A.; Takayama-Watanabe, E.; Vines, C.A.; Cherr, G.N. Sperm motility-initiating substance in newt egg-jelly induces differential initiation of sperm motility based on sperm intracellular calcium levels. Dev. Growth Differ. 2011, 53, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Ukita, M.; Itoh, T.; Watanabe, T.; Watanabe, A.; Onitake, K. Substances for the Initiation of Sperm Motility in Egg-Jelly of the Japanese Newt, Cynops pyrrhogaster. Zool. Sci. 1999, 16, 793–802. [Google Scholar] [CrossRef]
- Turani, B.; Aliko, V.; Faggio, C. Allurin and egg jelly coat impact on in-vitro fertilization success of endangered Albanian water frog, Pelophylax shqipericus. Nat. Prod. Res. 2020, 34, 830–837. [Google Scholar] [CrossRef]
- Reinhart, D.; Ridgway, J.; Chandler, D.E. Xenopus laevis fertilisation: Analysis of sperm motility in egg jelly using video light microscopy. Zygote 1998, 6, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Tholl, N.; Naqvi, S.; McLaughlin, E.; Boyles, S.; Bieber, A.L.; Chandler, D.E. Swimming of Xenopus laevis sperm exhibits multiple gears and its duration is extended by egg jelly constituents. Biol. Bull. 2011, 220, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Hosono, J.; Kanatani, H.; Katagiri, C. Toad egg-jelly as a source of divalent cations essential for fertilization. Dev. Biol. 1984, 105, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, G. Status of Sperm Functionality Assessment in Wildlife Species: From Fish to Primates. Animals 2021, 11, 1491. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Set Point |
|---|---|
| Camera | |
| Exposure (ms) | 4 |
| Gain | 300 |
| Integrate Enabled | False |
| Integrate Time (ms) | 500 |
| Cell Detection | |
| Elongation Maximum (%) | 30 |
| Elongation Minimum (%) | 1 |
| Enable Advanced Tail Detection | False |
| Head Brightness Minimum | 150 |
| Head Size Maximum (µm2) | 45 |
| Head Size Minimum (µm2) | 5 |
| Static Tail Filter | False |
| Tail Brightness Minimum | 50 |
| Tail Min Brightness auto Offset | 10 |
| Tail Min Brightness Mode | Auto—First Frame |
| Chamber | |
| Capillary Correction | 1.3 |
| Illumination | |
| Histogram Smooth Width | 0 |
| Max Photometer | 70 |
| Min Photometer | 50 |
| Kinematics | |
| Cell Travel Max (µm) | 10 |
| Enable Motile Static Collision Avoidance | False |
| Motile Cells Require a Tail | False |
| Motile Require Tails Max VSL (µms−1) | 0 |
| Progressive STR (%) | 0 |
| Progressive VAP (µms−1) | 5 |
| Slow VAP (µms−1) | 2 |
| Slow VSL (µms−1) | 0 |
| Static Algorithm | Width_Multiplier |
| Static VAP (µms−1) | 0 |
| Static VSL (µms−1) | 0 |
| Static Width Multiplier | 0.3 |
| Male Group | A | A | B | B | C | C | D | DE * |
|---|---|---|---|---|---|---|---|---|
| Number of males in pool (n) | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 5 |
| Collection pool (hours post-injection) | 1–3 | 4–6 | 1–3 | 4–6 | 1–3 | 4–6 | 1–3 | 4–6 |
| Sperm concentration (×106/mL) | 15.2 | 10.1 | 21.0 | 17.9 | 14.5 | 10.5 | 38.5 a | 63.0 b |
| Progressive motility (%) | 82% | 72% | 85% | 80% | 41% | 85% | 75% | 73% |
| Total motility (%) | 87% | 78% | 93% | 84% | 85% | 92% | 92% | 91% |
| Membrane intact sperm (%) | 92% | NA | 93% | NA | 97% | 97% | 89% | 91% |
| Pre-Freeze | Post-Thaw | Post-Thaw | |
|---|---|---|---|
| [CPA] initial | - | 20% DMF + 20% TRE | 12% DMF + 12% TRE |
| Dilution ratio | 0 | 1:1 | 1:5 |
| Replicates (n straws) | 3 | 5 | 4 |
| Total motility (%) | 90.7 ± 6.7% | 56.2 ± 8.3% | 21.7 ± 2.8% |
| Progressive motility (%) | 68.3 ± 19.4% | 19.6 ± 5.7% | 1.0 ± 0.8% |
| Intact cells (%) | 86.3 ± 3.5% | 56.4 ± 10.0% | 22.8 ± 19.4% |
| VAP (µms−1) | - | 10.4 ± 5.4 | 4.5 ± 3.3 |
| VCL (µms−1) | - | 19.7 ± 8.9 | 11.7± 11.4 |
| VSL (µms−1) | - | 7.7 ± 4.6 | 3.6 ± 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobbs, R.J.; Upton, R.; Calatayud, N.E.; Silla, A.J.; Daly, J.; McFadden, M.S.; O’Brien, J.K. Cryopreservation Cooling Rate Impacts Post-Thaw Sperm Motility and Survival in Litoria booroolongensis. Animals 2023, 13, 3014. https://doi.org/10.3390/ani13193014
Hobbs RJ, Upton R, Calatayud NE, Silla AJ, Daly J, McFadden MS, O’Brien JK. Cryopreservation Cooling Rate Impacts Post-Thaw Sperm Motility and Survival in Litoria booroolongensis. Animals. 2023; 13(19):3014. https://doi.org/10.3390/ani13193014
Chicago/Turabian StyleHobbs, Rebecca J., Rose Upton, Natalie E. Calatayud, Aimee J. Silla, Jonathan Daly, Michael S. McFadden, and Justine K. O’Brien. 2023. "Cryopreservation Cooling Rate Impacts Post-Thaw Sperm Motility and Survival in Litoria booroolongensis" Animals 13, no. 19: 3014. https://doi.org/10.3390/ani13193014
APA StyleHobbs, R. J., Upton, R., Calatayud, N. E., Silla, A. J., Daly, J., McFadden, M. S., & O’Brien, J. K. (2023). Cryopreservation Cooling Rate Impacts Post-Thaw Sperm Motility and Survival in Litoria booroolongensis. Animals, 13(19), 3014. https://doi.org/10.3390/ani13193014






