Establishment and Characterization of a Spermatogonial Stem Cell Line from Tiger Puffer Fish (Takifugu rubripes)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Sample Preparation
2.2. Primary Culture and Subculture
2.3. Cryopreservation and Recovery of Cells
2.4. Cell Growth Studies
2.5. Chromosome Karyotype Analysis
2.6. Transfection with GFP Reporter Gene
2.7. Alkaline Phosphatase Staining
2.8. Fluorescence In Situ Hybridization
2.9. Total DNA and RNA Extraction and Polymerase Chain Reaction
2.10. Induced Differentiation of TrSSC In Vitro
3. Results
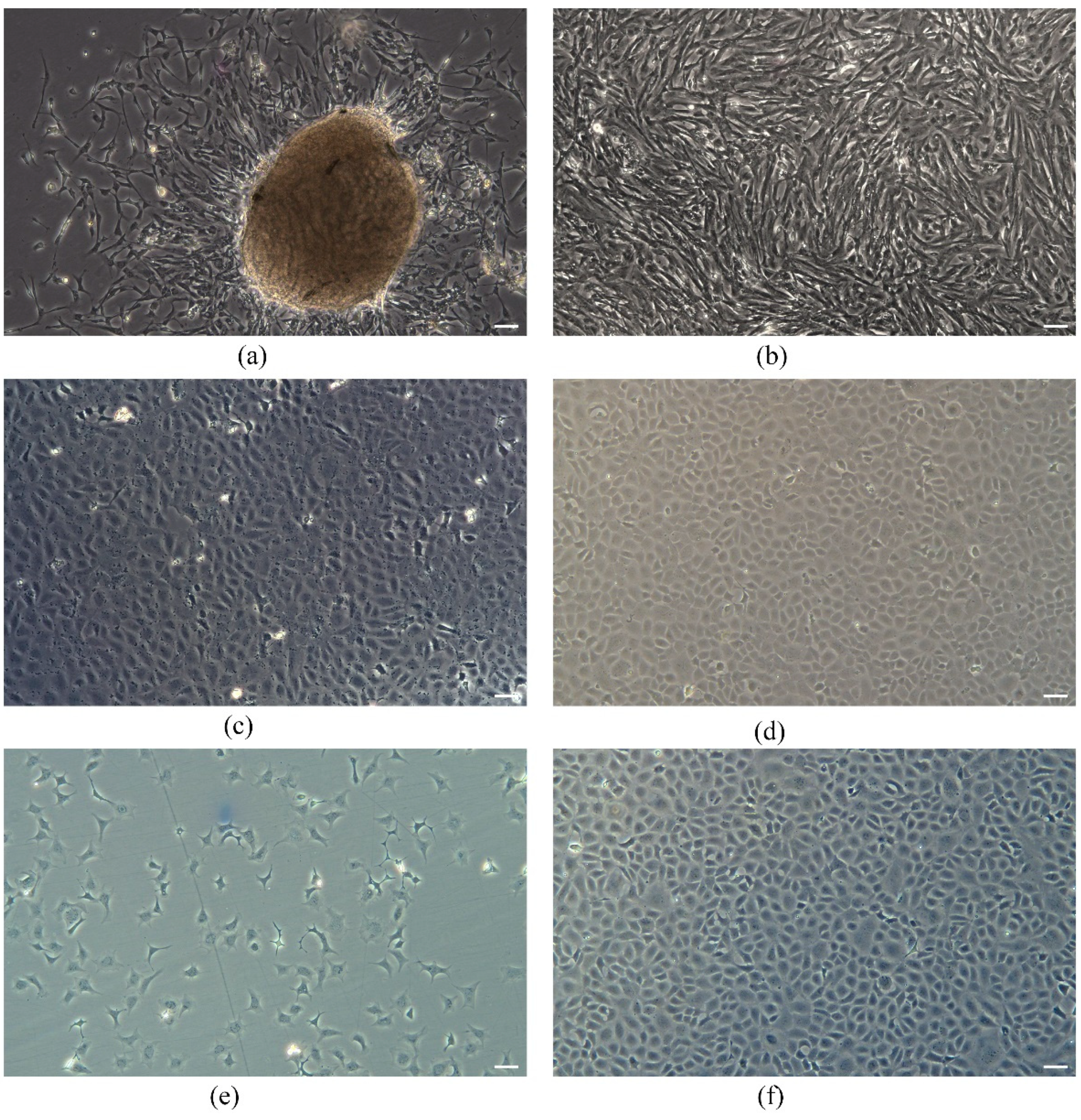
3.1. Establishment of a Spermatogonial Stem Cell Line
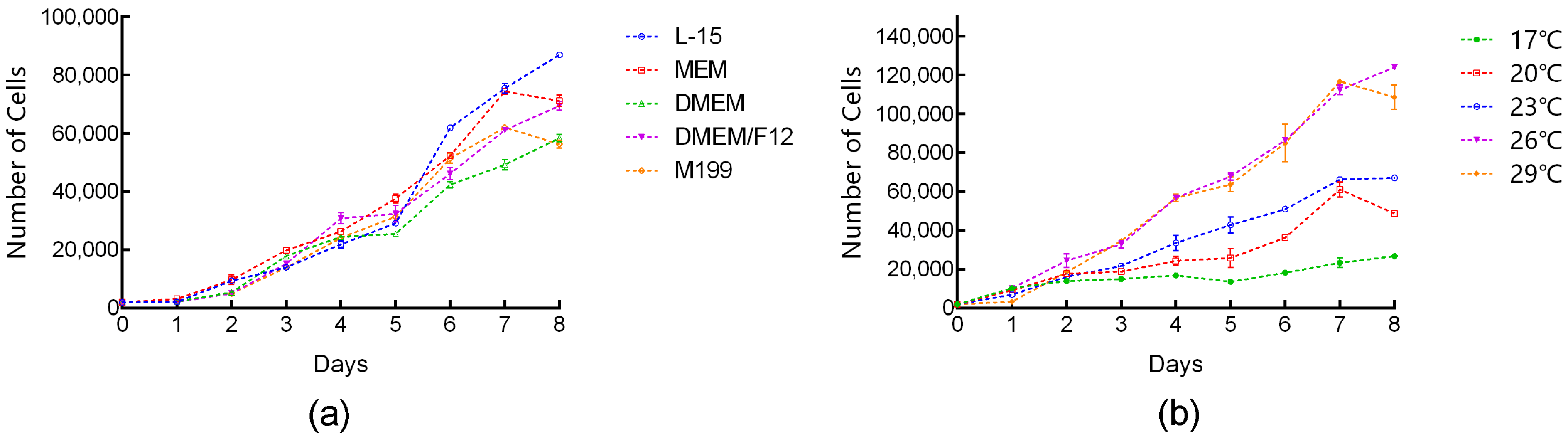
3.2. Effects of Temperature and Medium on Cell Growth
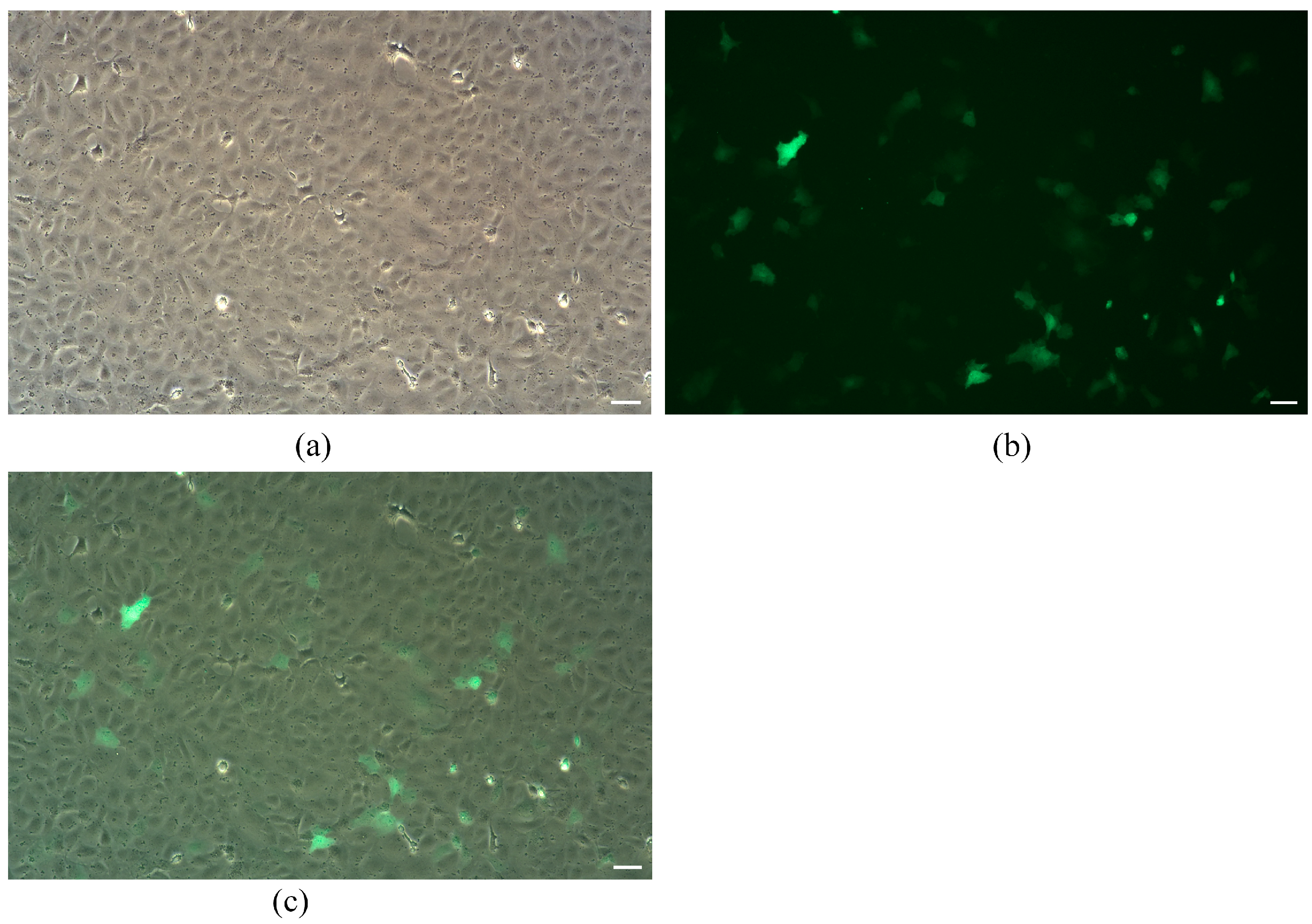
3.3. GFP Reporter Transfection
3.4. Chromosome Numbe
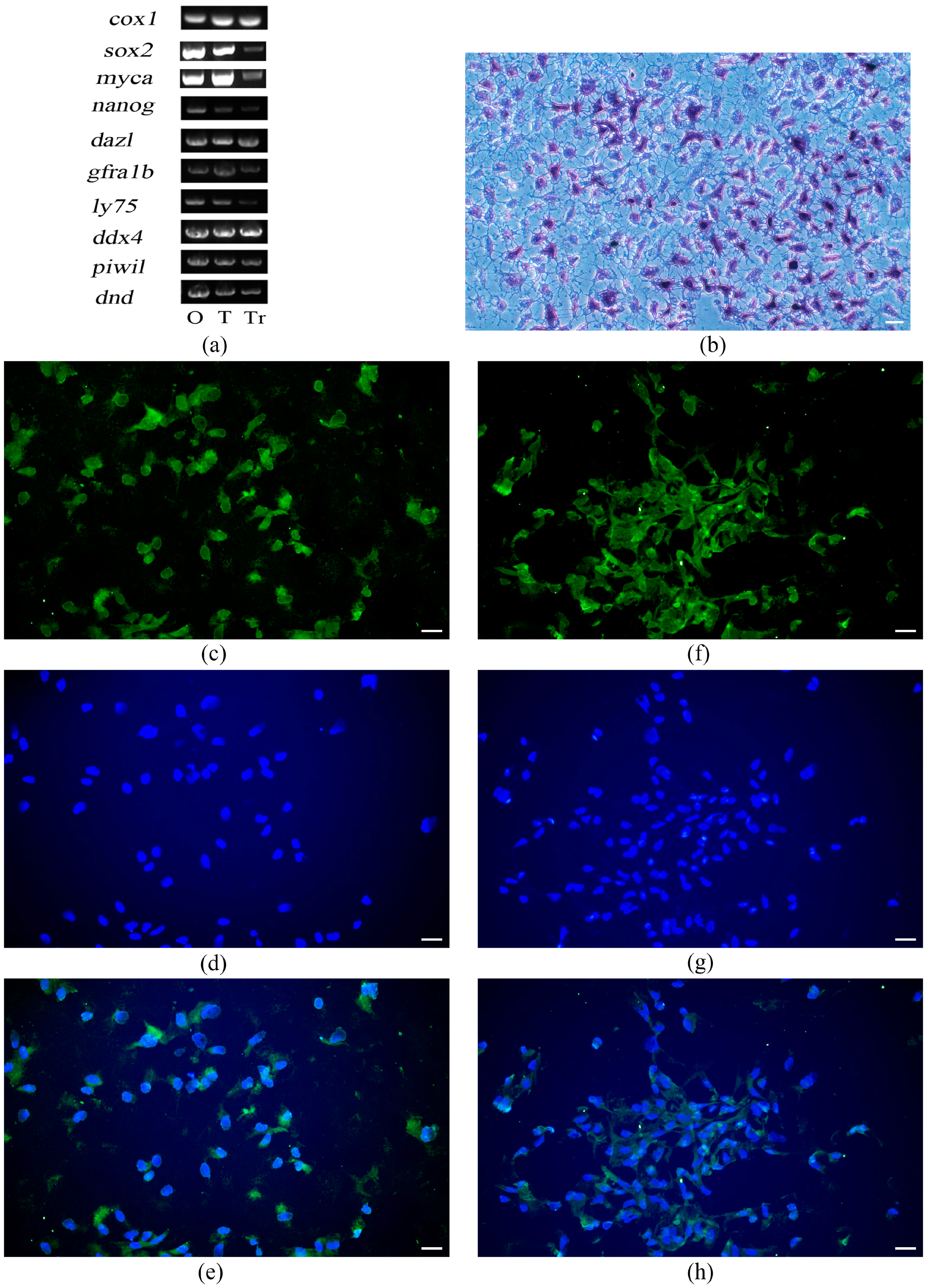
3.5. Characterization of the Spermatogonial Property of T. rubripes TrSSCs
3.6. Induced differentiation of TrSSC In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.; Li, M.; Gui, J.; Hong, Y. Fish germ cells. Sci. China Life Sci. 2010, 53, 435–446. [Google Scholar] [PubMed]
- de Rooij, D.G. Proliferation and differentiation of spermatogonial stem cells. Reproduction 2001, 121, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Meistrich, M.L.; van Beek, M.E.A.B. Spermatogonial stem cells. In Cell and Molecular Biology of the Testis; Desjardins, C., Ewing, L.L., Eds.; Oxford University Press: New York, NY, USA, 1993; pp. 266–295. [Google Scholar]
- Ohta, H.; Tohda, A.; Nishimune, Y. Proliferation and differentiation of spermatogonial stem cells in the w/wv mutant mouse testis. Biol. Reprod. 2003, 69, 1815–1821. [Google Scholar] [PubMed]
- Phillips, B.T.; Gassei, K.; Orwig, K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1663–1678. [Google Scholar]
- Hofmann, M.C.; Braydich-Stolle, L.; Dettin, L.; Johnson, E.; Dym, M. Immortalization of mouse germ line stem cells. Stem Cells 2005, 23, 200–210. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Miki, H.; Inoue, K.; Ogonuki, N.; Toyokuni, S.; Ogura, A.; Shinohara, T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol. Reprod. 2005, 72, 985–991. [Google Scholar] [CrossRef]
- van Pelt, A.M.; Roepers-Gajadien, H.L.; Gademan, I.S.; Creemers, L.B.; de Rooij, D.G.; van Dissel-Emiliani, F.M. Establishment of cell lines with rat spermatogonial stem cell characteristics. Endocrinology 2002, 143, 1845–1850. [Google Scholar] [CrossRef]
- Zheng, Y.; Feng, T.; Zhang, P.; Lei, P.; Li, F.; Zeng, W. Establishment of cell lines with porcine spermatogonial stem cell properties. J. Anim. Sci. Biotechnol. 2020, 11, 33. [Google Scholar] [CrossRef]
- Li, C.H.; Yan, L.Z.; Ban, W.Z.; Tu, Q.; Wu, Y.; Wang, L.; Bi, R.; Ji, S.; Ma, Y.H.; Nie, W.H.; et al. Long-term propagation of tree shrew spermatogonial stem cells in culture and successful generation of transgenic offspring. Cell Res. 2017, 27, 241–252. [Google Scholar] [CrossRef]
- Zhong, C.; Tao, Y.; Liu, M.; Wu, X.; Yang, Y.; Wang, T.; Meng, Z.; Xu, H.; Liu, X. Establishment of a Spermatogonial Stem Cell Line with Potential of Meiosis in a Hermaphroditic Fish, Epinephelus coioides. Cells 2022, 11, 2868. [Google Scholar] [CrossRef]
- Shikina, S.; Ihara, S.; Yoshizaki, G. Culture conditions for maintaining the survival and mitotic activity of rainbow trout transplantable type A spermatogonia. Mol. Reprod. Dev. Inc. Gamete Res. 2008, 75, 529–537. [Google Scholar]
- Shikina, S.; Yoshizaki, G. Improved in vitro culture conditions to enhance the survival, mitotic activity, and transplantability of rainbow trout type a spermatogonia. Biol. Reprod. 2010, 83, 268–276. [Google Scholar] [PubMed]
- Sun, X.; Tao, B.; Wang, Y.; Hu, W.; Sun, Y. Isolation and characterization of germline stem cells in protogynous hermaphroditic Monopterus albus. Int. J. Mol. Sci. 2022, 23, 5861. [Google Scholar] [CrossRef] [PubMed]
- Poursaeid, S.; Kalbassi, M.-R.; Hassani, S.-N.; Baharvand, H. Isolation, characterization, in vitro expansion and transplantation of Caspian trout (Salmo caspius) type a spermatogonia. Gen. Comp. Endocrinol. 2020, 289, 113341. [Google Scholar]
- Hong, Y.; Liu, T.; Zhao, H.; Xu, H.; Wang, W.; Liu, R.; Chen, T.; Deng, J.; Gui, J. Establishment of a normal medakafish spermatogonial cell line capable of sperm production in vitro. Proc. Natl. Acad. Sci. USA 2004, 101, 8011–8016. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kan, Y.; Zhong, Y.; Jawad, M.; Wei, W.; Gu, K.; Gui, L.; Li, M. Generation of a normal long-term-cultured Chinese hook snout carp spermatogonial stem cell line capable of sperm production in vitro. Biology 2022, 11, 1069. [Google Scholar]
- Cui, J.-Z.; Shen, X.-Y.; Gong, Q.-L.; Yang, G.-P.; Gu, Q.-Q. Identification of sex markers by cDNA-AFLP in Takifugu rubripes. Aquaculture 2006, 257, 30–36. [Google Scholar] [CrossRef]
- Shang, F.; Lu, Y.; Li, Y.; Han, B.; Wei, R.; Liu, S.; Liu, Y.; Liu, Y.; Wang, X. Transcriptome Analysis Identifies Key Metabolic Changes in the Brain of Takifugu rubripes in Response to Chronic Hypoxia. Genes 2022, 13, 1347. [Google Scholar]
- Jia, Y.; Jing, Q.; Zhai, J.; Guan, C.; Huang, B. Alternations in oxidative stress, apoptosis, and innate-immune gene expression at mRNA levels in subadult tiger puffer (Takifugu rubripes) under two different rearing systems. Fish Shellfish. Immunol. 2019, 92, 756–764. [Google Scholar]
- Yan, H.; Liu, Q.; Jiang, J.; Shen, X.; Zhang, L.; Yuan, Z.; Wu, Y.; Liu, Y. Identification of sex differentiation-related microRNA and long non-coding RNA in Takifugu rubripes gonads. Sci. Rep. 2021, 11, 7459. [Google Scholar]
- Li, W.; Xiong, Y.; Wang, Z.; Zhang, Q.; Shen, X.; Liu, Q.; Yan, H.; Gao, R.; Liu, Y.; Pang, H. Effects of different photoperiod conditions on survival, growth, and gonadal development of Takifugu rubripes adults. Aquaculture 2023, 564, 739048. [Google Scholar]
- Kim, S.-S.; Lee, K.-J. Dietary protein requirement of juvenile tiger puffer (Takifugu rubripes). Aquaculture 2009, 287, 219–222. [Google Scholar]
- Yoshikawa, H.; Ino, Y.; Shigenaga, K.; Katayama, T.; Kuroyanagi, M.; Yoshiura, Y. Production of tiger puffer Takifugu rubripes from cryopreserved testicular germ cells using surrogate broodstock technology. Aquaculture 2018, 493, 302–313. [Google Scholar]
- Chuda, H.; Matsuyama, M.; Ikeda, Y.; Matsuura, S. Development of the maturation-and ovulation-induction method in cultured tiger puffer Takifugu rubripes by hormonal treatments. Nippon. Suisan Gakkaishi (Jpn. Ed.) 1997, 63, 728–733. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Jia, S.; Xu, H.; Wang, X.; Lu, B.; Wu, W.; Huang, D.; Kong, L.; Kang, X.; Tian, F. A functionalized self-assembling peptide containing E7 and YIGSR sequences enhances neuronal differentiation of spermatogonial stem cells on aligned PCL fibers for spinal cord injury repair. Theranostics 2022, 12, 7567. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, Y.; Zhang, Y.; Wang, G.; He, Z.; Liu, Y.; Cao, W.; Wang, Y.; Chen, S.; Fu, Y. A New Cell Line Derived from the Spleen of the Japanese Flounder (Paralichthys olivaceus) and Its Application in Viral Study. Biology 2022, 11, 1697. [Google Scholar]
- Yoo, G.Y.; Lee, T.H.; Gil, H.W.; Lim, S.G.; Park, I.S. Cytogenetic analysis of hybrids and hybrid triploids between the river puffer, Takifugu obscurus, and the tiger puffer, Takifugu rubripes. Aquac. Res. 2018, 49, 637–650. [Google Scholar]
- Miyaki, K.; Tabeta, O.; Kayano, H. Karyotypes in six species of pufferfishes genus Takifugu (Tetraodontidae, Tetraodontiformes). Fish. Sci. 1995, 61, 594–598. [Google Scholar] [CrossRef]
- Zhong, Z.; Jiang, Y.; Zhao, L.; Wang, Y.; Zhang, Z. Establishment and characterization of the ovary cell line derived from two-spot puffer Takifugu bimaculatus and its application for gene editing and marine toxicology. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 264, 109528. [Google Scholar]
- Zhong, Z.; Wang, Y.; Feng, Y.; Xu, Y.; Zhao, L.; Jiang, Y.; Zhang, Z. The molecular regulation mechanism of dmrt1—Based on the establishment of the testis cell line derived from two-spot puffer Takifugu bimaculatus. Fish Physiol. Biochem. 2022, 48, 1475–1494. [Google Scholar]
- Singh, U.; Quintanilla, R.H.; Grecian, S.; Gee, K.R.; Rao, M.S.; Lakshmipathy, U. Novel live alkaline phosphatase substrate for identification of pluripotent stem cells. Stem Cell Rev. Rep. 2012, 8, 1021–1029. [Google Scholar] [PubMed]
- Patra, S.K.; Chakrapani, V.; Panda, R.P.; Mohapatra, C.; Jayasankar, P.; Barman, H.K. First evidence of molecular characterization of rohu carp Sox2 gene being expressed in proliferating spermatogonial cells. Theriogenology 2015, 84, 268–276.e1. [Google Scholar] [PubMed]
- Ateş, P.S.; Ünal, İ.; Üstündağ, Ü.V.; Alturfan, A.A.; Yiğitbaşı, T.; Emekli-Alturfan, E. Methylparaben induces malformations and alterations on apoptosis, oxidant–antioxidant status, ccnd1 and myca expressions in zebrafish embryos. J. Biochem. Mol. Toxicol. 2018, 32, e22036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, W.-W.; Mo, C.-Y.; Dong, M.-D.; Jia, K.-T.; Liu, W.; Yi, M.-S. Production of functional sperm from in vitro-cultured premeiotic spermatogonia in a marine fish. Zool. Res. 2022, 43, 537. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Z.; Liu, W.; Li, Z.; Wang, Y.; Zhou, L.; Yi, M.-S.; Gui, J.-F. Molecular characterization and expression pattern of a germ cell marker gene dnd in gibel carp (Carassius gibelio). Gene 2016, 591, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, R.; Takeuchi, Y.; Morita, T.; Ishida, M.; Yoshizaki, G. The Pacific bluefin tuna (Thunnus orientalis) dead end gene is suitable as a specific molecular marker of type A spermatogonia. Mol. Reprod. Dev. 2013, 80, 871–880. [Google Scholar] [CrossRef]
- Nobrega, R.H.; Greebe, C.D.; van de Kant, H.; Bogerd, J.; de Franca, L.R.; Schulz, R.W. Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS ONE 2010, 5, e12808. [Google Scholar]
- Wang, H.; Wang, B.; Liu, X.; Liu, Y.; Du, X.; Zhang, Q.; Wang, X. Identification and expression of piwil2 in turbot Scophthalmus maximus, with implications of the involvement in embryonic and gonadal development. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2017, 208, 84–93. [Google Scholar] [CrossRef]
- Houwing, S.; Kamminga, L.M.; Berezikov, E.; Cronembold, D.; Girard, A.; van den Elst, H.; Filippov, D.V.; Blaser, H.; Raz, E.; Moens, C.B. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell 2007, 129, 69–82. [Google Scholar]
- Li, H.; Xu, W.; Xiang, S.; Tao, L.; Fu, W.; Liu, J.; Liu, W.; Xiao, Y.; Peng, L. Defining the pluripotent marker genes for identification of teleost fish cell pluripotency during reprogramming. Front. Genet. 2022, 13, 819682. [Google Scholar]
- Wang, D.; Manali, D.; Wang, T.; Bhat, N.; Hong, N.; Li, Z.; Wang, L.; Yan, Y.; Liu, R.; Hong, Y. Identification of pluripotency genes in the fish medaka. Int. J. Biol. Sci. 2011, 7, 440. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, J.; Jiang, J.; Fan, L.; Wang, W.; Liu, J.; Zhang, Q.; Wang, X. Identification and characterization of a nanog homolog in Japanese flounder (Paralichthys olivaceus). Gene 2013, 531, 411–421. [Google Scholar] [PubMed]
- Zhong, C.; Liu, M.; Tao, Y.; Wu, X.; Yang, Y.; Wang, T.; Meng, Z.; Xu, H.; Liu, X. Pou5f1 and Nanog are reliable germ cell-specific genes in gonad of a protogynous hermaphroditic fish, orange-spotted grouper (Epinephelus coioides). Genes 2021, 13, 79. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhu, F.; Li, Z.; Hong, N.; Hong, Y. Dazl is a critical player for primordial germ cell formation in medaka. Sci. Rep. 2016, 6, 28317. [Google Scholar] [CrossRef]
- Chen, X.; Song, P.; Xia, J.; Guo, J.; Shi, Y.; Zhong, Y.; Li, M. Evolutionarily conserved boule and dazl identify germ cells of Coilia nasus. Aquac. Fish. 2023, 8, 244–251. [Google Scholar]
- Zhao, C.; Liu, Q.; Xu, S.; Xiao, Y.; Wang, W.; Yang, J.; Yang, Y.; Wang, Y.; Song, Z.; Li, J. Identification of type A spermatogonia in turbot (Scophthalmus maximus) using a new cell-surface marker of Lymphocyte antigen 75 (ly75/CD205). Theriogenology 2018, 113, 137–145. [Google Scholar]
- Nagasawa, K.; Shikina, S.; Takeuchi, Y.; Yoshizaki, G. Lymphocyte antigen 75 (Ly75/CD205) is a surface marker on mitotic germ cells in rainbow trout. Biol. Reprod. 2010, 83, 597–606. [Google Scholar] [CrossRef]
- Lacerda, S.M.d.S.N.; Costa, G.M.J.; de França, L.R. Biology and identity of fish spermatogonial stem cell. Gen. Comp. Endocrinol. 2014, 207, 56–65. [Google Scholar]
- Costa, G.M.; Avelar, G.F.; Rezende-Neto, J.V.; Campos-Junior, P.H.A.; Lacerda, S.M.; Andrade, B.S.; Thome, R.G.; Hofmann, M.-C.; Franca, L.R. Spermatogonial stem cell markers and niche in equids. PLoS ONE 2012, 7, e44091. [Google Scholar]
- Nagano, M.C.; Yeh, J.R. The identity and fate decision control of spermatogonial stem cells: Where is the point of no return? Curr. Top. Dev. Biol. 2013, 102, 61–95. [Google Scholar]
- Lacerda, S.M.S.N.; Costa, G.M.J.; da Silva, M.d.A.; Campos-Junior, P.H.A.; Segatelli, T.M.; Peixoto, M.T.D.; Resende, R.R.; de França, L.R. Phenotypic characterization and in vitro propagation and transplantation of the Nile tilapia (Oreochromis niloticus) spermatogonial stem cells. Gen. Comp. Endocrinol. 2013, 192, 95–106. [Google Scholar]
- Bosseboeuf, A.; Gautier, A.; Auvray, P.; Mazan, S.; Sourdaine, P. Characterization of spermatogonial markers in the mature testis of the dogfish (Scyliorhinus canicula L.). Reproduction 2014, 147, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Hayashi, M.; Kouguchi, T.; Yamaguchi, K.; Miwa, M.; Yoshizaki, G. Expression patterns of gdnf and gfrα1 in rainbow trout testis. Gene Expr. Patterns 2014, 14, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki-Takahashi, Y.; Shikina, S.; Watanabe, M.; Banba, A.; Yagisawa, M.; Takahashi, K.; Fujihara, R.; Okabe, T.; Valdez, D.M., Jr.; Yamauchi, A. Production of functional eggs and sperm from in vitro-expanded type A spermatogonia in rainbow trout. Commun. Biol. 2020, 3, 308. [Google Scholar] [CrossRef] [PubMed]
- Satoh, R.; Bando, H.; Sakai, N.; Kotani, T.; Yamashita, M. Function of leukaemia inhibitory factor in spermatogenesis of a teleost fish, the medaka Oryzias latipes. Zygote 2019, 27, 423–431. [Google Scholar] [PubMed]
- Kawasaki, T.; Saito, K.; Sakai, C.; Shinya, M.; Sakai, N. Production of zebrafish offspring from cultured spermatogonial stem cells. Genes Cells 2012, 17, 316–325. [Google Scholar] [CrossRef]
- Komeya, M.; Ogawa, T. Spermatogonial stem cells: Progress and prospects. Asian J. Androl. 2015, 17, 771. [Google Scholar]
- Yang, H.; Hao, D.; Liu, C.; Huang, D.; Chen, B.; Fan, H.; Liu, C.; Zhang, L.; Zhang, Q.; An, J. Generation of functional dopaminergic neurons from human spermatogonial stem cells to rescue parkinsonian phenotypes. Stem Cell Res. Ther. 2019, 10, 195. [Google Scholar]


| Gene | F-Primer (5′ to 3′) |
|---|---|
| cox1-F | GAGGCTTTGGGAACTGATTA |
| cox1-R | GGTATTGTGAGATTGCTGGG |
| ddx4-F | AGAGGGATTAGACAAGGTGG |
| ddx4-R | GGCTCAAAACCCATATCCAG |
| dnd-F | TGATTCCTCTGTTCACCACT |
| dnd-R | GCACAAGTTATTGAGGAGCA |
| piwil1-F | TCAAACATACAGAGGAACGC |
| piwil1-R | GATTTCCTGACCTTTGTGCT |
| gfra1b-F | GAAGAAGGAGAAGAACTGCC |
| gfra1b-R | CATCACCGTACCAATGAGG |
| ly75-F | ACGTTACCCACTTTGACAAG |
| ly75-R | CATATCTAACTGGTGCAGGC |
| nanog-F | AAGTGACAGATTTGAGCAGC |
| nanog-R | GTAGTTTGGATGCCACTAGC |
| dazl-F | ACTATTTGGTCGGTATGGGT |
| dazl-R | TCCACAATCCACAAAGTTCC |
| sox2-F | AGGAAGATGGCGCAAGAAAA |
| sox2-R | GACATGTGTAGCCTGGTCTG |
| myca-F | GCGAGGACATCTGGAAGAAG |
| myca-R | AGACGTGGCATCTCTTCAAC |
| vasa | DIG-ACGATTCTCTTTAGATGGCATTCCAGGTGTA |
| gfra1b | DIG-GAAGGTGCCACAGACGGGATACAACGGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.; Liu, Q.; He, Y.; Zhang, J.; Hou, J.; Ren, Y.; Ma, W.; Wang, Q.; Shao, C. Establishment and Characterization of a Spermatogonial Stem Cell Line from Tiger Puffer Fish (Takifugu rubripes). Animals 2023, 13, 2959. https://doi.org/10.3390/ani13182959
Tan L, Liu Q, He Y, Zhang J, Hou J, Ren Y, Ma W, Wang Q, Shao C. Establishment and Characterization of a Spermatogonial Stem Cell Line from Tiger Puffer Fish (Takifugu rubripes). Animals. 2023; 13(18):2959. https://doi.org/10.3390/ani13182959
Chicago/Turabian StyleTan, Leilei, Qian Liu, Yangbin He, Jingjing Zhang, Jilun Hou, Yuqin Ren, Wenxiu Ma, Qian Wang, and Changwei Shao. 2023. "Establishment and Characterization of a Spermatogonial Stem Cell Line from Tiger Puffer Fish (Takifugu rubripes)" Animals 13, no. 18: 2959. https://doi.org/10.3390/ani13182959
APA StyleTan, L., Liu, Q., He, Y., Zhang, J., Hou, J., Ren, Y., Ma, W., Wang, Q., & Shao, C. (2023). Establishment and Characterization of a Spermatogonial Stem Cell Line from Tiger Puffer Fish (Takifugu rubripes). Animals, 13(18), 2959. https://doi.org/10.3390/ani13182959





