Effects of Lactobacillus plantarum Postbiotics on Growth Performance, Immune Status, and Intestinal Microflora of Growing Minks
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Lactobacillus plantarum Probiotics
2.2. Animals, Facilities and Management
2.3. Experimental Design
2.4. Data Collection
2.4.1. Lactic Acid and Short-Chain Fatty Acids
2.4.2. Immunity
2.4.3. Intestinal Flora
2.5. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Immune Function
3.2.1. Serum Immune Indexes
3.2.2. Mucosal Immune Indexes
3.3. Intestinal Flora
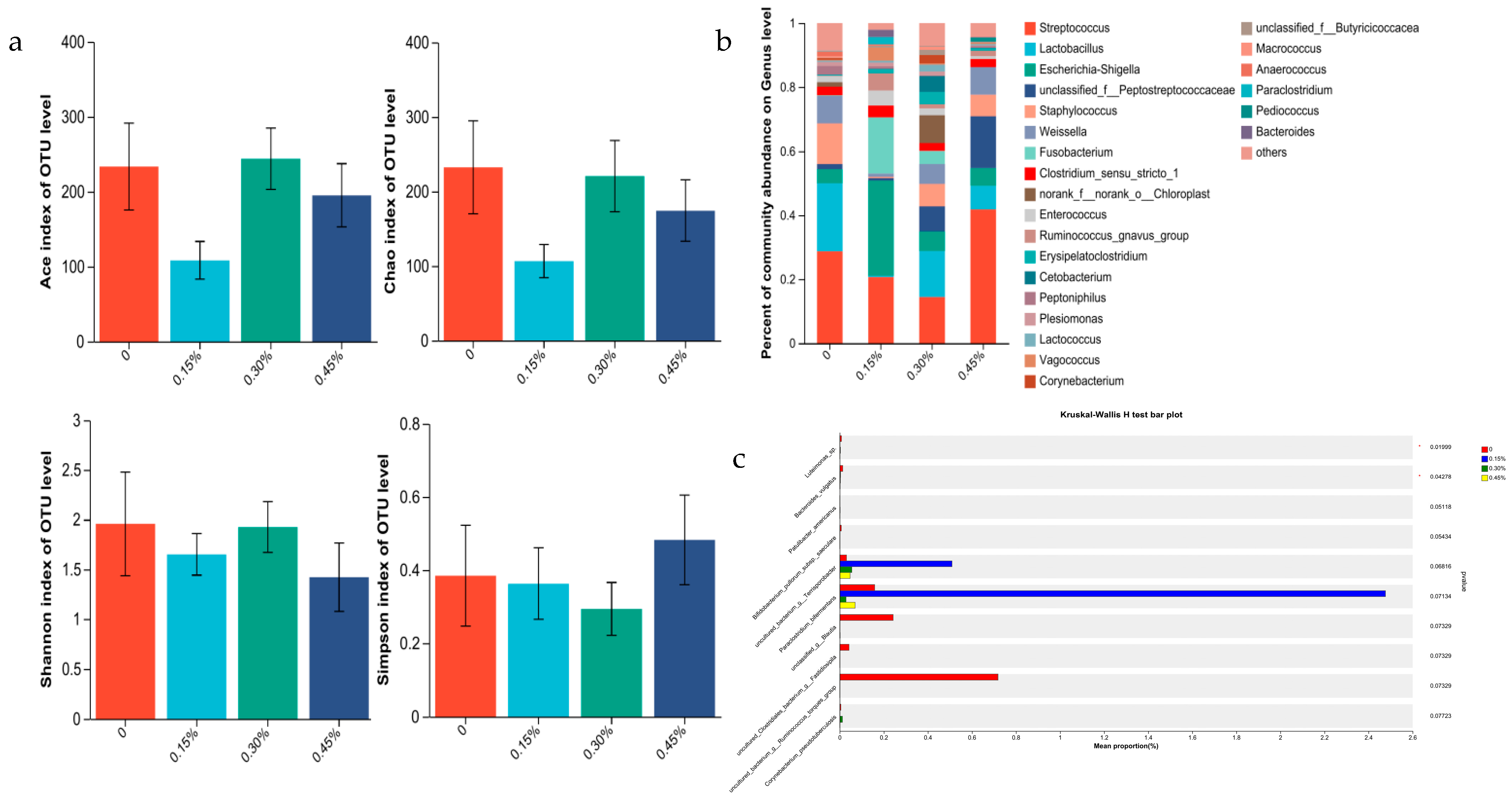
3.3.1. Intestine Microbiome of Male Minks
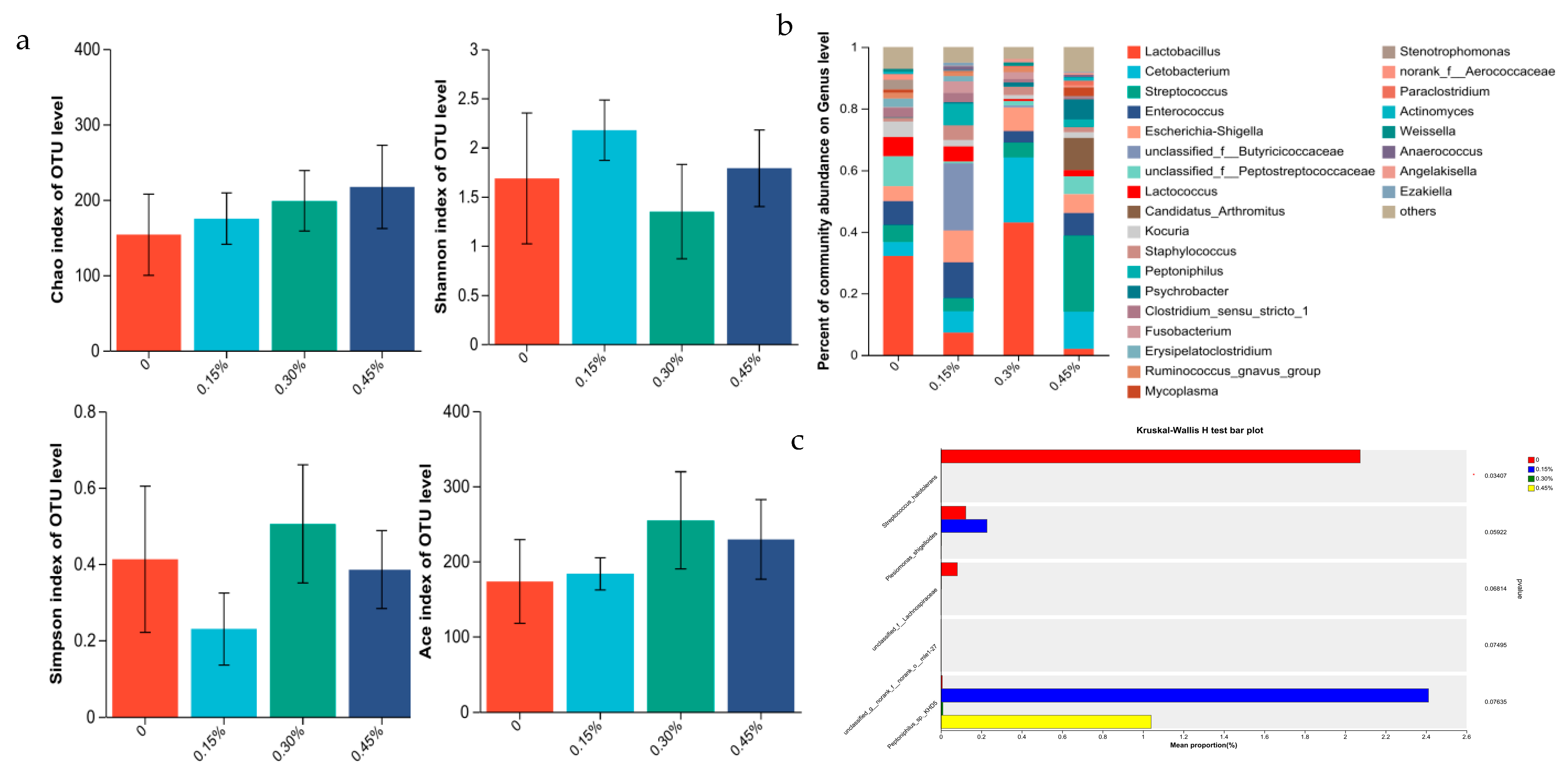
3.3.2. Intestine Microbiome of Female Minks
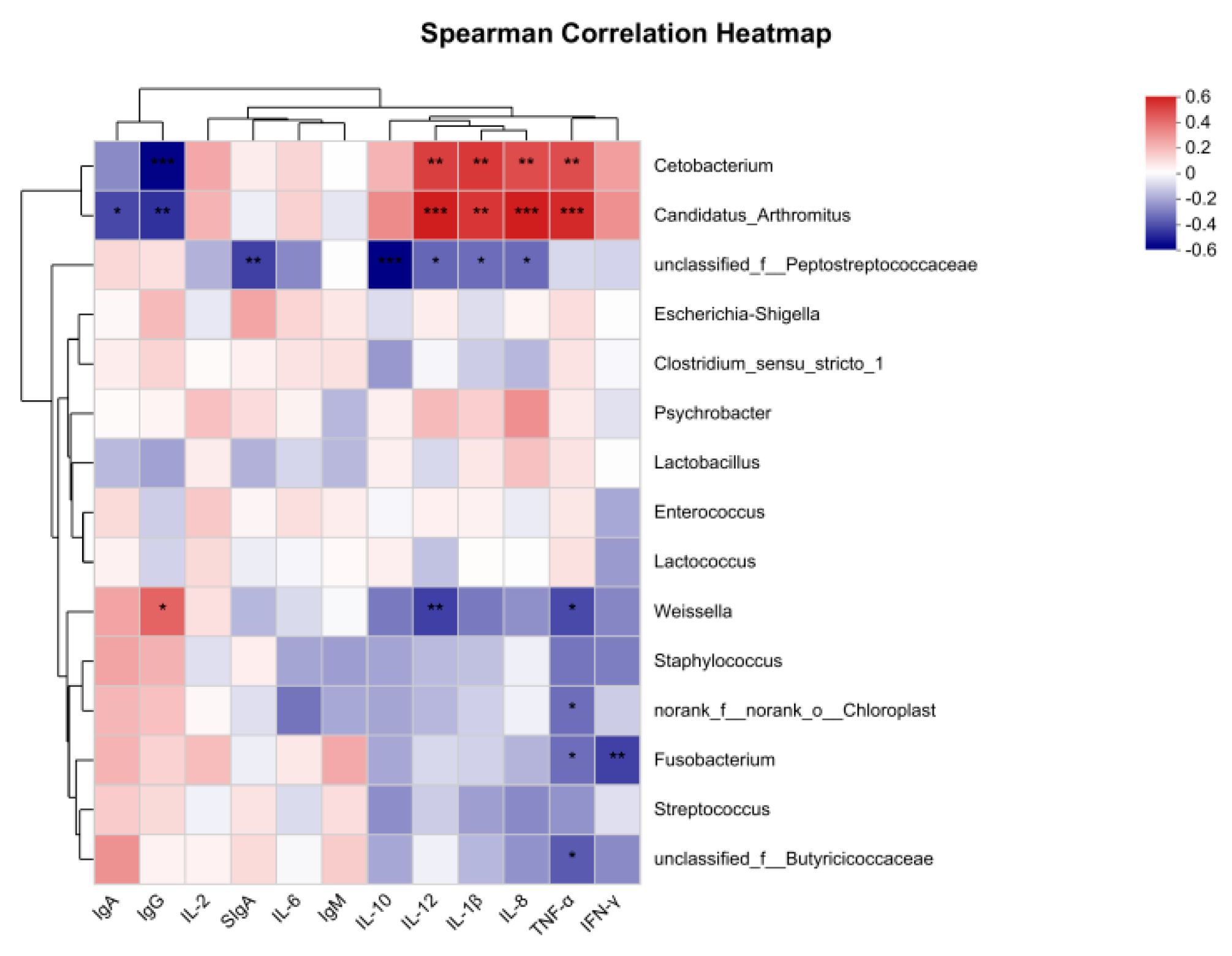
3.3.3. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marteau, P.R.; de Vrese, M.; Cellier, C.J.; Schrezenmeir, J. Protection from gastrointestinal diseases with the use of probiotics. Am. J. Clin. Nutr. 2001, 73, 430s–436s. [Google Scholar] [CrossRef] [PubMed]
- Ellen, M.S.; Daniel, J.M.; Gregor, R.; Glenn, R.G.; Robert, A.R. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar]
- Colin, H.; Francisco, G.; Gregor, R.; Glenn, R.G.; Daniel, J.M.; Bruno, P.; Lorenzo, M.; Berni, R.C.; Harry, J.F.; Seppo, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar]
- Chen, C.C.; Shih, Y.C.; Chiou, P.W.S.; Yu, B. Evaluating Nutritional Quality of Single Stage- and Two Stage-fermented Soybean Meal. Asian-Australas. J. Anim. Sci. 2010, 23, 598–606. [Google Scholar] [CrossRef]
- Am, J.M.; Joris, M.; Jeroen, D.; Stefaan, S.D. Fermented liquid feed for pigs: An ancient technique for the future. J. Anim. Sci. Biotechnol. 2015, 6, 4. [Google Scholar]
- Kullar, R.; Goldstein, E.J.C.; Johnson, S.; McFarland, L.V. Lactobacillus bacteremia and probiotics: A review. Microorganisms 2023, 11, 896. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.; Garcia-Varela, R.; Garcia, H.; Mata-Haro, V.; González-Córdova, A.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An evolving term within the functional foods field. Trends. Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Seppo, S.; Carmen, M.C.; Akihito, E.; Colin, H.; Sarah, L.; Eamonn, M.M.Q.; Ellen, M.S.; Raanan, S.; Jonathan, R.S.; Hania, S.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar]
- María Remes-Troche, J.; Coss-Adame, E.; Ángel Valdovinos-Díaz, M.; Gómez-Escudero, O.; Eugenia Icaza-Chávez, M.; Antonio Chávez-Barrera, J.; Zárate-Mondragón, F.; Antonio Velarde-Ruíz Velasco, J.; Rafael Aceves-Tavares, G.; Antonio Lira-Pedrín, M.; et al. Lactobacillus acidophilus LB: A useful pharmabiotic for the treatment of digestive disorders. Ther. Adv. Gastroenterol. 2020, 13, 1756284820971201. [Google Scholar] [CrossRef]
- Humam, M.A.; Loh, C.T.; Foo, L.H.; Samsudin, A.A.; Mustapha, M.N.; Zulkifli, I.; Izuddin, I.W. Effects of Feeding Different Postbiotics Produced by Lactobacillus plantarum on Growth Performance, Carcass Yield, Intestinal Morphology, Gut Microbiota Composition, Immune Status, and Growth Gene Expression in Broilers under Heat Stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef]
- Stefania, M.D.; Marzia, S.; Diana, M.; Miranda, P.; Giovanna, T.; Rita, P.; Donatella, P. Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS. Evid.-Based Complement. Altern. Med. 2018, 2018, 1756308. [Google Scholar]
- Shi, J.M.; Shi, D.B.; Xue, Y.L.; Luo, M.; Ruan, C.X.; Ruan, W.D. Research progress and application prospect of epigenetic element. Ejmnc 2022, 9, 800–807. [Google Scholar]
- Cuevas-González, P.F.; Liceaga, A.M.; Aguilar-Toalá, J.E. Postbiotics and paraprobiotics: From concepts to applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- LI, B.; Zhang, K.; Li, C.; Wang, X.; Chen, Y.; Yang, Y. Characterization and comparison of microbiota in the gastrointestinaltracts of the goat (capra hircus) during preweaning development. Front. Microbiol. 2019, 10, 2125. [Google Scholar]
- Jin, Y.Y.; Xu, B.; Wang, L.Y.; Sun, Q.Y.; Xi, Y.Y.; Yuan, Y.Z.; Wang, G.L.; Fu, C.; Li, S.Y. Effects of enzymatic hydrolysis of artemisia annua combined with Bacillus licheniformis on growth performance and cecal flora of broilers. Chin. J. Org. Chem. 2021, 33, 3810–3820. [Google Scholar]
- Yi, X.G. Effects of heat-inactivated Lactobacillus plantarum on growth performance, intestinal morphology and immune-related gene expression in broilers. China Feed 2020, 34–38. [Google Scholar] [CrossRef]
- Yaseen, K.K.; Chwen, T.L.; Ling, H.F.; Henny, A.; Asmara, A.S. Effects of dietary postbiotic and inulin on growth performance, IGF1 and GHR mRNA expression, faecal microbiota and volatile fatty acids in broilers. BMC Vet. Res. 2016, 12, 163. [Google Scholar]
- Wu, F.; Liu, H.; Ma, S.L.; Xu, X.X.; Wu, L.F.; Pan, B.H. Effects of supplemental epigenin on growth performance and intestinal flora structure of weaned piglets without resistance. Chin. J. Anim. Sci. 2021, 57 (Suppl. 1), 253–256. [Google Scholar]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Emma, S.; Kim, P.D.; Tom, W.D.V. Postbiotics and Their Health Modulatory Biomolecules. Biomolecules 2022, 12, 1640. [Google Scholar]
- Leoschke, W.L. Nutrition and Nutritional Physiology of the Mink: A Historical Perspective; Trafford Publishing: Bloomington, IN, USA, 2011. [Google Scholar]
- Ehrenstein, M.R.; Cook, H.T.; Neuberger, M.S. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J. Exp. Med. 2000, 191, 1253–1258. [Google Scholar] [CrossRef]
- Bienenstock, J.; Gauldie, J.; Perey, D.Y. Synthesis of IgG, IgA, IgM by chicken tissues: Immunofluorescent and 14C amino acid incorporation studies. J. Immunol. 1973, 111, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Rol, N.; Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011, 4, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kaetzel, C.S. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol. Lett. 2014, 162, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Wu, X.T.; Yang, Y. Effect of probiotic lignocellulose on mucosal immune barrier of chicken cecum. J. Shanxi Agr. Sci. 2021, 49, 668–674. [Google Scholar]
- Phil-Dong, M.; Soo, J.L.; Hee-Yun, K.; Na-Ra, H.; Inyeong, K.; Hyung-Min, K.; Hyun-Ja, J. Heat-treated Lactobacillus plantarum increases the immune responses through activation of natural killer cells and macrophages on in vivo and in vitro models. J. Med. Microbiol. 2019, 68, 467–474. [Google Scholar]
- Bidisha, P.; Stephen, B.; Wendy, D.; Casey, M.; Carolina, S.; Christine, S.; Trygve, O.T. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin. Epigenetics 2015, 7, 112. [Google Scholar]
- Shenderov, B.A. Gut indigenous microbiota and epigenetics. Microb. Ecol. Health Dis. 2012, 23, 618. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Lu, Y.C.; Ou, C.C.; Lin, S.L.; Tsai, C.C.; Huang, C.T.; Lin, M.Y. Lactobacillus plantarum MYL26 induces endotoxin tolerance phenotype in Caco-2 cells. BMC Microbiol. 2013, 13, 190. [Google Scholar] [CrossRef]
- Cario, E.; Gerken, G.; Podolsky, D. Toll-Like Receptor 2 Controls Mucosal Inflammation by Regulating Epithelial Barrier Function. Gastroenterology 2007, 132, 1359–1374. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, P.S.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, A.D.; Kritchevsky, B.S.; et al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: From C. elegans to mice. GeroScience 2020, 42, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-kappaB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Geun, H.K.; Yeon, S.L.; Na-Ra, K.; Yeon, M.K.; Min, J.L.; Tae-Hoo, Y.; Kyun, S.C.; Kyun, D.C. Inhibitory effects of Lactobacillus plantarum lipoteichoic acid (LTA) on Staphylococcus aureus LTA-induced tumor necrosis factor-alpha production. J. Microbiol. Biotechnol. 2008, 18, 1191–1196. [Google Scholar]
- Veit, H.; Simon, R.; Stefanie, B.; Anne, K.; Bernd, J.; Thomas, G.; Stefan, E.; Gunther, H. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar]
- Kadowaki, N.; Ho, S.; Antonenko, S.; Malefyt, R.W.; Kastelein, R.A.; Bazan, F.; Liu, Y.J. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001, 194, 863–870. [Google Scholar] [CrossRef]
- Schneberger, D.; Caldwell, S.; Kanthan, R.; Singh, B. Expression of Toll-like receptor 9 in mouse and human lungs. J. Anat. 2013, 222, 495–503. [Google Scholar] [CrossRef]
- Lee, J.; Mo, J.H.; Katakura, K.; Alkalay, I.; Rucker, A.N.; Liu, Y.T.; Lee, H.K.; Shen, C.; Cojocaru, G.; Shenouda, S.; et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat. Cell Biol. 2006, 8, 1327–1336. [Google Scholar] [CrossRef]
- Kundu, P.; Blacher, E.; Elinav, E.; Pettersson, S. Our Gut Microbiome: The Evolving Inner Self. Cell 2017, 171, 1481–1493. [Google Scholar] [CrossRef]
- Bamba, T.; Matsuda, H.; Endo, M.; Fujiyama, Y. The pathogenic role of Bacteroides vulgatus in patients with ulcerative colitis. J. Gastroenterol. 1995, 30, 45–47. [Google Scholar]
- Lange, A.; Beier, S.; Steimle, A.; Autenrieth, I.B.; Huson, D.H.; Frick, J.S. Extensive Mobilome-Driven Genome Diversification in Mouse Gut-Associated Bacteroides vulgatus mpk. Genome Biol. Evol. 2016, 8, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.H.; Miller, J.M.; Rowley, S.J.; Hou, S.; Donachie, S.P. Draft Genome Sequence of a Novel Luteimonas sp. Strain from Coral Mucus, Hawai’i. Genome Announc. 2016, 4, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Korcz, E.; Kerényi, Z.; Varga, L. Dietary fibers, prebiotics, and exopolysaccharides produced by lactic acid bacteria: Potential health benefits with special regard to cholesterol-lowering effects. Food Funct. 2018, 9, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.Z.; Ho, Y.W.; Abdullah, N.; Jalaludin, S. Growth performance, intestinal microbial populations, and serum cholesterol of broilers fed diets containing Lactobacillus cultures. Poult. Sci. 1998, 77, 1259–1265. [Google Scholar] [CrossRef]
- Loh, T.C.; Choe, D.W.; Foo, H.L.; Qurni, A.S.; Hair, M.B. Effects of feeding different postbiotic metabolite combinations produced by Lactobacillus plantarum strains on egg quality and production performance, faecal parameters and plasma cholesterol in laying hens. BMC Vet. Res. 2014, 10, 149. [Google Scholar] [CrossRef]
- Thu, T.V.; Chwen, L.T.; Foo, H.L.; Yaakub, H.; Bejo, M.H. Effects of liquid metabolite combinations produced by Lactobacillus plantarum on growth performance, faeces characteristics, intestinal morphology and diarrhoea incidence in postweaning piglets. Trop. Anim. Health Prod. 2011, 43, 69–75. [Google Scholar] [CrossRef]
- Eslami, M.; Yousefi, B.; Kokhaei, P.; Moghadas, A.J.; Moghadam, B.S.; Arabkari, V.; Niazi, Z. Are probiotics useful for therapy of Helicobacter pylori diseases? Comp. Immunol. Microbiol. Infect. Dis. 2019, 64, 99–108. [Google Scholar] [CrossRef]
- Arancha, H.; Noelia, M.; Víctor, L.; Miguel, A.A.; Abelardo, M.; Borja, S. An extracellular Serine/Threonine-rich protein from Lactobacillus plantarum NCIMB 8826 is a novel aggregation-promoting factor with affinity to mucin. Appl. Environ. Microbiol. 2013, 79, 6059–6066. [Google Scholar]
- Tsuchiya, C.; Sakata, T.; Sugita, H. Novel ecological niche of Cetobacterium somerae, an anaerobic bacterium in the intestinal tracts of freshwater fish. Lett. Appl. Microbiol. 2008, 46, 43–48. [Google Scholar] [CrossRef]
- Qi, X.Z.; Zhang, Y.; Zhang, Y.L.; Luo, F.; Song, K.; Wang, G.X.; Ling, F. Vitamin B12 produced by Cetobacterium somerae improves host resistance against pathogen infection through strengthening the interactions within gut microbiota. Microbiome 2023, 11, 135. [Google Scholar] [CrossRef]
- Mannu, L.; Paba, A.; Daga, E.; Comunian, R.; Zanetti, S.; Duprè, L.; Sechi, L.A. Comparison of the incidence of virulence determinants and antibiotic resistance between Enterococcus faecium strains of dairy, animal and clinical origin. Int. J. Food Microbiol. 2003, 88, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Woo, G.J. Transfer of tetracycline resistance genes with aggregation substance in food-borne Enterococcus faecalis. Curr. Microbiol. 2015, 70, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.N. Discovery, Genome and Virulence Gene Analysis of Three New Species of Animal-Derived Streptococcus. Ph.D. Thesis, Shanxi University, Taiyuan, China, 2018. [Google Scholar]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Duarte, M.E.; Kim, S.W. Postbiotics effects of Lactobacillus fermentate on intestinal health, mucosa-associated microbiota, and growth efficiency of nursery pigs challenged with F18+ Escherichia coli. J. Anim. Sci. 2022, 100, skac210. [Google Scholar] [CrossRef] [PubMed]
| Items | Content |
|---|---|
| Sea fishes | 30.00 |
| Unhatched fertilized egg | 24.00 |
| Chicken ribs | 10.00 |
| Chicken head | 15.00 |
| Extruded corn | 7.00 |
| Chicken livers | 5.00 |
| Wheat bran | 3.00 |
| Spary-dried blood cells | 2.00 |
| Soybean meal | 3.00 |
| Premix 1 | 1.00 |
| Total | 100.00 |
| Nutrient levels | |
| DM | 41.91 |
| ME (MJ/kg) 2 | 16.59 |
| Ether extract | 21.55 |
| Crude protein | 34.66 |
| Calcium | 1.10 |
| Phosphorus | 1.21 |
| PLP | SEM | Gender | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.15% | 0.30% | 0.45% | Male | Female | PPLP | PGender | PGender×PLP | |||
| Wt/g | |||||||||||
| Initial (wk 0) | 1099.97 | 1128.10 | 1117.87 | 1122.38 | 32.65 | 1310.67 | 923.48 | 23.85 | 0.94 | <0.001 | 0.64 |
| Final (wk 8) | 1859.02 | 1967.36 | 2026.89 | 1941.19 | 68.22 | 2394.49 | 1502.74 | 48.43 | 0.36 | <0.001 | 0.58 |
| ADG, g | |||||||||||
| Wk 0 to wk 4 | 15.41 | 15.67 | 19.32 | 15.71 | 1.18 | 20.39 | 12.66 | 0.84 | 0.06 | <0.001 | 0.05 |
| wk 4 to wk 8 | 11.70 | 14.30 | 13.15 | 13.54 | 1.22 | 18.31 | 8.03 | 0.83 | 0.47 | <0.001 | 0.95 |
| Wk 0 to wk 8 | 13.55 | 14.99 | 16.23 | 14.62 | 0.89 | 19.35 | 10.34 | 0.62 | 0.17 | <0.001 | 032 |
| ADFI, g | |||||||||||
| wk 0 to wk 4 | 283.76 | 306.49 | 302.57 | 294.90 | 10.74 | 335.37 | 258.49 | 7.37 | 0.42 | <0.001 | 0.71 |
| wk 4 to wk 8 | 284.99 | 296.86 | 297.36 | 292.27 | 9.54 | 343.36 | 242.38 | 6.54 | 0.75 | <0.001 | 0.99 |
| wk 0 to wk 4 | 284.37 | 301.67 | 299.96 | 293.58 | 9.31 | 339.36 | 250.43 | 6.38 | 0.51 | <0.001 | 0.91 |
| G:F | |||||||||||
| wk 0 to wk 4 | 20.32 | 20.76 | 17.61 | 20.27 | 1.35 | 17.57 | 21.91 | 0.93 | 0.30 | 0.002 | 0.38 |
| wk 4 to wk 8 | 29.58 | 24.75 | 27.00 | 25.69 | 2.14 | 20.71 | 32.80 | 1.47 | 0.38 | <0.001 | 0.94 |
| wk 0 to wk 8 | 22.63 | 21.89 | 20.36 | 21.56 | 0.98 | 18.10 | 25.12 | 0.69 | 0.41 | <0.001 | 0.68 |
| PLP | SEM | Gender | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.15% | 0.30% | 0.45% | Male | Female | PPLP | PGender | PGender×PLP | |||
| IgA/(μg/mL) | 328.10 b | 346.33 ab | 360.42 a | 343.85 ab | 7.72 | 361.52 | 327.83 | 4.78 | 0.005 | <0.001 | 0.18 |
| IgM/(μg/mL) | 1569.73 b | 1712.11 ab | 1914.27 a | 1496.05 b | 81.29 | 1719.12 | 1626.96 | 65.05 | 0.003 | 0.24 | 0.07 |
| IgG/(g/L) | 46.38 | 45.07 | 42.68 | 43.76 | 2.52 | 51.29 | 37.65 | 1.08 | 0.37 | <0.001 | 0.57 |
| PLP | SEM | Gender | SEM | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.15% | 0.30% | 0.45% | Male | Female | PPLP | PGender | PGender×PLP | |||
| SIgA/(ng/mL) | 2105.11 b | 2234.11 b | 2370.64 a | 2229.69 b | 45.75 | 2241.54 | 2228.23 | 37.22 | 0.004 | 0.78 | 0.91 |
| IL-1β/(pg/mL) | 228.35 | 229.31 | 226.17 | 218.09 | 9.68 | 200.85 | 250.10 | 4.06 | 0.62 | <0.001 | 0.85 |
| IL-8/(pg/mL) | 129.29 a | 124.81 a | 111.10 b | 125.97 a | 6.14 | 107.20 | 138.39 | 3.17 | 0.02 | <0.001 | 0.59 |
| IL-2/(pg/mL) | 314.12 a | 304.31 a | 289.14 ab | 251.76 b | 17.23 | 255.21 | 324.45 | 10.88 | 0.02 | <0.001 | 0.82 |
| IL-6/(pg/mL) | 31.96 | 28.27 | 29.21 | 29.26 | 1.82 | 27.01 | 32.34 | 1.11 | 0.47 | 0.003 | 0.95 |
| IL-12/(pg/mL) | 22.30 | 23.13 | 21.97 | 21.28 | 1.34 | 18.82 | 25.53 | 0.61 | 0.54 | <0.001 | 0.47 |
| IL-10/(pg/mL) | 92.18 | 88.94 | 91.57 | 86.62 | 3.94 | 81.54 | 98.11 | 2.33 | 0.50 | <0.001 | 0.002 |
| TNF-α/(pg/mL) | 992.95 a | 799.21 b | 784.09 b | 892.03 ab | 99.96 | 576.28 | 1157.86 | 36.58 | 0.01 | <0.001 | 0.01 |
| IFN-γ/(pg/mL) | 738.87 ab | 698.74 ab | 635.42 b | 819.48 a | 46.43 | 619.48 | 826.78 | 28.53 | 0.01 | <0.001 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhen, S.; Cao, L.; Sun, F.; Wang, L. Effects of Lactobacillus plantarum Postbiotics on Growth Performance, Immune Status, and Intestinal Microflora of Growing Minks. Animals 2023, 13, 2958. https://doi.org/10.3390/ani13182958
Li Y, Zhen S, Cao L, Sun F, Wang L. Effects of Lactobacillus plantarum Postbiotics on Growth Performance, Immune Status, and Intestinal Microflora of Growing Minks. Animals. 2023; 13(18):2958. https://doi.org/10.3390/ani13182958
Chicago/Turabian StyleLi, Yalin, Shibo Zhen, Lin Cao, Fengxue Sun, and Lihua Wang. 2023. "Effects of Lactobacillus plantarum Postbiotics on Growth Performance, Immune Status, and Intestinal Microflora of Growing Minks" Animals 13, no. 18: 2958. https://doi.org/10.3390/ani13182958
APA StyleLi, Y., Zhen, S., Cao, L., Sun, F., & Wang, L. (2023). Effects of Lactobacillus plantarum Postbiotics on Growth Performance, Immune Status, and Intestinal Microflora of Growing Minks. Animals, 13(18), 2958. https://doi.org/10.3390/ani13182958




