Zinc Oxide Nanoparticles (ZnO-NPs) Induce Cytotoxicity in the Zebrafish Olfactory Organs via Activating Oxidative Stress and Apoptosis at the Ultrastructure and Genetic Levels
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry and ZnO-NPs Exposure
2.2. Scanning Electron Microscopy (SEM) Preparations
2.3. Transmission Electron Microscopy (TEM) and Semi-Thin Sections Preparations
2.4. Histological Assessments by Light Microscope
2.5. Total RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.6. Protein Extraction and Western Blot Analysis
2.7. Statistical Analysis and Graphs Preparation
3. Results
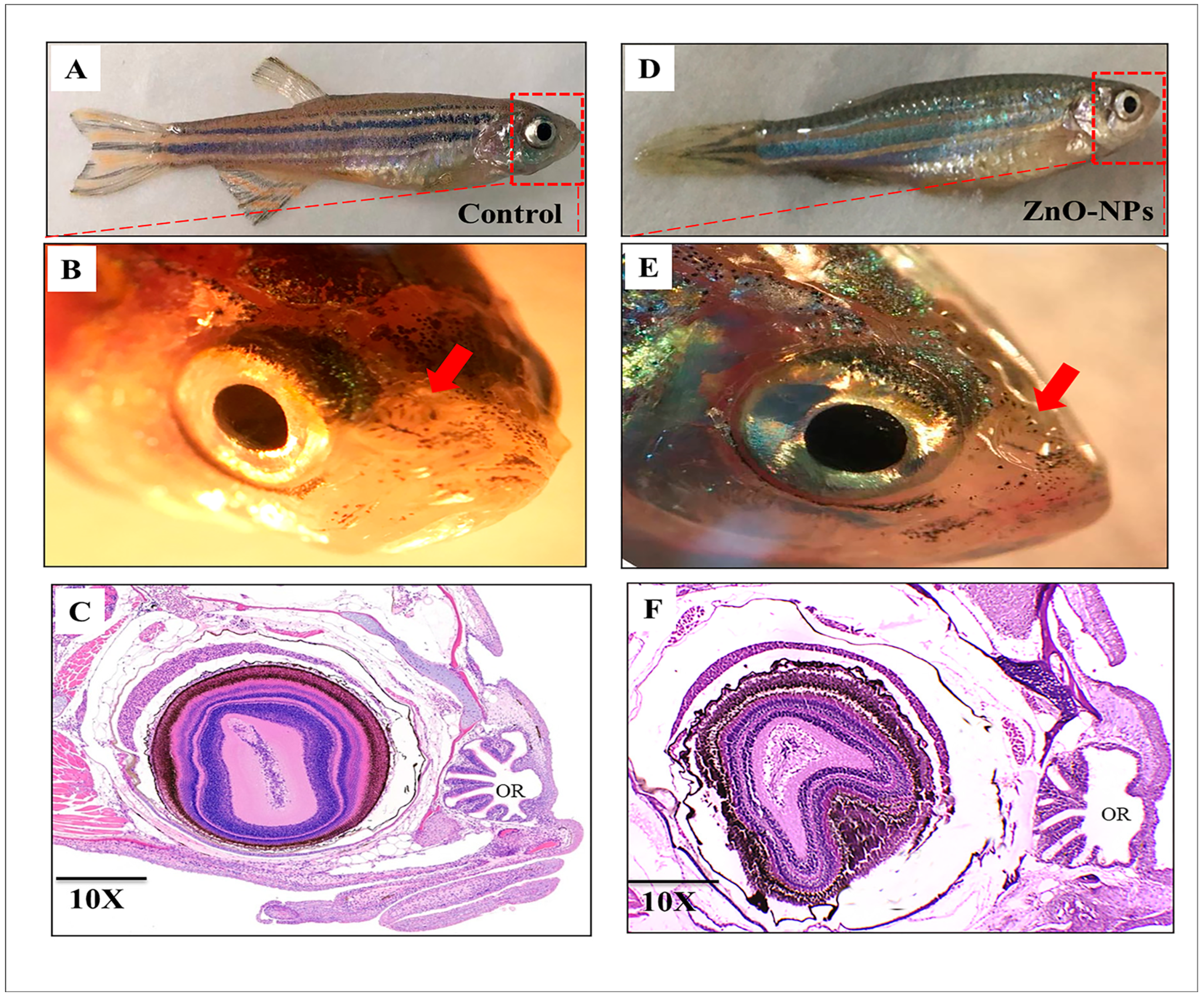
3.1. Macrostructure of Zebrafish Olfactory Organ
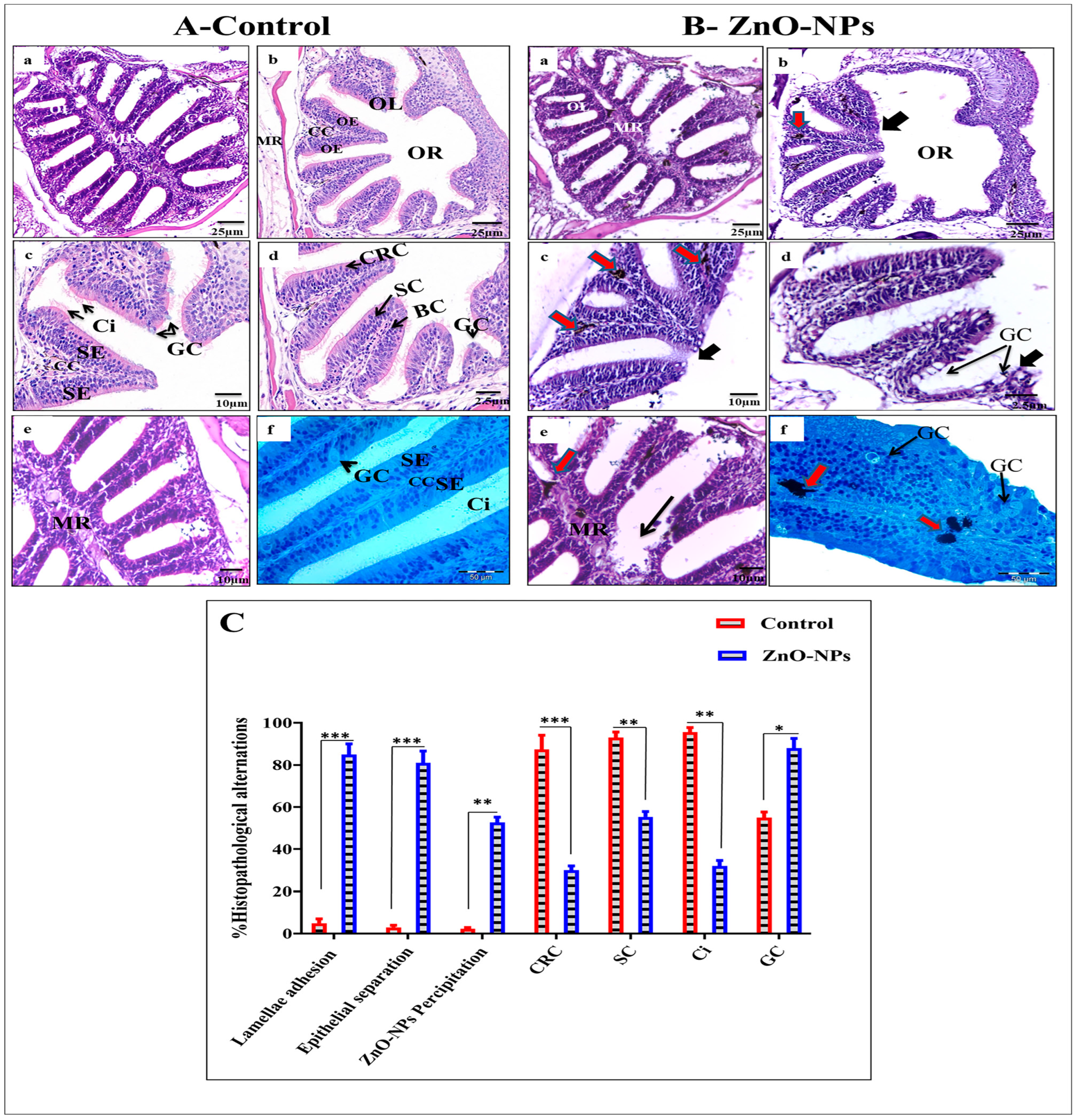
3.2. ZnO-NPs Alter the Histological Architecture of the Olfactory Rosette
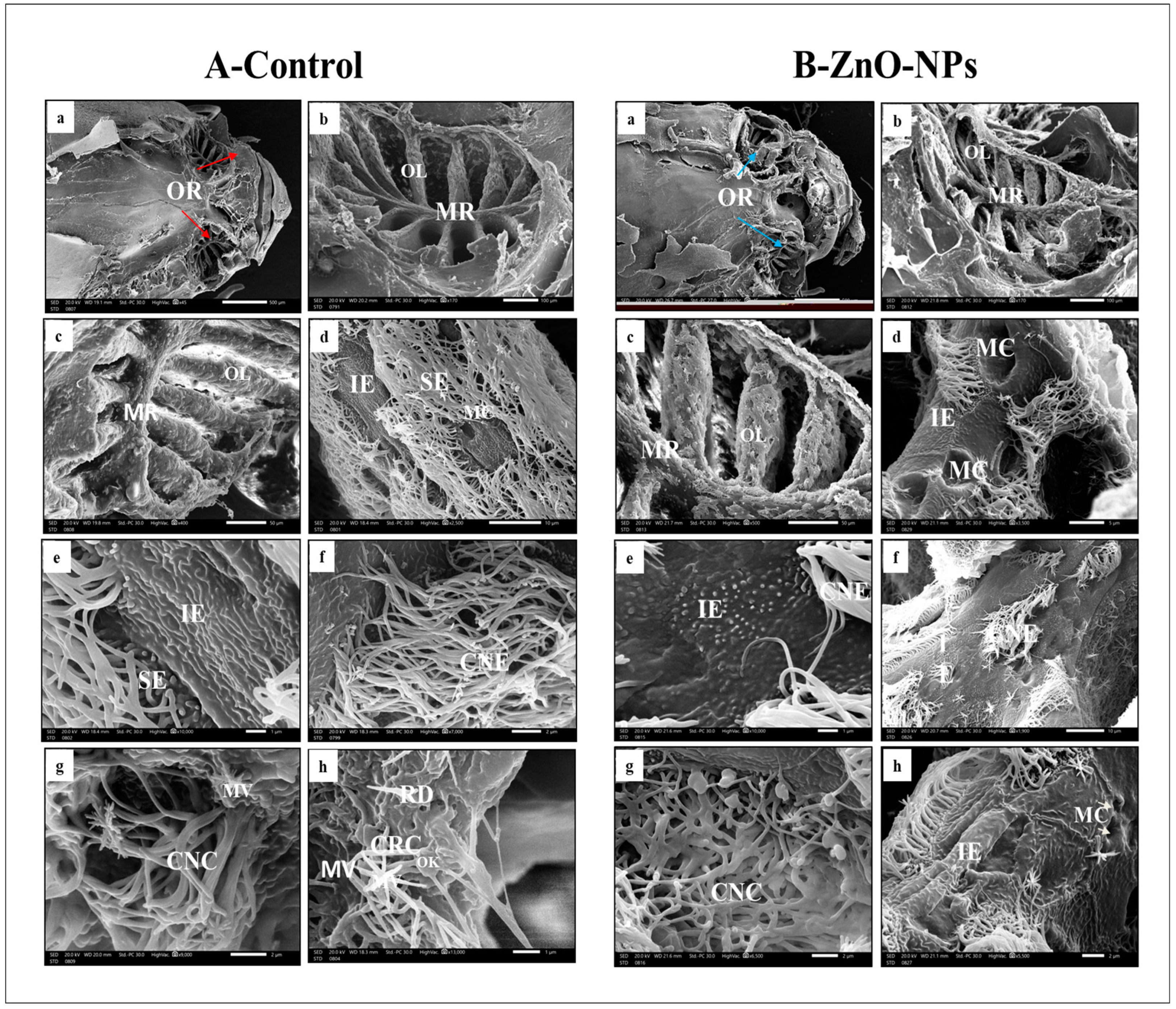
3.3. ZnO-NPs-Treated Rosette Exhibited Lamellar Sensory Area Destruction at the Scanning Electron Microscope (SEM) Level
- i.
- Ciliated receptor cells, their dendritic part protrudes slightly beyond the supporting cells’ boundary, forming a small terminal swelling hillock-like apex, the olfactory knob. From this knob, six to eight long cilia or flagella arise radially projecting into the lumen of the olfactory cavity (Figure 3A(h)).
- ii.
- Microvillous receptor cells with apical surfaces full of numerous shorter microvilli protruding into the olfactory space (Figure 3A (g,h)).
- iii.
- Rod cell shows rod-like projection and protrudes from its free surface by an olfactory knob. Rod neurons can be observed randomly between the ciliated sensory cells (Figure 3A(h)).
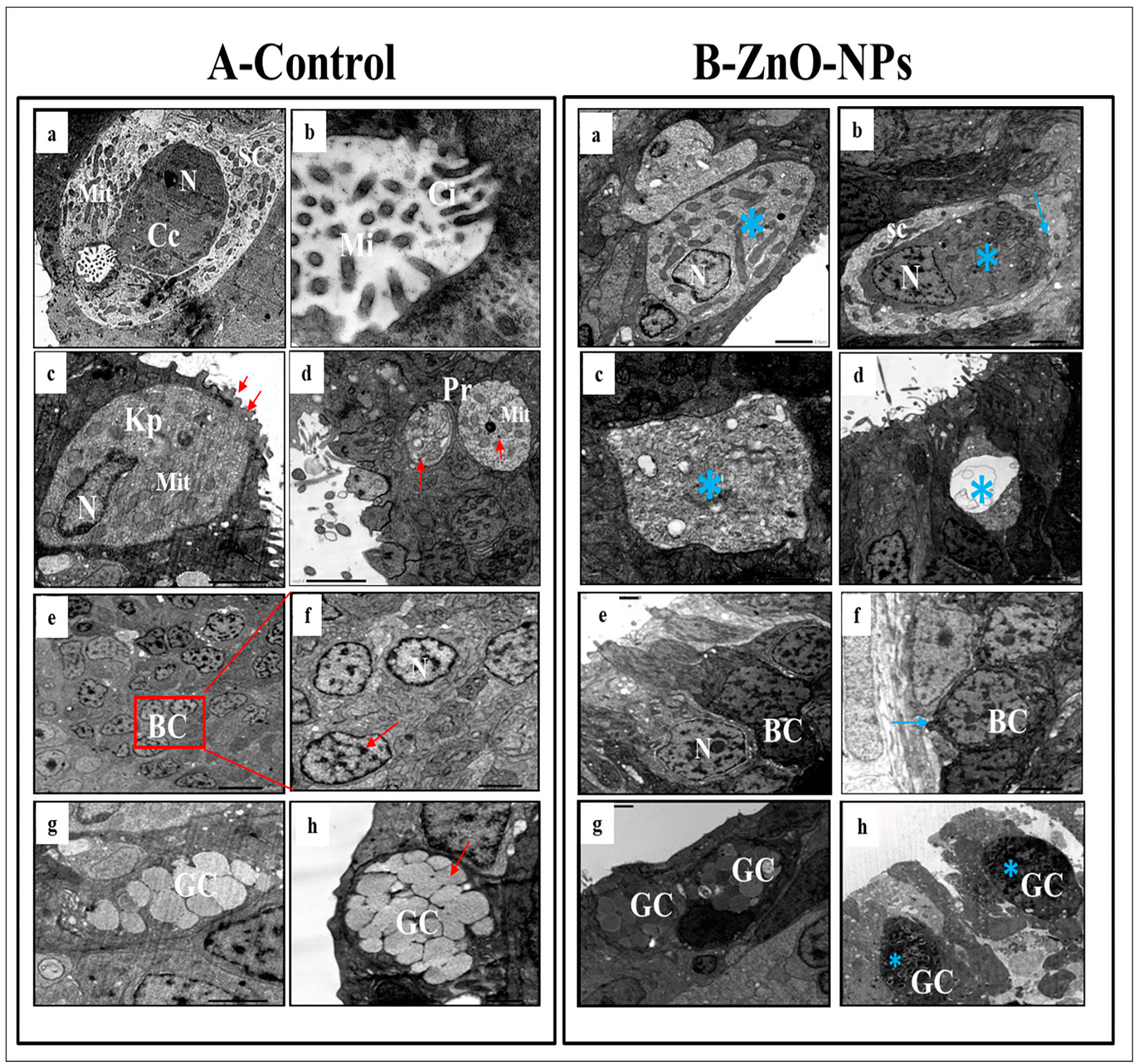
3.4. ZnO-NPs Induce Sensory Cell Apoptosis and Cytoplasmic Organelles Destruction at the Transmission Electron Microscope (TEM) Level
- i.
- Ciliated non-sensory cells are club-shaped with a narrow, deep surface and a relatively flat, broad, free surface. They do not have dendrites and axons but have numerous long rootlet cilia arising as a tuft oriented in the same direction from the cell’s surface (Figure 4A(a)). At the most apical cytoplasmic region, plentiful oval or rounded mitochondria are found without preferential orientation (Figure 4A(a,b)). Each cilium’s basal structure comprises a pair of centrioles and one rootlet (Figure 4A(c)). These cilia are kino-cilia, showing a typical axonemal pattern (9 + 2) of microtubules (mi) (Figure 4A(c,d)).
- ii.
- Ciliated receptor cells are long, slender-shaped bipolar sensory cells (neurons). Their most basally located somata are highly granulated cytoplasm packed with elongated or rounded mitochondria. A basal axonal process penetrates the basal lamina, and apical long dendritic processes extend toward the surface of the olfactory epithelium. These processes (cilia) randomly arise from an olfactory knob. Each cilium has a basal body without any rootlets but is associated with centrioles with several neuro-filaments present just beneath the plasma membrane of the olfactory knob (Figure 4A(e)).
- iii.
- Microvillous receptor cells are also elongated bipolar cells (neurons), their bodies featuring short dendrites and visible nuclei located at intermediate depths of the olfactory epithelium. Their apical surface is provided with a tuft of shorter dendrites, microvilli, which project radially outwards in the olfactory lumen from a concave olfactory knob under the surface level of the adjacent supporting cells. Some basal bodies are arranged in two rows at the microvilli’s base. Numerous elongated or rounded mitochondria are scattered in the cell cytoplasm (Figure 4A(f)).
- iv.
- Rod receptor cells are elongated bipolar cells (neurons) but differ from the other receptor cells in having a single thick cilium, rod-like projection, so they are known as rod receptor cells. This rod-like projection extends from a knob-like apex and has a pair of basal bodies at the base of its axonemal microtubules. As in all receptor cells, the cell body of a rod cell is fully packed with mitochondria scattered within its granulated cytoplasm (Figure 4A(g)).
- v.
- Rodlet cells are ovoid-shaped cells enclosed by a distinctive thick cuticula-like wall, found against the inner aspect of the cell plasma membrane, with a narrow apex pore. So, there is not any junction with neighboring cells. These cells have a typical animal cell structure with a basally located nucleus of various forms and shapes. Their cytoplasm is crowded with rounded to elongated vesicular mitochondria as well as few vesicular vacuoles and free ribosomes in addition to clusters of its most striking club or rod-shaped electron-opaque vesicles, rodlets (Figure 4A(h)).
- vi.
- Crypt cells are olfactory sensory neurons (OSNs) with elongated pear-shaped somata found near the apical surface of the sensory epithelium of examined zebrafish.
- vii.
- Kappe cells are found in the most apical epithelial positions close to the olfactory lumen. Their cell bodies are elongated pear-shaped, similar to crypt cells, but their inward superficial cap apical end is fortified with few cilia and microvilli. Their less flattened nuclei are positioned in the basal part of the cell body and swim in the ground cytoplasm with fully packed rounded mitochondria (Figure 5A(c)).
- viii.
- Morphologically, a pear-shaped cell is similar to Kappe cells but rounded and variable in size. It is located in the apical part of the epithelium, and its cytoplasm has numerous rounded mitochondria-free ribosomes (Figure 5A(d)).
- ix.
- Basal cells are intermingled with the basal portion of the other cell types in the olfactory epithelium. They are small polyhedral cells having a distinct globular shape and darkly stained centrally located nuclei. Basal cells are grouped and interposed by the bases of the sensory and non-sensory supporting cells, forming a discontinuous layer in the deeper part of the epithelium just above the basal lamina and did not reach the free surface of the epithelium (Figure 5A(e,f)).
- x.
- Goblet mucous cells are restricted to the non-sensory epithelium. They are oval-shaped, having basally located nuclei, and about two-thirds of the cell body is filled with large mucous granules. They are surrounded by ciliated non-sensory or epidermal cells bearing micro-ridges. Mature goblet cells secrete their mucous granules into the lumen of the olfactory cavity over the non-sensory epidermal cell surface (Figure 5A(g,h)).
3.5. ZnO-NPs Induce Apoptosis in the Olfactory Epithelium via Mediating Oxidative Stress and DNA Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanoparticle Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012, 14, 1109. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Pezzuto, F.; Scarano, A. Cytotoxic and Bacteriostatic Activity of Nanostructured TiO2 Coatings. Pol. J. Microbiol. 2016, 65, 225–229. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Mescola, A.; Iseppi, R.; Canton, R.; Rossi, G.; Stocchi, R.; Loschi, A.R.; Alessandrini, A.; Rea, S.; Sabia, C. Antibacterial Effect of Aluminum Surfaces Untreated and Treated with a Special Anodizing Based on Titanium Oxide Approved for Food Contact. Biology 2020, 9, 456. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Rosace, G.; Rea, S.; Stocchi, R.; Morales-Medina, J.C.; Canton, R.; Mescola, A.; Condo, C.; Loschi, A.R.; Sabia, C. Time-Course Study of the Antibacterial Activity of an Amorphous SiO(x)C(y)H(z) Coating Certified for Food Contact. Antibiotics 2021, 10, 901. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Mescola, A.; Rosace, G.; Stocchi, R.; Rossi, G.; Alessandrini, A.; Preziuso, S.; Scarano, A.; Rea, S.; Loschi, A.R.; et al. Antibacterial Effect of Stainless Steel Surfaces Treated with a Nanotechnological Coating Approved for Food Contact. Microorganisms 2021, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Berube, D.M. Rhetorical gamesmanship in the nano debates over sunscreens and nanoparticles. J. Nanoparticle Res. 2008, 10, 23–37. [Google Scholar] [CrossRef][Green Version]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, N.; Mendler, S.; Buhr, C.R.; Ritz, U.; Kammerer, P.W.; Brieger, J. Zinc Oxide Nanoparticles Exhibit Favorable Properties to Promote Tissue Integration of Biomaterials. Biomedicines 2021, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nayak, T.R.; Hong, H.; Cai, W. Biomedical applications of zinc oxide nanomaterials. Curr. Mol. Med. 2013, 13, 1633–1645. [Google Scholar] [CrossRef]
- Ahamed, M.; Khan, M.A.M.; Akhtar, M.J.; Alhadlaq, H.A.; Alshamsan, A. Role of Zn doping in oxidative stress mediated cytotoxicity of TiO2 nanoparticles in human breast cancer MCF-7 cells. Sci. Rep. 2016, 6, 30196. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative Toxicity of Nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a Freshwater Microalga (Pseudokirchneriella subcapitata): The Importance of Particle Solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Chandan, N.K.; Wakchaure, G.C.; Singh, N.P. Synergistic effect of zinc nanoparticles and temperature on acute toxicity with response to biochemical markers and histopathological attributes in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 229, 108678. [Google Scholar] [CrossRef]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.K.; Jung, M.; Kim, K.H.; Chung, N.; Ahn, E.K.; Lim, Y.; Lee, K.H. Cellular toxicity of various inhalable metal nanoparticles on human alveolar epithelial cells. Inhal. Toxicol. 2007, 19 (Suppl. 1), 59–65. [Google Scholar] [CrossRef]
- Sardoiwala, M.N.; Kaundal, B.; Choudhury, S.R. Toxic impact of nanomaterials on microbes, plants and animals. Environ. Chem. Lett. 2018, 16, 147–160. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Khalil, S.R.; Zheng, C.; Abou-Zeid, S.M.; Farag, M.R.; Elsabbagh, H.S.; Siddique, M.S.; Azzam, M.M.; Di Cerbo, A.; Elkhadrawey, B.A. Modulatory effect of thymol on the immune response and susceptibility to Aeromonas hydrophila infection in Nile tilapia fish exposed to zinc oxide nanoparticles. Aquat. Toxicol. 2023, 259, 106523. [Google Scholar] [CrossRef]
- Mawed, S.A.; Centoducati, G.; Farag, M.R.; Alagawany, M.; Abou-Zeid, S.M.; Elhady, W.M.; El-Saadony, M.T.; Di Cerbo, A.; Al-Zahaby, S.A. Dunaliella salina Microalga Restores the Metabolic Equilibrium and Ameliorates the Hepatic Inflammatory Response Induced by Zinc Oxide Nanoparticles (ZnO-NPs) in Male Zebrafish. Biology 2022, 11, 1447. [Google Scholar] [CrossRef] [PubMed]
- Di Cerbo, A.; Canello, S.; Guidetti, G.; Fiore, F.; Corsi, L.; Rubattu, N.; Testa, C.; Cocco, R. Adverse food reactions in dogs due to antibiotic residues in pet food: A preliminary study. Vet. Ital. 2018, 54, 137–146. [Google Scholar] [CrossRef]
- Arunachalam, M.; Raja, M.; Vijayakumar, C.; Malaiammal, P.; Mayden, R.L. Natural history of zebrafish (Danio rerio) in India. Zebrafish 2013, 10, 1–14. [Google Scholar] [CrossRef]
- Schilling, T.F.; Webb, J. Considering the zebrafish in a comparative context. J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 515–522. [Google Scholar] [CrossRef]
- Becker, C.G.; Becker, T. Adult zebrafish as a model for successful central nervous system regeneration. Restor. Neurol. Neurosci. 2008, 26, 71–80. [Google Scholar] [PubMed]
- Choi, J.S.; Kim, R.O.; Yoon, S.; Kim, W.K. Developmental Toxicity of Zinc Oxide Nanoparticles to Zebrafish (Danio rerio): A Transcriptomic Analysis. PLoS ONE 2016, 11, e0160763. [Google Scholar] [CrossRef]
- Kteeba, S.M.; El-Ghobashy, A.E.; El-Adawi, H.I.; El-Rayis, O.A.; Sreevidya, V.S.; Guo, L.; Svoboda, K.R. Exposure to ZnO nanoparticles alters neuronal and vascular development in zebrafish: Acute and transgenerational effects mitigated with dissolved organic matter. Environ. Pollut. 2018, 242, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Brooker, R.M.; Dixson, D.L. Assessing the Role of Olfactory Cues in the Early Life History of Coral Reef Fish: Current Methods and Future Directions. In Chemical Signals in Vertebrates 13; Springer: Cham, Switzerland, 2016; pp. 17–31. [Google Scholar]
- Kondo, K.; Kikuta, S.; Ueha, R.; Suzukawa, K.; Yamasoba, T. Age-Related Olfactory Dysfunction: Epidemiology, Pathophysiology, and Clinical Management. Front. Aging Neurosci. 2020, 12, 208. [Google Scholar] [CrossRef]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfaction and Aging: A Mini-Review. Gerontology 2015, 61, 485–490. [Google Scholar] [CrossRef]
- Hummel, T.; Whitcroft, K.L.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.M.; Damm, M.; Frasnelli, J.; Gudziol, H.; Gupta, N.; et al. Position paper on olfactory dysfunction. Rhinology 2016, 56, 1–30. [Google Scholar] [CrossRef]
- Liu, B.; Luo, Z.; Pinto, J.M.; Shiroma, E.J.; Tranah, G.J.; Wirdefeldt, K.; Fang, F.; Harris, T.B.; Chen, H. Relationship Between Poor Olfaction and Mortality among Community-Dwelling Older Adults: A Cohort Study. Ann. Intern. Med. 2019, 170, 673–681. [Google Scholar] [CrossRef]
- Schubert, C.R.; Fischer, M.E.; Pinto, A.A.; Klein, B.E.K.; Klein, R.; Tweed, T.S.; Cruickshanks, K.J. Sensory Impairments and Risk of Mortality in Older Adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2017, 72, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Razmara, P.; Lari, E.; Mohaddes, E.; Zhang, Y.; Goss, G.G.; Pyle, G.G. The effect of copper nanoparticles on olfaction in rainbow trout (Oncorhynchus mykiss). Environ. Sci. Nano 2019, 6, 2094–2104. [Google Scholar] [CrossRef]
- Bilberg, K.; Døving, K.B.; Beedholm, K.; Baatrup, E. Silver nanoparticles disrupt olfaction in Crucian carp (Carassius carassius) and Eurasian perch (Perca fluviatilis). Aquat. Toxicol. 2011, 104, 145–152. [Google Scholar] [CrossRef]
- Gao, L.; Yang, S.T.; Li, S.; Meng, Y.; Wang, H.; Lei, H. Acute toxicity of zinc oxide nanoparticles to the rat olfactory system after intranasal instillation. J. Appl. Toxicol. 2013, 33, 1079–1088. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Mawed, S.A.; Marini, C.; Alagawany, M.; Farag, M.R.; Reda, R.M.; El-Saadony, M.T.; Elhady, W.M.; Magi, G.E.; Di Cerbo, A.; El-Nagar, W.G. Zinc Oxide Nanoparticles (ZnO-NPs) Suppress Fertility by Activating Autophagy, Apoptosis, and Oxidative Stress in the Developing Oocytes of Female Zebrafish. Antioxidants 2022, 11, 1567. [Google Scholar] [CrossRef] [PubMed]
- Ruzhinskaya, N.N.; Gdovskii, P.A.; Devitsina, G.V. Chloride Cells, A Constituent of the Fish Olfactory Epithelium. J. Evol. Biochem. Physiol. 2001, 37, 89–94. [Google Scholar] [CrossRef]
- Slaoui, M.; Fiette, L. Histopathology procedures: From tissue sampling to histopathological evaluation. Drug Saf. Eval. Methods Protoc. 2011, 691, 69–82. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Canello, S.; Guidetti, G.; Laurino, C.; Palmieri, B. Unusual antibiotic presence in gym trained subjects with food intolerance; a case report. Nutr. Hosp. 2014, 30, 395–398. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Palmieri, B. Review: The market of probiotics. Pak. J. Pharm. Sci. 2015, 28, 2199–2206. [Google Scholar]
- Mazzeranghi, F.; Zanotti, C.; Di Cerbo, A.; Verstegen, J.P.; Cocco, R.; Guidetti, G.; Canello, S. Clinical efficacy of nutraceutical diet for cats with clinical signs of cutaneus adverse food reaction (CAFR). Pol. J. Vet. Sci. 2017, 20, 269–276. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Rubino, V.; Morelli, F.; Ruggiero, G.; Landi, R.; Guidetti, G.; Canello, S.; Terrazzano, G.; Alessandrini, A. Mechanical phenotyping of K562 cells by the Micropipette Aspiration Technique allows identifying mechanical changes induced by drugs. Sci. Rep. 2018, 8, 1219. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Palmieri, B. The economic impact of second opinion in pathology. Saudi Med. J. 2012, 33, 1051–1052. [Google Scholar]
- Iannitti, T.; Palmieri, B.; Aspiro, A.; Di Cerbo, A. A preliminary study of painless and effective transdermal botulinum toxin A delivery by jet nebulization for treatment of primary hyperhidrosis. Drug Des. Dev. Ther. 2014, 8, 931–935. [Google Scholar] [CrossRef]
- Destefanis, S.; Giretto, D.; Muscolo, M.C.; Di Cerbo, A.; Guidetti, G.; Canello, S.; Giovazzino, A.; Centenaro, S.; Terrazzano, G. Clinical evaluation of a nutraceutical diet as an adjuvant to pharmacological treatment in dogs affected by Keratoconjunctivitis sicca. BMC Vet. Res. 2016, 12, 214. [Google Scholar] [CrossRef]
- Farag, M.R.; Zizzadoro, C.; Alagawany, M.; Abou-Zeid, S.M.; Mawed, S.A.; El Kholy, M.S.; Di Cerbo, A.; Azzam, M.M.; Mahdy, E.A.A.; Khedr, M.H.E.; et al. In ovo protective effects of chicoric and rosmarinic acids against Thiacloprid-induced cytotoxicity, oxidative stress, and growth retardation on newly hatched chicks. Poult. Sci. 2023, 102, 102487. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.R.; Abo-Al-Ela, H.G.; Alagawany, M.; Azzam, M.M.; El-Saadony, M.T.; Rea, S.; Di Cerbo, A.; Nouh, D.S. Effect of Quercetin Nanoparticles on Hepatic and Intestinal Enzymes and Stress-Related Genes in Nile Tilapia Fish Exposed to Silver Nanoparticles. Biomedicines 2023, 11, 663. [Google Scholar] [CrossRef]
- Farag, M.R.; Moselhy, A.A.A.; El-Mleeh, A.; Aljuaydi, S.H.; Ismail, T.A.; Di Cerbo, A.; Crescenzo, G.; Abou-Zeid, S.M. Quercetin Alleviates the Immunotoxic Impact Mediated by Oxidative Stress and Inflammation Induced by Doxorubicin Exposure in Rats. Antioxidants 2021, 10, 1906. [Google Scholar] [CrossRef]
- Farag, M.R.; Khalil, S.R.; Zaglool, A.W.; Hendam, B.M.; Moustafa, A.A.; Cocco, R.; Di Cerbo, A.; Alagawany, M. Thiacloprid Induced Developmental Neurotoxicity via ROS-Oxidative Injury and Inflammation in Chicken Embryo: The Possible Attenuating Role of Chicoric and Rosmarinic Acids. Biology 2021, 10, 1100. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Landi, R.; Rubino, V.; Di Cerbo, A.; Giovazzino, A.; Palatucci, A.T.; Centenaro, S.; Guidetti, G.; Canello, S.; Cortese, L.; et al. Oxytetracycline induces DNA damage and epigenetic changes: A possible risk for human and animal health? PeerJ 2017, 5, e3236. [Google Scholar] [CrossRef]
- Guildford, A.L.; Poletti, T.; Osbourne, L.H.; Di Cerbo, A.; Gatti, A.M.; Santin, M. Nanoparticles of a different source induce different patterns of activation in key biochemical and cellular components of the host response. J. R. Soc. Interface 2009, 6, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Lebeaupin, C.; Vallee, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef]
- Hentig, J.T.; Byrd-Jacobs, C.A. Exposure to Zinc Sulfate Results in Differential Effects on Olfactory Sensory Neuron Subtypes in Adult Zebrafish. Int. J. Mol. Sci. 2016, 17, 1445. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, Z.; Tian, W.; He, X.; Ma, Y.; Zhao, Y.; Chai, Z. Toxicity of zinc oxide nanoparticles to zebrafish embryo: A physicochemical study of toxicity mechanism. J. Nanoparticle Res. 2010, 12, 1645–1654. [Google Scholar] [CrossRef]
- Du, J.; Wang, S.; You, H.; Jiang, R.; Zhuang, C.; Zhang, X. Developmental toxicity and DNA damage to zebrafish induced by perfluorooctane sulfonate in the presence of ZnO nanoparticles. Environ. Toxicol. 2016, 31, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Liu, H.; Zhang, S.; Liu, X.; Hayat, T.; Alsaedi, A.; Wang, X. Mechanism of toxic effects of Nano-ZnO on cell cycle of zebrafish (Danio rerio). Chemosphere 2019, 229, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kteeba, S.M.; El-Adawi, H.I.; El-Rayis, O.A.; El-Ghobashy, A.E.; Schuld, J.L.; Svoboda, K.R.; Guo, L. Zinc oxide nanoparticle toxicity in embryonic zebrafish: Mitigation with different natural organic matter. Environ. Pollut. 2017, 230, 1125–1140. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Lee, B.; Kim, K.-T. Mechanisms and effects of zinc oxide nanoparticle transformations on toxicity to zebrafish embryos. Environ. Sci. Nano 2021, 8, 1690–1700. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wu, Y.; You, H.; Lv, L. Acute ZnO nanoparticles exposure induces developmental toxicity, oxidative stress and DNA damage in embryo-larval zebrafish. Aquat. Toxicol. 2013, 136–137, 49–59. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio). Nanotechnology 2009, 20, 195103. [Google Scholar] [CrossRef]
- Wyatt, T.D. Pheromones and signature mixtures: Defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 2010, 196, 685–700. [Google Scholar] [CrossRef]
- Eisthen, H.L. Evolution of vertebrate olfactory systems. Brain Behav. Evol. 1997, 50, 222–233. [Google Scholar] [CrossRef]
- Hamdani, E.H.; Doving, K.B. Specific projection of the sensory crypt cells in the olfactory system in crucian carp, Carassius carassius. Chem. Senses 2006, 31, 63–67. [Google Scholar] [CrossRef]
- Døving, K.; Kasumyan, A. Chemoreception. In Fish Larval Physiology; CRC Press: Boca Raton, FL, USA, 2008; pp. 331–394. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Sechi, S.; Canello, S.; Guidetti, G.; Fiore, F.; Cocco, R. Behavioral Disturbances: An Innovative Approach to Monitor the Modulatory Effects of a Nutraceutical Diet. J. Vis. Exp. 2017, 119, e54878. [Google Scholar] [CrossRef]
- Sechi, S.; Di Cerbo, A.; Canello, S.; Guidetti, G.; Chiavolelli, F.; Fiore, F.; Cocco, R. Effects in dogs with behavioural disorders of a commercial nutraceutical diet on stress and neuroendocrine parameters. Vet. Rec. 2017, 180, 18. [Google Scholar] [CrossRef] [PubMed]
- Kermen, F.; Franco, L.; Wyatt, C.; Yaksi, E. Neural circuits mediating olfactory-driven behavior in fish. Front. Neural Circuits 2013, 7, 62. [Google Scholar] [CrossRef]
- Biechl, D.; Tietje, K.; Gerlach, G.; Wullimann, M.F. Crypt cells are involved in kin recognition in larval zebrafish. Sci. Rep. 2016, 6, 24590. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, M.; Bettini, S.; Milani, L.; Maurizii, M.G.; Franceschini, V. Differential response of olfactory sensory neuron populations to copper ion exposure in zebrafish. Aquat. Toxicol. 2017, 183, 54–62. [Google Scholar] [CrossRef]
- Hansen, A.; Zeiske, E. The peripheral olfactory organ of the zebrafish, Danio rerio: An ultrastructural study. Chem. Senses 1998, 23, 39–48. [Google Scholar] [CrossRef]
- Hansen, A.; Zielinski, B.S. Diversity in the olfactory epithelium of bony fishes: Development, lamellar arrangement, sensory neuron cell types and transduction components. J. Neurocytol. 2005, 34, 183–208. [Google Scholar] [CrossRef]
- Miyasaka, N.; Wanner, A.A.; Li, J.; Mack-Bucher, J.; Genoud, C.; Yoshihara, Y.; Friedrich, R.W. Functional development of the olfactory system in zebrafish. Mech. Dev. 2013, 130, 336–346. [Google Scholar] [CrossRef]
- Olivares, J.; Schmachtenberg, O. An update on anatomy and function of the teleost olfactory system. PeerJ 2019, 7, e7808. [Google Scholar] [CrossRef]
- Calvo-Ochoa, E.; Byrd-Jacobs, C.A. The Olfactory System of Zebrafish as a Model for the Study of Neurotoxicity and Injury: Implications for Neuroplasticity and Disease. Int. J. Mol. Sci. 2019, 20, 1639. [Google Scholar] [CrossRef] [PubMed]
- Gayoso, J.; Castro, A.; Anadon, R.; Manso, M.J. Crypt cells of the zebrafish Danio rerio mainly project to the dorsomedial glomerular field of the olfactory bulb. Chem. Senses 2012, 37, 357–369. [Google Scholar] [CrossRef]
- Ahuja, G.; Nia, S.B.; Zapilko, V.; Shiriagin, V.; Kowatschew, D.; Oka, Y.; Korsching, S.I. Kappe neurons, a novel population of olfactory sensory neurons. Sci. Rep. 2014, 4, 4037. [Google Scholar] [CrossRef]
- Hansen, A.; Finger, T.E. Phyletic distribution of crypt-type olfactory receptor neurons in fishes. Brain Behav Evol 2000, 55, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Rolen, S.H.; Anderson, K.; Morita, Y.; Caprio, J.; Finger, T.E. Correlation between olfactory receptor cell type and function in the channel catfish. J. Neurosci. 2003, 23, 9328–9339. [Google Scholar] [CrossRef]
- Lipschitz, D.L.; Michel, W.C. Amino acid odorants stimulate microvillar sensory neurons. Chem. Senses 2002, 27, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Miyasaka, N.; Yoshihara, Y. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J. Neurosci. 2005, 25, 4889–4897. [Google Scholar] [CrossRef]
- Hara, T.J.; Zhang, C. Topographic bulbar projections and dual neural pathways of the primary olfactory neurons in salmonid fishes. Neuroscience 1998, 82, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Suzuki, N. Whole-cell response characteristics of ciliated and microvillous olfactory receptor neurons to amino acids, pheromone candidates and urine in rainbow trout. Chem. Senses 2001, 26, 1145–1156. [Google Scholar] [CrossRef]
- Oka, Y.; Korsching, S.I. Shared and unique G alpha proteins in the zebrafish versus mammalian senses of taste and smell. Chem. Senses 2011, 36, 357–365. [Google Scholar] [CrossRef]
- Cancalon, P. Degeneration and regeneration of olfactory cells induced by ZnSO4 and other chemicals. Tissue Cell 1982, 14, 717–733. [Google Scholar] [CrossRef]
- Bazaes, A.; Schmachtenberg, O. Odorant tuning of olfactory crypt cells from juvenile and adult rainbow trout. J. Exp. Biol. 2012, 215, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, S.; Bottaro, M.; Gallus, L.; Girosi, L.; Vacchi, M.; Tagliafierro, G. Observations of crypt neuron-like cells in the olfactory epithelium of a cartilaginous fish. Neurosci. Lett. 2006, 403, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, E.H.; Lastein, S.; Gregersen, F.; Doving, K.B. Seasonal variations in olfactory sensory neurons—Fish sensitivity to sex pheromones explained? Chem. Senses 2008, 33, 119–123. [Google Scholar] [CrossRef]
- Wakisaka, N.; Miyasaka, N.; Koide, T.; Masuda, M.; Hiraki-Kajiyama, T.; Yoshihara, Y. An Adenosine Receptor for Olfaction in Fish. Curr. Biol. 2017, 27, 1437–1447.E4. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.Y.; Jesuthasan, S.J.; Baxendale, S.; van Hateren, N.J.; Marzo, M.; Hill, C.J.; Whitfield, T.T. Olfactory Rod Cells: A Rare Cell Type in the Larval Zebrafish Olfactory Epithelium with a Large Actin-Rich Apical Projection. Front. Physiol. 2021, 12, 626080. [Google Scholar] [CrossRef]
- Elsaesser, R.; Paysan, J. The sense of smell, its signalling pathways, and the dichotomy of cilia and microvilli in olfactory sensory cells. BMC Neurosci. 2007, 8, S1. [Google Scholar] [CrossRef]
- Yamamoto, M. Comparative Morphology of the Peripheral Olfactory Organ in Teleosts. In Chemoreception in Fishes; Hara, T.J., Ed.; Elsevier: New York, NY, USA, 1982; pp. 39–59. [Google Scholar]
- Ichikawa, M.; Ueda, K. Fine structure of the olfactory epithelium in the goldfish, Carassius auratus. Cell Tissue Res. 1977, 183, 445–455. [Google Scholar] [CrossRef]
- Rhein, L.D.; Cagan, R.H.; Orkand, P.M.; Dolack, M.K. Surface specializations of the olfactory epithelium of rainbow trout, Salmo gairdneri. Tissue Cell 1981, 13, 577–587. [Google Scholar] [CrossRef]
- Hernadi, L. Fine structural characterization of the olfactory epithelium and its response to divalent cations Cd2+ in the fish Alburnus alburnus (Teleostei, Cyprinidae): A scanning and transmission electron microscopic study. Neurobiology 1993, 1, 11–31. [Google Scholar]
- Datta, N.C.; Bandopadhyay, S. Ultrastructure of cell types of the olfactory epithelium in a catfish, Heteropneustesfossilis (Bloch). J. Biosci. 1997, 22, 233–245. [Google Scholar] [CrossRef]
- Ismail, M.H.; Salem, M.A.; Nassar, S.A.; Mawed, S.A. Comparative morphological and histological studies on the olfactory organ in two models of bony fishes (Mugil cephalus and Malapterurus electricus): Light and electron microscopic studies. Egypt. J. Zool. 2013, 60, 223–244. [Google Scholar] [CrossRef]
- Waryani, B.; Zhao, Y.; Zhang, C.; Abbasi, A.R.; Ferrando, S.; Dai, R.; Soomro, A.N.; Baloch, W.A.; Abbas, G. Surface architecture of the olfactory epithelium of two Chinese cave loaches (Cypriniformes: Nemacheilidae: Oreonectes). Ital. J. Zool. 2015, 82, 179–185. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.Y.; Huang, Z.Q.; Ning, T.; Xiang, X.H.; Li, C.Q.; Chen, S.Y.; Xiao, H. Microscopic and Submicroscopic Gradient Variation of Olfactory Systems among Six Sinocyclocheilus Species Living in Different Environments. Zool. Sci. 2018, 35, 411–420. [Google Scholar] [CrossRef]
- Zielinski, B.S.; Hara, T.J. Ciliated and microvillar receptor cells degenerate and then differentiate in the olfactory epithelium of rainbow trout following olfactory nerve section. Microsc. Res. Tech. 1992, 23, 22–27. [Google Scholar] [CrossRef]
- Pashchenko, N.I.; Kasumyan, A.O. Scanning electron microscopy of development of the olfactory organ in ontogeny of grass carp Ctenopharyngodon idella. J. Ichthyol. 2015, 55, 880–899. [Google Scholar] [CrossRef]
- DePasquale, J.A. Tropomyosin and alpha-actinin in teleost rodlet cells. Acta Zool. 2021, 102, 323–332. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Capuano, S.; Simoni, E.; Previati, M.; Giari, L. Rodlet cells and the sensory systems in zebrafish (Danio rerio). Anat. Rec. 2007, 290, 367–374. [Google Scholar] [CrossRef]
- Al-Hussaini, A.H. On the Nature of Certain Pear-Shaped Cells in the Intestinal Epithelium of Fish. Bull. Inst. Egypte 1964, 40, 23–31. [Google Scholar]
- Grunberg, W.; Hager, G. Ultrastructural and histochemical aspects of the rodlet cells from the bulbus arteriosus of Cyprinus carpio L. (Pisces: Cyprinidae) (author’s transl). Anat. Anzeiger. 1978, 143, 277–290. [Google Scholar]
- Dezfuli, B.S.; Simoni, E.; Rossi, R.; Manera, M. Rodlet cells and other inflammatory cells of Phoxinus phoxinus infected with Raphidascaris acus (Nematoda). Dis. Aquat. Org. 2000, 43, 61–69. [Google Scholar] [CrossRef]
- Blaiotta, G.; Murru, N.; Di Cerbo, A.; Succi, M.; Coppola, R.; Aponte, M. Commercially standardized process for probiotic “Italico” cheese production. LWT-Food Sci. Technol. 2017, 79, 601–608. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Capuano, S.; Manera, M. A description of rodlet cells from the alimentary canal of Anguilla anguilla and their relationship with parasitic helminths. J. Fish Biol. 1998, 53, 1084–1095. [Google Scholar] [CrossRef]
- Fishelson, L.; Becker, K. Rodlet cells in the head and trunk kidney of the domestic carp (Cyprinus carpio): Enigmatic gland cells or coccidian parasites? Naturwissenschaften 1999, 86, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Iger, Y.; Abraham, M. Rodlet cells in the epidermis of fish exposed to stressors. Tissue Cell 1997, 29, 431–438. [Google Scholar] [CrossRef]
- Scarano, A.; Murmura, G.; Vantaggiato, G.; Lauritano, D.; Silvestre-Rangil, J.; Di Cerbo, A.; Lorusso, F. Delayed expansion of atrophic mandible (deam): A case report. Oral Implant. 2017, 10, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Pironi, F.; Maynard, B.; Simoni, E.; Bosi, G. Rodlet cells, fish immune cells and a sentinel of parasitic harm in teleost organs. Fish Shellfish Immunol. 2022, 121, 516–534. [Google Scholar] [CrossRef] [PubMed]
- Viswaprakash, N.; Dennis, J.C.; Globa, L.; Pustovyy, O.; Josephson, E.M.; Kanju, P.; Morrison, E.E.; Vodyanoy, V.J. Enhancement of odorant-induced responses in olfactory receptor neurons by zinc nanoparticles. Chem. Senses 2009, 34, 547–557. [Google Scholar] [CrossRef]
- Lazzari, M.; Bettini, S.; Ciani, F.; Franceschini, V. Light and transmission electron microscopy study of the peripheral olfactory organ of the guppy, Poecilia reticulata (Teleostei, Poecilidae). Microsc. Res. Tech. 2007, 70, 782–789. [Google Scholar] [CrossRef]
- Lazzari, M.; Bettini, S.; Milani, L.; Maurizii, M.G.; Franceschini, V. Differential nickel-induced responses of olfactory sensory neuron populations in zebrafish. Aquat. Toxicol. 2019, 206, 14–23. [Google Scholar] [CrossRef]
- Lazzari, M.; Bettini, S.; Milani, L.; Maurizii, M.G.; Franceschini, V. Response of Olfactory Sensory Neurons to Mercury Ions in Zebrafish: An Immunohistochemical Study. Microsc. Microanal. 2022, 28, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Razmara, P.; Pyle, G.G. Recovery of rainbow trout olfactory function following exposure to copper nanoparticles and copper ions. Aquat. Toxicol. 2022, 245, 106109. [Google Scholar] [CrossRef]
- Ghosh, D.; Mandal, D.K. Mercuric chloride induced toxicity responses in the olfactory epithelium of Labeo rohita (Hamilton): A light and electron microscopy study. Fish Physiol. Biochem. 2014, 40, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Ghosh, D.; Mandal, D.K. Induction of Metallothionein in the Olfactory Epithelium of Channa punctatus (Bloch) in Response to Cadmium Exposure: An Immunohistochemical Study. Proc. Zool. Soc. 2012, 65, 40–44. [Google Scholar] [CrossRef]
- Saliani, M.; Jalal, R.; Goharshadi, E. Mechanism of oxidative stress involved in the toxicity of ZnO nanoparticles against eukaryotic cells. Nanomed. J. 2016, 3, 1–14. Available online: https://profdoc.um.ac.ir/paper-abstract-1053987.html (accessed on 29 August 2023).
- Verma, S.K.; Panda, P.K.; Jha, E.; Suar, M.; Parashar, S.K.S. Altered physiochemical properties in industrially synthesized ZnO nanoparticles regulate oxidative stress; induce in vivo cytotoxicity in embryonic zebrafish by apoptosis. Sci. Rep. 2017, 7, 13909. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noe, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Hwang, J.; Jin, J.; Jeon, S.; Moon, S.H.; Park, M.Y.; Yum, D.Y.; Kim, J.H.; Kang, J.E.; Park, M.H.; Kim, E.J.; et al. SOD1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis. Redox Biol. 2020, 37, 101760. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Preedia Babu, E.; Subastri, A.; Suyavaran, A.; Lokeshwara Rao, P.; Suresh Kumar, M.; Jeevaratnam, K.; Thirunavukkarasu, C. Extracellularly synthesized ZnO nanoparticles interact with DNA and augment gamma radiation induced DNA damage through reactive oxygen species. RSC Adv. 2015, 5, 62067–62077. [Google Scholar] [CrossRef]
- Barzilai, A.; Yamamoto, K. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Panda, K.K.; Golari, D.; Venugopal, A.; Achary, V.M.M.; Phaomei, G.; Parinandi, N.L.; Sahu, H.K.; Panda, B.B. Green Synthesized Zinc Oxide (ZnO) Nanoparticles Induce Oxidative Stress and DNA Damage in Lathyrus sativus L. Root Bioassay System. Antioxidants 2017, 6, 35. [Google Scholar] [CrossRef]
- Payne, C.M.; Bernstein, C.; Bernstein, H. Apoptosis overview emphasizing the role of oxidative stress, DNA damage and signal-transduction pathways. Leuk. Lymphoma 1995, 19, 43–93. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Pezzuto, F.; Guidetti, G.; Canello, S.; Corsi, L. Tetracyclines: Insights and updates of their use in human and animal pathology and their potential toxicity. Open Biochem. J. 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Chen, Z.; Wan, X.; Hou, Q.; Shi, S.; Wang, L.; Chen, P.; Zhu, X.; Zeng, C.; Qin, W.; Zhou, W.; et al. GADD45B mediates podocyte injury in zebrafish by activating the ROS-GADD45B-p38 pathway. Cell Death Dis. 2016, 7, e2068. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Najafzadeh, M.; Jacob, B.K.; Dhawan, A.; Anderson, D. Zinc oxide nanoparticles affect the expression of p53, Ras p21 and JNKs: An ex vivo/in vitro exposure study in respiratory disease patients. Mutagenesis 2015, 30, 237–245. [Google Scholar] [CrossRef]
- Macleod, K.F.; Sherry, N.; Hannon, G.; Beach, D.; Tokino, T.; Kinzler, K.; Vogelstein, B.; Jacks, T. p53-dependent and independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 1995, 9, 935–944. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Leong, D.T. Effect of zinc oxide nanomaterials-induced oxidative stress on the p53 pathway. Biomaterials 2013, 34, 10133–10142. [Google Scholar] [CrossRef]
- Ng, K.W.; Khoo, S.P.; Heng, B.C.; Setyawati, M.I.; Tan, E.C.; Zhao, X.; Xiong, S.; Fang, W.; Leong, D.T.; Loo, J.S. The role of the tumor suppressor p53 pathway in the cellular DNA damage response to zinc oxide nanoparticles. Biomaterials 2011, 32, 8218–8225. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.J.; Zhivotovsky, B. DNA damage-induced apoptosis. Oncogene 2004, 23, 2797–2808. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006, 12, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, X.; Gao, Y.; Ji, Y. ZnO Nanoparticles Treatment Induces Apoptosis by Increasing Intracellular ROS Levels in LTEP-a-2 Cells. BioMed Res. Int. 2015, 2015, 423287. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Lei, X.; Zhang, Q. Moderate activation of IKK2-NF-kB in unstressed adult mouse liver induces cytoprotective genes and lipogenesis without apparent signs of inflammation or fibrosis. BMC Gastroenterol. 2015, 15, 94. [Google Scholar] [CrossRef]
- Du, J.; Cai, J.; Wang, S.; You, H. Oxidative stress and apotosis to zebrafish (Danio rerio) embryos exposed to perfluorooctane sulfonate (PFOS) and ZnO nanoparticles. Int. J. Occup. Med. Environ. Health 2017, 30, 213–229. [Google Scholar] [CrossRef]
- Gotoh, T.; Oyadomari, S.; Mori, K.; Mori, M. Nitric oxide-induced apoptosis in RAW 264.7 macrophages is mediated by endoplasmic reticulum stress pathway involving ATF6 and CHOP. J. Biol. Chem. 2002, 277, 12343–12350. [Google Scholar] [CrossRef]
- McCullough, K.D.; Martindale, J.L.; Klotz, L.O.; Aw, T.Y.; Holbrook, N.J. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol. Cell. Biol. 2001, 21, 1249–1259. [Google Scholar] [CrossRef]

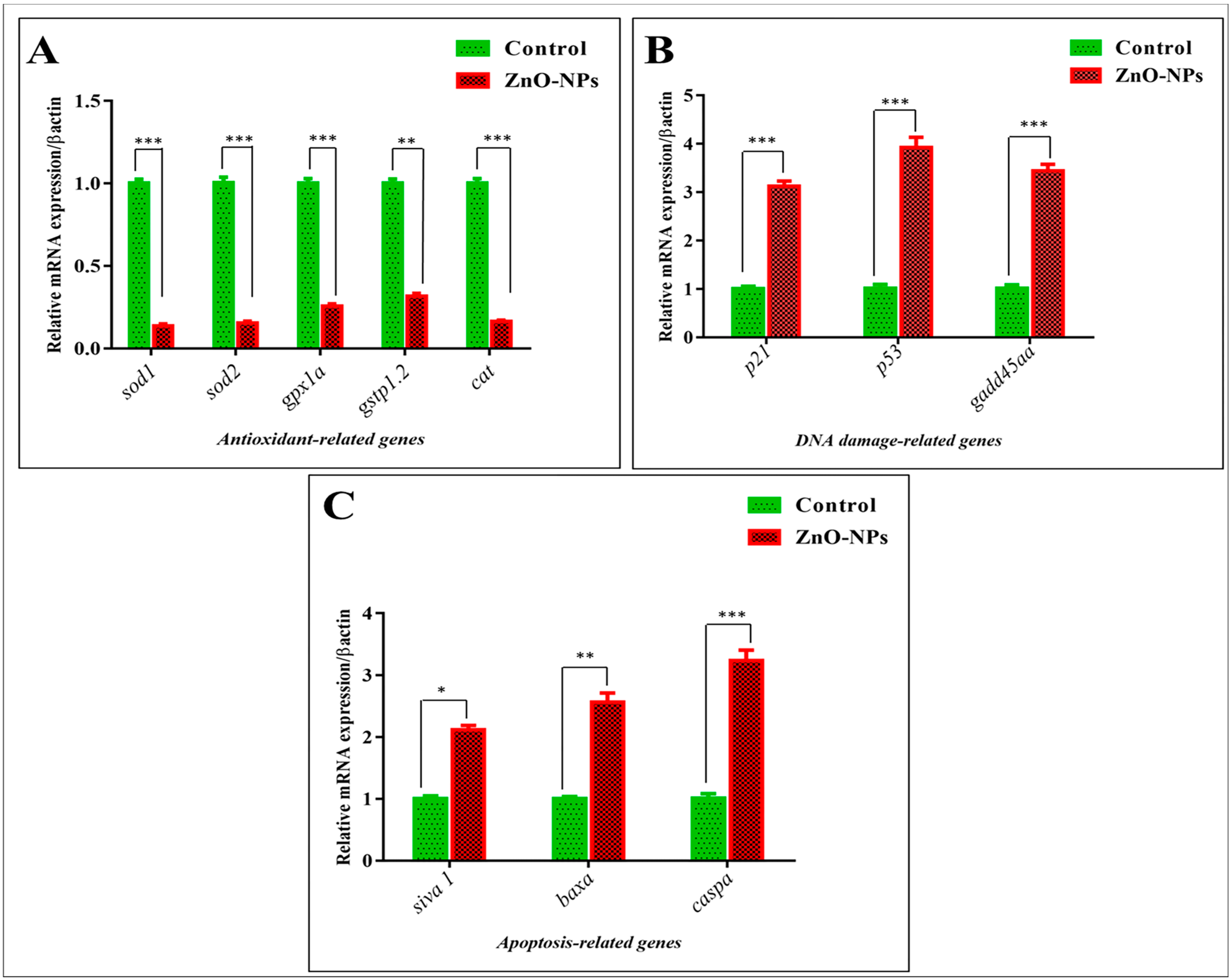
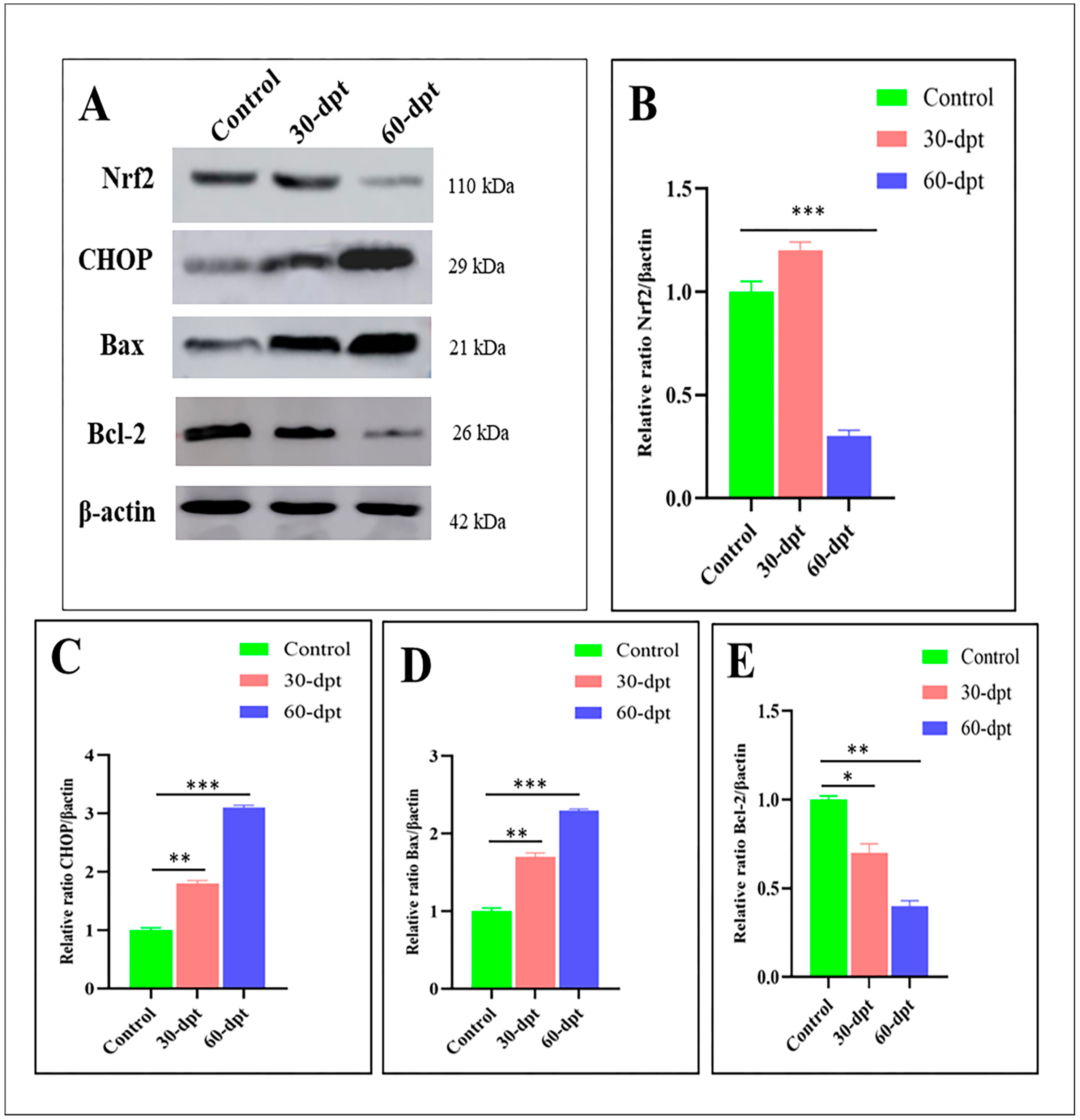
| Gene | Name | Primers | Accession (Gene ID) |
|---|---|---|---|
| sod1 | Superoxide dismutase1 | F: 5′ CGCACTTCAACCCTCATGAC 3′ R: 5′ TGAATCACCATGGTCCTCCC 3′ | NM_131294 |
| sod2 | Superoxide dismutase2 | F:5’ CCTCCAGACAGAAGCA 3’ R:5’ CTGAAATGAGCCAAAGT 3’ | NM_199976 |
| gpx1a | glutathione peroxidase 1a | F:5’ GCACAACAGTCAGGGAT 3’ R:5’ TCAGGAACGCAAACAG 3’ | NM_001007281 |
| gstp1.2 | glutathione S-transferase pi 1.2 | F: 5′ CCAACCACCTCAAATGCT 3′ R: 5′ ACGGGAAAGAGTCCAGACAG 3′ | NM_131734 |
| cat | Catalase | F: 5’ TGTGGAAGGAGGGTCG 3’ R: 5′ CTTTGGCTTTGGAGTAG 3′ | NM_130912 |
| p21 | cyclin-dependent kinase inhibitor 1A (cdkn1a) | F: 5′ CCTACGTTCACTCGGTAATGGG 3′ R: 5′ CACTAGACGCTTCTTGGCTTGG 3′ | NM_001128420 |
| p53 | Tumor protein p53 (tp53), transcript variant 2 | F: 5′ GCAGTCTGGCACAGCAAAATCTGT 3′ R: 5′ TCAGCCACATGCTCGGACTTCTTA 3′ | NM_131327 |
| gadd45aa | Growth arrest and DNA-damage-inducible, alpha, a | F: 5′ GCTGCGAGAACGACATCAACA 3′ R: 5′ GGGCACCCACTGATCCATACA 3′ | NM_200576 |
| siva1 | Apoptosis-inducing factor | F: 5′ CCGCTACCGACAGGAGATCTACGA 3′ R: 5′ GGTGTGGAGCGCGCTCTGTGCAGT 3′ | NM_001327928 |
| baxa | BCL2 associated X, apoptosis regulator | F: 5 GACAGGGATGCTGAAGTGA 3′ R: 5′ TGAGTCGGCTGAAGATTAGA 3′ | NM_131562 |
| caspa | caspase a (caspa) | F: 5′ GACGGTGAGCCTGATGAGCCAA 3′ R: 5′ CCTGAACAGTTCCTCGATGTGA 3′ | NM_131505 |
| Actin b1 | actin, beta 1 (actb1) | F: 5’ ATGGATGAGGAAATCGCTGC 3’ R:5’ CTTTCTGTCCCATGCCAACC 3’ | NM_131031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zahaby, S.A.; Farag, M.R.; Alagawany, M.; Taha, H.S.A.; Varoni, M.V.; Crescenzo, G.; Mawed, S.A. Zinc Oxide Nanoparticles (ZnO-NPs) Induce Cytotoxicity in the Zebrafish Olfactory Organs via Activating Oxidative Stress and Apoptosis at the Ultrastructure and Genetic Levels. Animals 2023, 13, 2867. https://doi.org/10.3390/ani13182867
Al-Zahaby SA, Farag MR, Alagawany M, Taha HSA, Varoni MV, Crescenzo G, Mawed SA. Zinc Oxide Nanoparticles (ZnO-NPs) Induce Cytotoxicity in the Zebrafish Olfactory Organs via Activating Oxidative Stress and Apoptosis at the Ultrastructure and Genetic Levels. Animals. 2023; 13(18):2867. https://doi.org/10.3390/ani13182867
Chicago/Turabian StyleAl-Zahaby, Sheren A., Mayada R. Farag, Mahmoud Alagawany, Heba S. A. Taha, Maria Vittoria Varoni, Giuseppe Crescenzo, and Suzan Attia Mawed. 2023. "Zinc Oxide Nanoparticles (ZnO-NPs) Induce Cytotoxicity in the Zebrafish Olfactory Organs via Activating Oxidative Stress and Apoptosis at the Ultrastructure and Genetic Levels" Animals 13, no. 18: 2867. https://doi.org/10.3390/ani13182867
APA StyleAl-Zahaby, S. A., Farag, M. R., Alagawany, M., Taha, H. S. A., Varoni, M. V., Crescenzo, G., & Mawed, S. A. (2023). Zinc Oxide Nanoparticles (ZnO-NPs) Induce Cytotoxicity in the Zebrafish Olfactory Organs via Activating Oxidative Stress and Apoptosis at the Ultrastructure and Genetic Levels. Animals, 13(18), 2867. https://doi.org/10.3390/ani13182867








