Edwardsiella tarda in Tambaqui (Colossoma macropomum): A Pathogenicity, Antimicrobial Susceptibility, and Genetic Analysis of Brazilian Isolates
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. E. tarda Isolate Collection
2.2. Bacteriological Examinations
2.3. Edwardsiella Tarda Identification
2.3.1. MALDI-TOF MS Real-Time Identification
2.3.2. Molecular Confirmation of Identification
2.4. Genetic Typing Using Repetitive Extragenic Palindromic-PCR (rep-PCR)
2.5. Antimicrobial Susceptibility Testing
2.6. Pathogenicity Evaluation
2.6.1. Fish and Experimental Infection
2.6.2. Histological Analysis
3. Results
3.1. Bacterial Identification
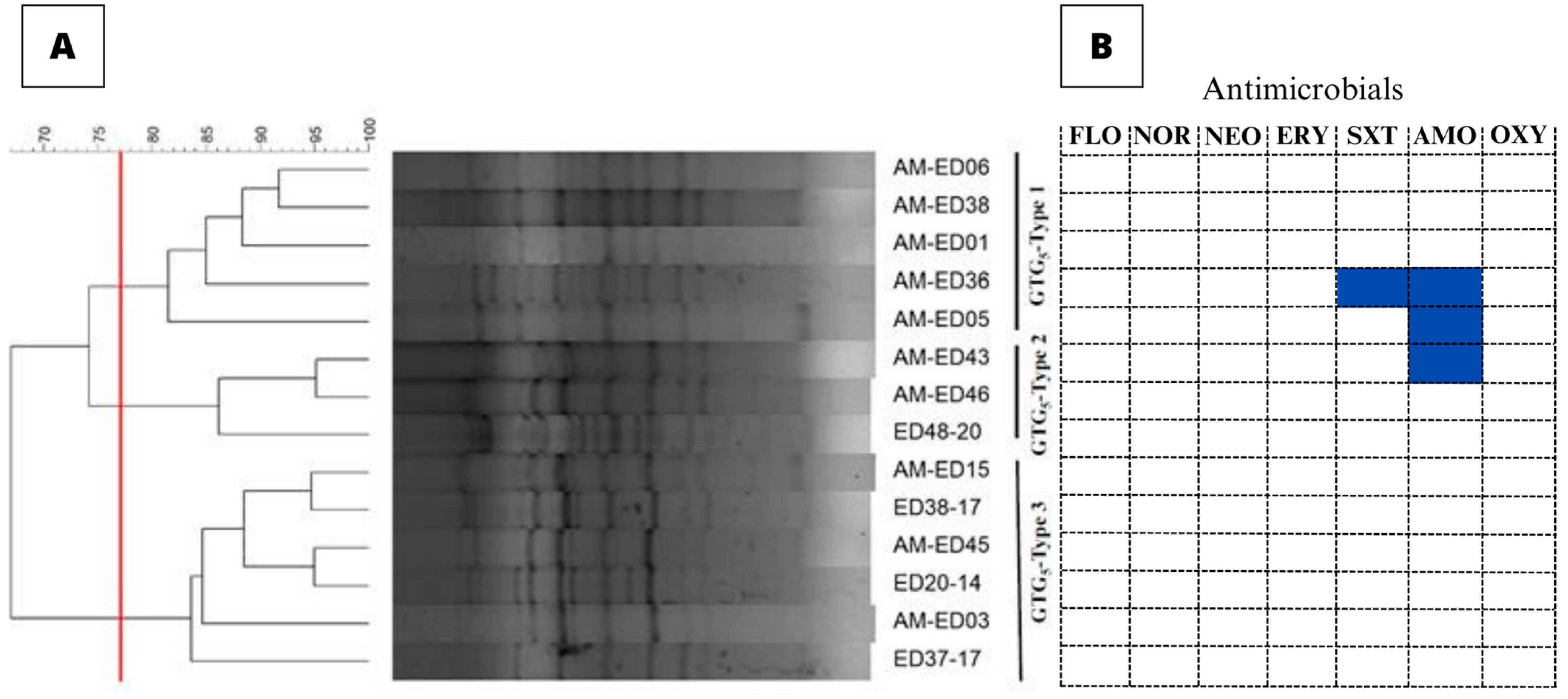
3.2. Genetic Typing of the E. tarda Isolates
3.3. Antimicrobial Susceptibility
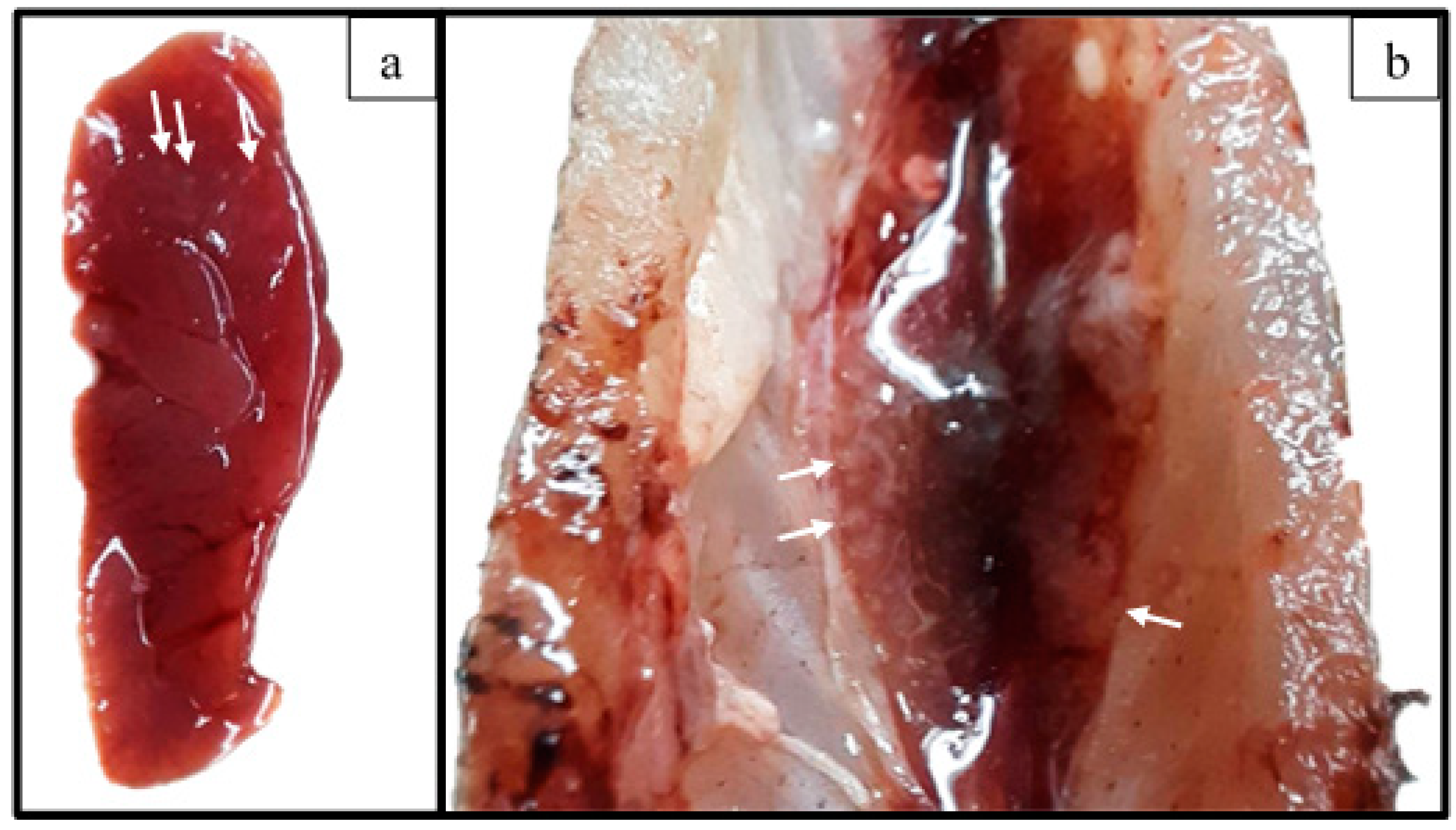
3.4. Challenge Assay
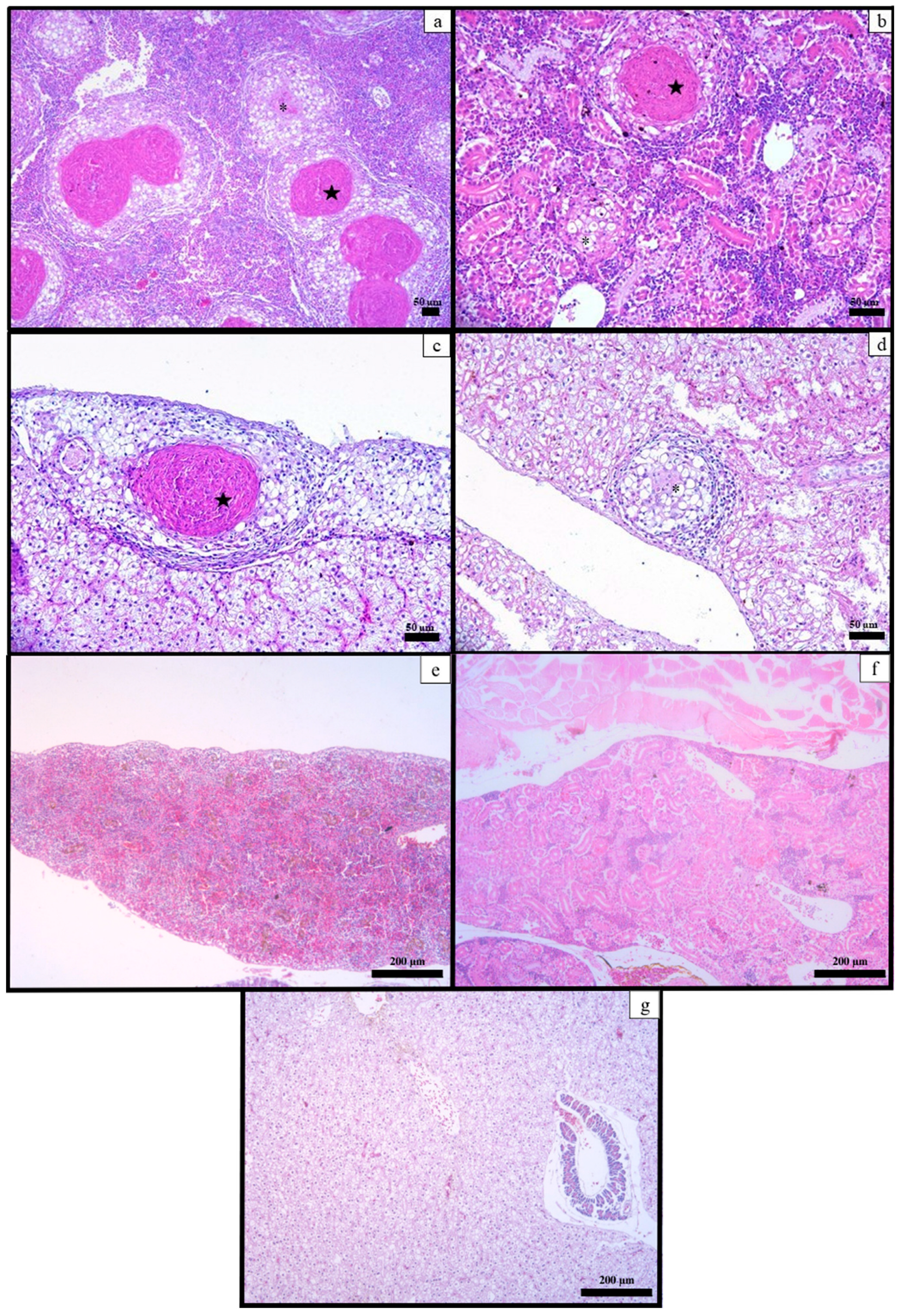
3.5. Histological Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valladão, G.M.R.; Gallani, S.U.; Pilarski, F. South American Fish for Continental Aquaculture. Rev. Aquac. 2018, 10, 351–369. [Google Scholar] [CrossRef]
- IBGE, Instituto Brasileiro de Geografia e Estatística. Produção Da Pecuária Municipal 2019; IBGE: Rio de Janeiro, Brasil, 2020.
- Associação Brasileira da Piscicultura. Anuário Brasileiro Da Piscicultura Peixe BR 2022; Associação Brasileira da Piscicultura: São Paulo, Brasil, 2022. [Google Scholar]
- Castro, L.D.A.; Jerônimo, G.T.; da Silva, R.M.; Santos, M.J.; Ramos, C.A.; Porto, S.M.D.A. Occurrence, Pathogenicity, and Control of Acanthocephalosis Caused by Neoechinorhynchus buttnerae: A Review. Rev. Bras. De Parasitol. Veterinária 2020, 29, e008320. [Google Scholar] [CrossRef]
- Gallani, S.U.; Valladão, G.M.R.; Assane, I.M.; de Alves, L.O.; Kotzent, S.; Hashimoto, D.T.; Pilarski, F. Motile Aeromonas Septicemia in Tambaqui Colossoma macropomum: Pathogenicity, Lethality and New Insights for Control and Disinfection in Aquaculture. Microb. Pathog. 2020, 149, 104512. [Google Scholar] [CrossRef]
- Mielke, T.D.; Francisco, C.J.; Dorella, F.A.; Figueiredo, H.C.P.; Tavares, G.C.; Gallani, S.U. The Strategic Use of Water Additives for Tambaqui Colossoma macropomum Transport: New Insights of Bacteriosis and Productivity Approach. Aquaculture 2022, 558, 738406. [Google Scholar] [CrossRef]
- Abayneh, T.; Colquhoun, D.J.; Sørum, H. Edwardsiella piscicida sp. nov., a Novel Species Pathogenic to Fish. J. Appl. Microbiol. 2013, 114, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Lai, Q.; Liu, Q.; Wu, H.; Xiao, J.; Shao, Z.; Wang, Q.; Zhang, Y. Phylogenomics Characterization of a Highly Virulent Edwardsiella Strain ET080813T Encoding Two Distinct T3SS and Three T6SS Gene Clusters: Propose a Novel Species as Edwardsiella anguillarum sp. nov. Syst. Appl. Microbiol. 2015, 38, 36–47. [Google Scholar] [CrossRef]
- Reichley, S.R.; Ware, C.; Steadman, J.; Gaunt, P.S.; García, J.C.; LaFrentz, B.R.; Thachil, A.; Waldbieser, G.C.; Stine, C.B.; Buján, N.; et al. Comparative Phenotypic and Genotypic Analysis of Edwardsiella Isolates from Different Hosts and Geographic Origins, with Emphasis on Isolates Formerly Classified as E. tarda, and Evaluation of Diagnostic Methods. J. Clin. Microbiol. 2017, 55, 3466–3491. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Aoki, T.; Jung, T.S. Pathogenesis of and Strategies for Preventing Edwardsiella tarda Infection in Fish. Vet. Res. 2012, 43, 67. [Google Scholar] [CrossRef]
- Iveson, J.B. Strontium Chloride B and E.E. Enrichment Broth Media for the Isolation of Edwardsiella, Salmonella and Arizona Species from Tiger Snakes. J. Hyg. 1971, 69, 323–330. [Google Scholar] [CrossRef]
- Miniero Davies, Y.; Xavier de Oliveira, M.G.; Paulo Vieira Cunha, M.; Soares Franco, L.; Pulecio Santos, S.L.; Zanolli Moreno, L.; Túlio de Moura Gomes, V.; Zanolli Sato, M.I.; Schiavo Nardi, M.; Micke Moreno, A.; et al. Edwardsiella tarda Outbreak Affecting Fishes and Aquatic Birds in Brazil. Vet. Q. 2018, 38, 99–105. [Google Scholar] [CrossRef]
- Mauel, M.J.; Miller, D.L.; Frazier, K.S.; Hines, M.E. Bacterial Pathogens Isolated from Cultured Bullfrogs (Rana castesbeiana). J. Vet. Diagn. Investig. 2002, 14, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, S.; Kuriyama, A.; Hashimoto, T. Edwardsiella tarda Bacteremia, Okayama, Japan, 2005–2016. Emerg. Infect. Dis. 2019, 25, 1817–1823. [Google Scholar] [CrossRef]
- Lima, L.C.; Fernandes, A.A.; Costa, A.A.P.; Velasco, F.O.; Leite, R.C.; Hackett, J.L. Isolation and Characterizaton of Edwardsiella tarda from Pacu Myleus micans. Arq. Bras. Med. Vet. Zootec. 2008, 60, 275–277. [Google Scholar] [CrossRef]
- Ramos, E.F.; Sandoval, C.N.; Morales, C.S.; Contreras, S.G.; Manchego, S.A. Lesiones Histopatológicas y Aislamiento Bacteriológico En Gamitanas (Colossoma macropomum) Aparentemente Sanas. Rev. De Investig. Vet. Del Perú 2016, 27, 188–195. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, X.H. Edwardsiella tarda: An Intriguing Problem in Aquaculture. Aquaculture 2014, 431, 129–135. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, Q.; Liu, Q.; Wang, X.; Liu, H.; Zhang, Y. Isolation and Identification of Fish Pathogen Edwardsiella tarda from Mariculture in China. Aquac. Res. 2008, 40, 13–17. [Google Scholar] [CrossRef]
- Suzuki, K.; Yanai, M.; Hayashi, Y.; Otsuka, H.; Kato, K.; Soma, M. Edwardsiella tarda Bacteremia with Psoas and Epidural Abscess as a Food-Borne Infection: A Case Report and Literature Review. Intern. Med. 2018, 57, 893–897. [Google Scholar] [CrossRef]
- Michael, J.; Abbott, S.L. Infections Associated with the Genus Edwardsiella: The Role of Edwardsiella tarda in Human Disease. Clin. Infect. Dis. 1993, 17, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Hong Nhung, P.; Ohkusu, K.; Mishima, N.; Noda, M.; Monir Shah, M.; Sun, X.; Hayashi, M.; Ezaki, T. Phylogeny and Species Identification of the Family Enterobacteriaceae Based on DnaJ Sequences. Diagn. Microbiol. Infect. Dis. 2007, 58, 153–161. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Costa, F.A.A.; Leal, C.A.G.; Leite, R.C.; Figueiredo, H.C.P. Genotyping of Streptococcus dysgalactiae Strains Isolated from Nile Tilapia, Oreochromis niloticus (L.). J. Fish Dis. 2013, 37, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Dice, L.R. Measures of the Amount of Ecologic Association between Species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Hunter, P.R.; Gaston, M.A. Numerical Index of the Discriminatory Ability of Typing Systems: An Application of Simpson’s Index of Diversity. J. Clin. Microbiol. 1988, 26, 2466. [Google Scholar] [CrossRef]
- CLSI, Clinical and Laboratory Standards Institute. VET03 Methods for Antimicrobial Broth Dilution and Disk Diffusion Susceptibility Testing of Bacteria Isolated from Aquatic Animals; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Kronvall, G.; Smith, P. Normalized Resistance Interpretation, the NRI Method. APMIS 2016, 124, 1023–1030. [Google Scholar] [CrossRef]
- Kronvall, G.; Kahlmeter, G.; Myhre, E.; Galas, M.F. A New Method for Normalized Interpretation of Antimicrobial Resistance from Disk Test Results for Comparative Purposes. Clin. Microbiol. Infect. 2003, 9, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Ruane, N.M.; Douglas, I.; Carroll, C.; Kronvall, G.; Fleming, G.T.A. Impact of Inter-Lab Variation on the Estimation of Epidemiological Cut-off Values for Disc Diffusion Susceptibility Test Data for Aeromonas salmonicida. Aquaculture 2007, 272, 168–179. [Google Scholar] [CrossRef]
- Smith, P. Eight Rules for Improving the Quality of Papers on the Antimicrobial Susceptibility of Bacteria Isolated from Aquatic Animals. Dis. Aquat. Organ. 2020, 139, 87–92. [Google Scholar] [CrossRef]
- Smith, P.; Finnegan, W.; Ngo, T.; Kronvall, G. Influence of Incubation Temperature and Time on the Precision of MIC and Disc Diffusion Antimicrobial Susceptibility Test Data. Aquaculture 2018, 490, 19–24. [Google Scholar] [CrossRef]
- Schwarz, S.; Silley, P.; Simjee, S.; Woodford, N.; van Duijkeren, E.; Johnson, A.P.; Gaastra, W. Assessing the Antimicrobial Susceptibility of Bacteria Obtained from Animals. Vet. Microbiol. 2010, 141, 601–604. [Google Scholar] [CrossRef]
- Luna, L.G. Manual of Histologic Staining Methods of the Armed Forces Institute of Pathology, 3rd ed.; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Wang, X.; Wang, F.; Chen, G.; Yang, B.; Chen, J.; Fang, Y.; Wang, K.; Hou, Y. Edwardsiella tarda Induces Enteritis in Farmed Seahorses (Hippocampus erectus): An Experimental Model and Its Evaluation. Fish Shellfish Immunol. 2020, 98, 391–400. [Google Scholar] [CrossRef]
- Shetty, M.; Maiti, B.; Venugopal, M.N.; Karunasagar, I.; Karunasagar, I. First Isolation and Characterization of Edwardsiella tarda from Diseased Striped Catfish, Pangasianodon hypophthalmus (Sauvage). J. Fish Dis. 2014, 37, 265–271. [Google Scholar] [CrossRef]
- Sebastião, F.A.; Furlan, L.R.; Hashimoto, D.T.; Pilarski, F. Identification of Bacterial Fish Pathogens in Brazil by Direct Colony PCR and 16S RRNA Gene Sequencing. Adv. Microbiol. 2015, 5, 409–424. [Google Scholar] [CrossRef]
- Buján, N.; Mohammed, H.; Balboa, S.; Romalde, J.L.; Toranzo, A.E.; Arias, C.R.; Magariños, B. Genetic Studies to Re-Affiliate Edwardsiella tarda Fish Isolates to Edwardsiella piscicida and Edwardsiella anguillarum Species. Syst. Appl. Microbiol. 2018, 41, 30–37. [Google Scholar] [CrossRef]
- Bera, K.K.; Kumar, S.; Paul, T.; Prasad, K.P.; Shukla, S.P.; Kumar, K. Triclosan Induces Immunosuppression and Reduces Survivability of Striped Catfish Pangasianodon hypophthalmus during the Challenge to a Fish Pathogenic Bacterium Edwardsiella tarda. Environ. Res. 2020, 186, 109575. [Google Scholar] [CrossRef]
- Yu, J.E.; Yoo, A.Y.; Choi, K.H.; Cha, J.; Kwak, I.; Kang, H.Y. Identification of Antigenic Edwardsiella tarda Surface Proteins and Their Role in Pathogenesis. Fish Shellfish Immunol. 2013, 34, 673–682. [Google Scholar] [CrossRef]
- Hossain, M.M.; Kawai, K.; Oshima, S. Immunogenicity of Pressure Inactivated Edwardsiella tarda Bacterin to Anguilla japonica (Japanese Eel). Pak. J. Biol. Sci. 2011, 14, 755–767. [Google Scholar] [CrossRef][Green Version]
- Qin, L.; Xu, J.; Wang, Y.G. Edwardsiellosis in Farmed Turbot, Scophthalmus maximus (L.), Associated with an Unusual Variant of Edwardsiella tarda: A Clinical, Aetiological and Histopathological Study. J. Fish Dis. 2014, 37, 103–111. [Google Scholar] [CrossRef]
- Miyazaki, T.; Kaige, N. Comparative Histopathology of Edwardsiellosis in Fishes. Fish Pathol. 1985, 20, 219–227. [Google Scholar] [CrossRef]
- Iregui, C.A.; Guarín, M.; Tibatá, V.M.; Ferguson, H.W. Novel Brain Lesions Caused by Edwardsiella tarda in a Red Tilapia (Oreochromis spp.). J. Vet. Diagn. Investig. 2012, 24, 446–449. [Google Scholar] [CrossRef]
- Darwish, A.; Plumb, J.A.; Newton, J.C. Histopathology and Pathogenesis of Experimental Infection with Edwardsiella tarda in Channel Catfish. J. Aquat. Anim. Health 2000, 12, 255–266. [Google Scholar] [CrossRef]
- Rajme-Manzur, D.; Gollas-Galván, T.; Vargas-Albores, F.; Martínez-Porchas, M.; Hernández-Oñate, M.Á.; Hernández-López, J. Granulomatous Bacterial Diseases in Fish: An Overview of the Host’s Immune Response. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 261, 111058. [Google Scholar] [CrossRef]
- Castro, N.; Toranzo, A.E.; Bastardo, A.; Barja, J.L.; Magariños, B. Intraspecific Genetic Variability of Edwardsiella tarda Strains from Cultured Turbot. Dis. Aquat. Organ. 2011, 95, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.J.; Quiniou, S.M.; Cody, T.; Tabuchi, M.; Ware, C.; Cipriano, R.C.; Mauel, M.J.; Soto, E. Comparative Analysis of Edwardsiella Isolates from Fish in the Eastern United States Identifies Two Distinct Genetic Taxa amongst Organisms Phenotypically Classified as E. tarda. Vet. Microbiol. 2013, 165, 358–372. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, M.; Xiao, J.; Wu, H.; Wang, X.; Lv, Y.; Xu, L.; Zheng, H.; Wang, S.; Zhao, G.; et al. Genome Sequence of the Versatile Fish Pathogen Edwardsiella tarda Provides Insights into Its Adaptation to Broad Host Ranges and Intracellular Niches. PLoS ONE 2009, 4, e7646. [Google Scholar] [CrossRef]
- Lim, Y.-J.; Kim, D.-H.; Roh, H.J.; Park, M.-A.; Park, C.-I.; Smith, P. Epidemiological Cut-off Values for Disc Diffusion Data Generated by Standard Test Protocols from Edwardsiella tarda and Vibrio harveyi. Aquac. Int. 2016, 24, 1153–1161. [Google Scholar] [CrossRef]
- Shin, D.-M.; Hossain, S.; Wimalasena, S.; Heo, G.-J. Antimicrobial Resistance and Virulence Factors of Edwardsiella tarda Isolated from Pet Turtles. Pak. Vet. J. 2017, 37, 85–89. [Google Scholar]
- Preena, P.G.; Dharmaratnam, A.; Swaminathan, T.R. Antimicrobial Resistance Analysis of Pathogenic Bacteria Isolated from Freshwater Nile Tilapia (Oreochromis niloticus) Cultured in Kerala, India. Curr. Microbiol. 2020, 77, 3278–3287. [Google Scholar] [CrossRef]
- Preena, P.G.; Dharmaratnam, A.; Raj, N.S.; Raja, S.A.; Nair, R.R.; Swaminathan, T.R. Antibiotic-Resistant Enterobacteriaceae from Diseased Freshwater Goldfish. Arch. Microbiol. 2020, 203, 219–231. [Google Scholar] [CrossRef]
- Preena, P.G.; Arathi, D.; Raj, N.S.; Kumar, T.V.A.; Raja, S.A.; Reshma, R.N.; Swaminathan, T.R. Diversity of Antimicrobial-Resistant Pathogens from a Freshwater Ornamental Fish Farm. Lett. Appl. Microbiol. 2019, 71, 108–116. [Google Scholar] [CrossRef]
- Lee, S.W.; Wendy, W. Antibiotic and Heavy Metal Resistance of Aeromonas hydrophila and Edwardsiella tarda Isolated from Red Hybrid Tilapia (Oreochromis spp.) Coinfected with Motile Aeromonas Septicemia and Edwardsiellosis. Vet. World 2017, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Stock, I.; Wiedemann, B. Natural Antibiotic Susceptibilities of Edwardsiella tarda, E. ictaluri, and E. hoshinae. Antimicrob. Agents Chemother. 2001, 45, 2255. [Google Scholar] [CrossRef] [PubMed]

| Isolate | Culture Collection | Clinical Status of the Fish | State | Year of Isolation | Organ | MALDI-TOF Score Value |
|---|---|---|---|---|---|---|
| ED20-14 | Aquavet | Diseased | MG | 2014 | Kidney | 2.494 |
| ED37-17 | Aquavet | Healthy | RO | 2017 | Kidney | 2.358 |
| ED38-17 | Aquavet | Healthy | RO | 2017 | Kidney | 2.534 |
| AM-ED01 | LAMAO | Healthy | AM | 2018 | Brain | 2.210 |
| AM-ED03 | LAMAO | Healthy | AM | 2018 | Brain | 2.459 |
| AM-ED05 | LAMAO | Diseased | AM | 2019 | Kidney | 2.153 |
| AM-ED06 | LAMAO | Healthy | AM | 2019 | Brain | 2.598 |
| AM-ED15 | LAMAO | Diseased | AM | 2019 | Brain | 2.521 |
| AM-ED36 | LAMAO | Healthy | AM | 2019 | Spleen | 2.539 |
| AM-ED38 | LAMAO | Diseased | AM | 2019 | Kidney | 2.600 |
| AM-ED43 | LAMAO | Diseased | AM | 2019 | Liver | 2.286 |
| AM-ED45 | LAMAO | Healthy | AM | 2019 | Intestines | 2.292 |
| AM-ED46 | LAMAO | Healthy | AM | 2020 | Kidney | 2.347 |
| ED48-20 | Aquavet | Healthy | AM | 2020 | Intestines | 2.316 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, F.Y.T.; Rocha, V.P.; Janampa-Sarmiento, P.C.; Costa, H.L.; Egger, R.C.; Passos, N.C.; de Assis, C.H.S.; Carneiro, S.P.; Santos, Á.F.; Silva, B.A.; et al. Edwardsiella tarda in Tambaqui (Colossoma macropomum): A Pathogenicity, Antimicrobial Susceptibility, and Genetic Analysis of Brazilian Isolates. Animals 2023, 13, 2910. https://doi.org/10.3390/ani13182910
Reis FYT, Rocha VP, Janampa-Sarmiento PC, Costa HL, Egger RC, Passos NC, de Assis CHS, Carneiro SP, Santos ÁF, Silva BA, et al. Edwardsiella tarda in Tambaqui (Colossoma macropomum): A Pathogenicity, Antimicrobial Susceptibility, and Genetic Analysis of Brazilian Isolates. Animals. 2023; 13(18):2910. https://doi.org/10.3390/ani13182910
Chicago/Turabian StyleReis, Francisco Yan Tavares, Victória Pontes Rocha, Peter Charrie Janampa-Sarmiento, Henrique Lopes Costa, Renata Catão Egger, Naísa Cristine Passos, Carlos Henrique Santos de Assis, Sarah Portes Carneiro, Ágna Ferreira Santos, Brendhal Almeida Silva, and et al. 2023. "Edwardsiella tarda in Tambaqui (Colossoma macropomum): A Pathogenicity, Antimicrobial Susceptibility, and Genetic Analysis of Brazilian Isolates" Animals 13, no. 18: 2910. https://doi.org/10.3390/ani13182910
APA StyleReis, F. Y. T., Rocha, V. P., Janampa-Sarmiento, P. C., Costa, H. L., Egger, R. C., Passos, N. C., de Assis, C. H. S., Carneiro, S. P., Santos, Á. F., Silva, B. A., Dorella, F. A., Leibowitz, M. P., Luz, R. K., Pierezan, F., Gallani, S. U., Tavares, G. C., & Figueiredo, H. C. P. (2023). Edwardsiella tarda in Tambaqui (Colossoma macropomum): A Pathogenicity, Antimicrobial Susceptibility, and Genetic Analysis of Brazilian Isolates. Animals, 13(18), 2910. https://doi.org/10.3390/ani13182910






