Examining the Veterinary Electronic Antimicrobial Prescriptions for Dogs and Cats in the Campania Region, Italy: Corrective Strategies Are Imperative
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pharma-Surveillance Information System—Vet Info
2.2. Data Collection
2.3. Data Management and Statistical Analysis
3. Results
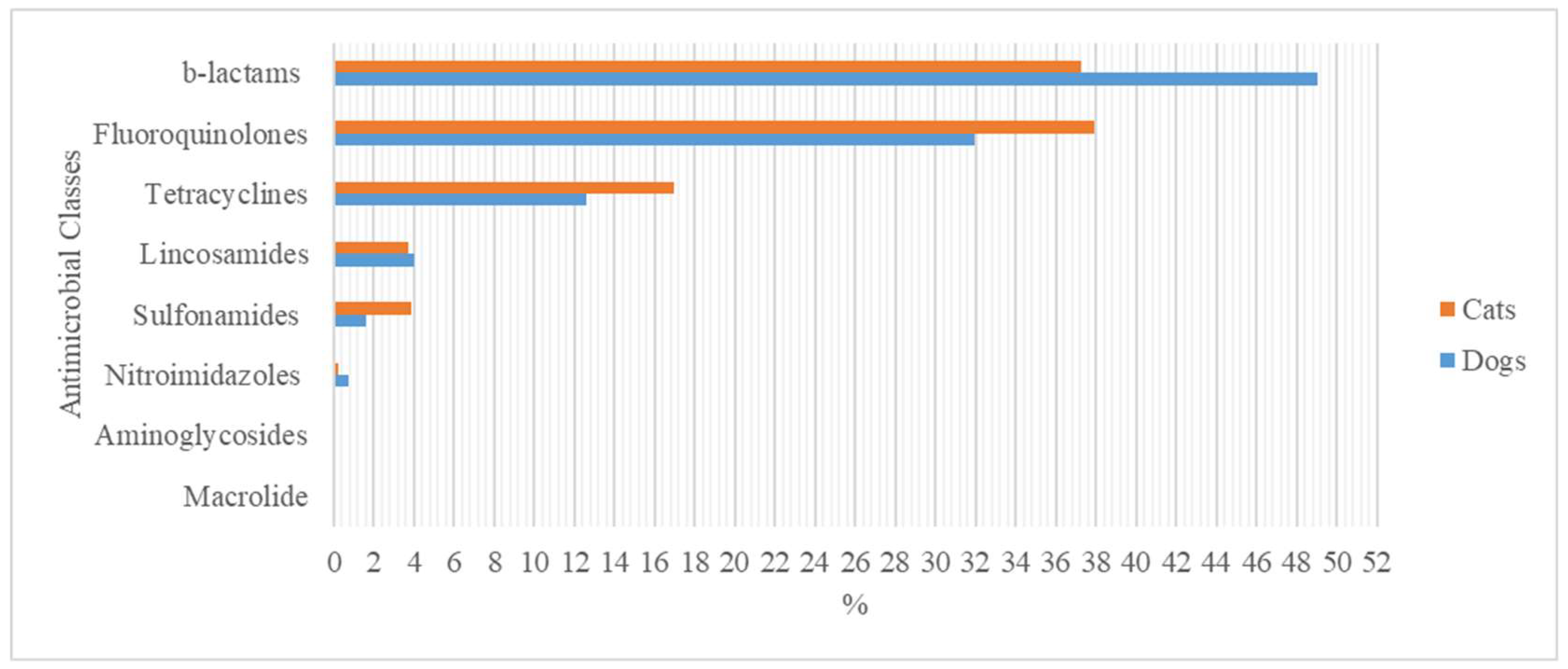
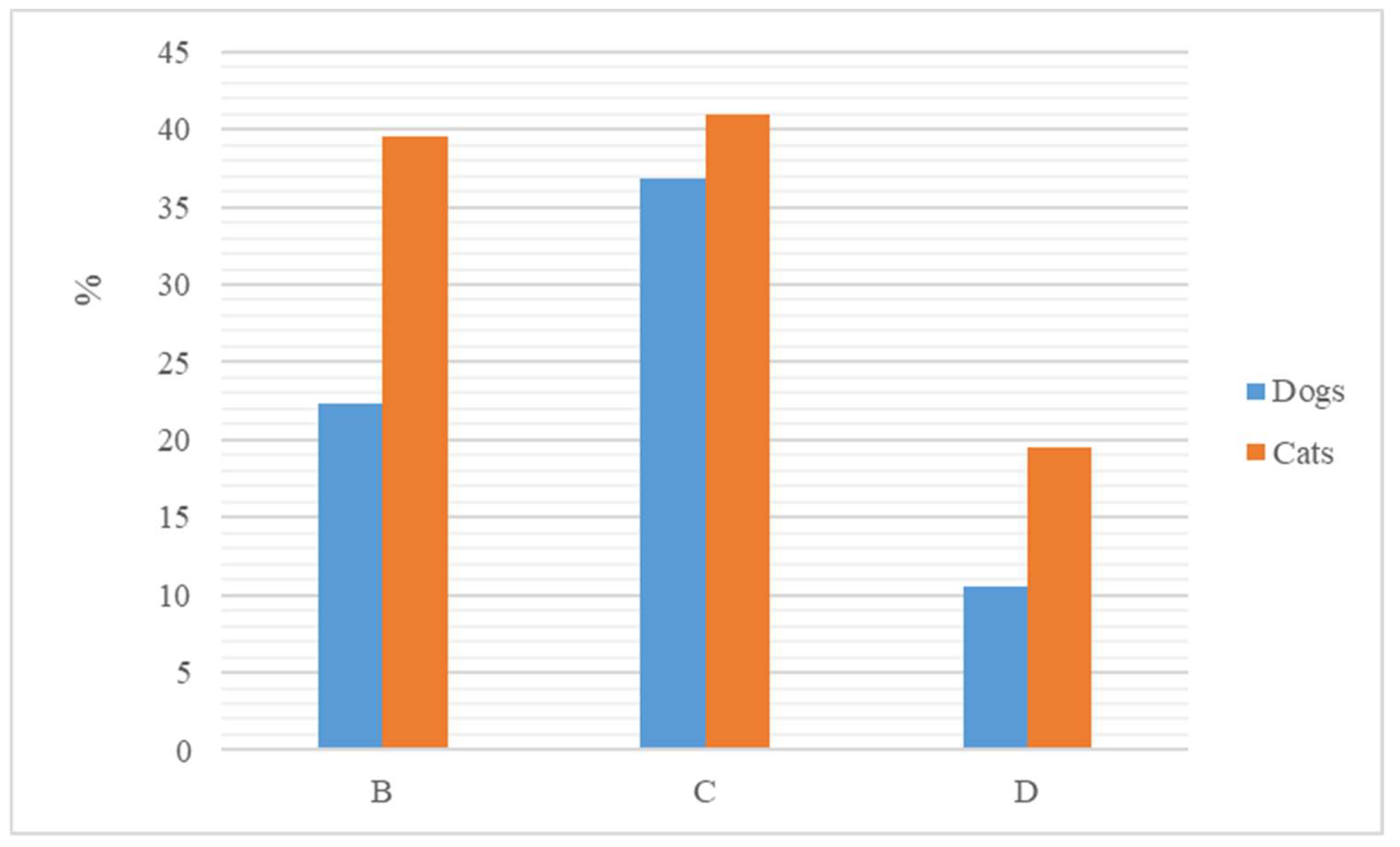
3.1. Antimicrobial Agents in the Canine Specie
3.2. Antimicrobial Agents in the Feline Specie
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2020/2021. EFSA J. 2023, 21, 232. [Google Scholar] [CrossRef]
- Vercelli, C.; Gambino, G.; Amadori, M.; Re, G. Implications of Veterinary Medicine in the Comprehension and Stewardship of Antimicrobial Resistance Phenomenon. From the Origin till Nowadays. Vet. Anim. Sci. 2022, 16, 100249. [Google Scholar] [CrossRef] [PubMed]
- Caniça, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M.A.P. Antibiotic Resistance in Foodborne Bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Galarce, N.; Arriagada, G.; Sánchez, F.; Venegas, V.; Cornejo, J.; Lapierre, L. Antimicrobial use in companion animals: Assessing veterinarians’ prescription patterns through the first national survey in Chile. Animals 2021, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Mouiche, M.M.M.; Mpouam, S.E.; Moffo, F.; Nkassa, C.M.N.; Mbah, C.K.; Mapiefou, N.P.; Awah-Ndukum, J. Prescription Pattern of Antimicrobial Use in Small Animal Veterinary Practice in Cameroon. Top. Companion Anim. Med. 2021, 44, 100540. [Google Scholar] [CrossRef] [PubMed]
- Singleton, D.A.; Rayner, A.; Brant, B.; Smyth, S.; Noble, P.J.M.; Radford, A.D.; Pinchbeck, G.L. A Randomised Controlled Trial to Reduce Highest Priority Critically Important Antimicrobial Prescription in Companion Animals. Nat. Commun. 2021, 12, 1593. [Google Scholar] [CrossRef]
- Schnepf, A.; Kramer, S.; Wagels, R.; Volk, H.A.; Kreienbrock, L. Evaluation of Antimicrobial Usage in Dogs and Cats at a Veterinary Teaching Hospital in Germany in 2017 and 2018. Front. Vet. Sci. 2021, 8, 689018. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Categorisation of Antibiotics in the European Union; EMA/CVMP/CHMP/682198; European Medicines Agency (EMA): Amsterdam, The Netherlands, 2017. [Google Scholar]
- Committee for Medicinal Products for Veterinary Use (CVMP). Reflection Paper on Antimicrobials in the Environment. 2018. Available online: www.ema.europa.eu/contact (accessed on 23 July 2022).
- Goggs, R.; Menard, J.M.; Altier, C.; Cummings, K.J.; Jacob, M.E.; Lalonde-Paul, D.F.; Papich, M.G.; Norman, K.N.; Fajt, V.R.; Scott, H.M.; et al. Patterns of Antimicrobial Drug Use in Veterinary Primary Care and Specialty Practice: A 6-Year Multi-Institution Study. J. Vet. Sci. 2021, 35, 1496–1508. [Google Scholar] [CrossRef]
- Chirollo, C.; Nocera, F.P.; Piantedosi, D.; Fatone, G.; Della Valle, G.; De Martino, L.; Cortese, L. Data on before and after the Traceability System of Veterinary Antimicrobial Prescriptions in Small Animals at the University Veterinary Teaching Hospital of Naples. Animals 2021, 11, 913. [Google Scholar] [CrossRef]
- Escher, M.; Vanni, M.; Intorre, L.; Caprioli, A.; Tognetti, R.; Scavia, G. Use of Antimicrobials in Companion Animal Practice: A Retrospective Study in a Veterinary Teaching Hospital in Italy. J. Antimicrob. Chemother. 2011, 66, 920–927. [Google Scholar] [CrossRef]
- Hubbuch, A.; Schmitt, K.; Lehner, C.; Hartnack, S.; Schuller, S.; Schüpbach-Regula, G.; Mevissen, M.; Peter, R.; Müntener, C.; Naegeli, H.; et al. Antimicrobial prescriptions in cats in Switzerland before and after the introduction of an online antimicrobial stewardship tool. BMC Vet. Res. 2020, 16, 229. [Google Scholar] [CrossRef]
- Hardefeldt, L.Y.; Selinger, J.; Stevenson, M.A.; Gilkerson, J.R.; Crabb, H.; Billman-Jacobe, H.; Thursky, K.; Bailey, K.E.; Awad, M.; Browning, G.F. Population wide assessment of antimicrobial use in dogs and cats using a novel data source—A cohort study using pet insurance data. Vet. Microbiol. 2018, 225, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Hur, B.A.; Hardefeldt, L.Y.; Verspoor, K.M.; Baldwin, T.; Gilkerson, J.R. Describing the antimicrobial usage patterns of companion animal veterinary practices; free text analysis of more than 4.4 million consultation records. PLoS ONE 2020, 15, e0230049. [Google Scholar]
- Murphy, C.P.; Reid-Smith, R.J.; Boerlin, P.; Weese, J.S.; Prescott, J.F.; Janecko, N.; McEwen, S.A. Out-Patient Antimicrobial Drug Use in Dogs and Cats for New Disease Events from Community Companion Animal Practices in Ontario. Can. Vet. J. 2012, 53, 291–298. [Google Scholar]
- Buckland, E.L.; O’Neill, D.; Summers, J.; Mateus, A.; Church, D.; Redmond, L.; Brodbelt, D. Characterisation of Antimicrobial Usage in Cats and Dogs Attending UK Primary Care Companion Animal Veterinary Practices. Vet. Rec. 2016, 179, 489. [Google Scholar] [CrossRef]
- Van Cleven, A.; Sarrazin, S.; de Rooster, H.; Paepe, D.; Van der Meeren, S.; Dewulf, J. Antimicrobial Prescribing Behaviour in Dogs and Cats by Belgian Veterinarians. Vet. Rec. 2018, 182, 324. [Google Scholar] [CrossRef] [PubMed]
- Lhermie, G.; La Ragione, R.M.; Weese, J.S.; Olsen, J.E.; Christensen, J.P.; Guardabassi, L. Indications for the Use of Highest Priority Critically Important Antimicrobials in the Veterinary Sector. J. Antimicrob. Chemother. 2020, 75, 1671–1680. [Google Scholar] [CrossRef]
- Walker, B.; Sánchez-Vizcaíno, F.; Barker, E.N. Effect of an Antimicrobial Stewardship Intervention on the Prescribing Behaviours of Companion Animal Veterinarians: A Pre–Post Study. Vet. Rec. 2022, 190, e1485. [Google Scholar] [CrossRef]
- Coates, A.R.M.; Hu, Y.; Holt, J.; Yeh, P. Antibiotic combination therapy against resistant bacterial infections: Synergy, rejuvenation and resistance reduction. Expert Rev. Anti-Infect. Ther. 2020, 18, 5–15. [Google Scholar] [CrossRef]
- Allerton, F.; Prior, C.; Bagcigil, A.F.; Broens, E.; Callens, B.; Damborg, P.; Dewulf, J.; Filippitzi, M.E.; Carmo, L.P.; Gómez-Raja, J.; et al. Overview and Evaluation of Existing Guidelines for Rational Antimicrobial Use in Small-Animal Veterinary Practice in Europe. Antibiotics 2021, 10, 409. [Google Scholar] [CrossRef]
- Bajwa, J. Canine Otitis Externa—Treatment and Complications. Can. Vet. J. 2019, 60, 97–99. [Google Scholar] [PubMed]
| Antibiotics | Rationale for the Treatment Chosen | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SK | RES | GI | GU | OPH | ORT | MAM | SEP | SUR | MET | CAR | NEU | ONC | PAR | Other | Tot. | |
| Amikacin *C | 1 | 1 | 7 | 9 | ||||||||||||
| Amoxicillin *D | 9 | 5 | 2 | 5 | 3 | 1 | 4 | 1 | 68 | 98 | ||||||
| Amoxicillin–Clavulanic acid *C | 1834 | 815 | 352 | 556 | 57 | 179 | 107 | 181 | 15 | 55 | 13 | 49 | 37 | 6 | 8068 | 12,324 |
| Ampicillin *D | 9 | 9 | ||||||||||||||
| Benzylpenicillin *D | 1 | 1 | 2 | |||||||||||||
| Benzylpenicillin–Dihydrostreptomycin * | 38 | 20 | 8 | 8 | 3 | 5 | 16 | 1 | 2 | 1 | 1 | 95 | 198 | |||
| Cefadroxil **C | 714 | 166 | 31 | 95 | 12 | 76 | 30 | 45 | 1 | 9 | 35 | 11 | 1 | 1387 | 2613 | |
| Cefalexin **C | 1482 | 213 | 90 | 149 | 16 | 265 | 32 | 82 | 11 | 10 | 2 | 105 | 6 | 7 | 3961 | 6431 |
| Cefovecin **B | 3 | 1 | 8 | 12 | ||||||||||||
| Chlortetracycline **D | 6 | 16 | 1 | 1 | 1 | 86 | 104 | 215 | ||||||||
| Clindamycin **C | 231 | 24 | 37 | 17 | 5 | 220 | 4 | 80 | 8 | 111 | 7 | 5 | 1011 | 1760 | ||
| Doxicicline D | 188 | 697 | 106 | 80 | 159 | 84 | 3 | 351 | 114 | 5 | 31 | 9 | 157 | 3260 | 5244 | |
| Enrofloxacin *B | 1004 | 571 | 188 | 1866 | 26 | 104 | 36 | 175 | 2 | 59 | 9 | 37 | 23 | 16 | 6283 | 10,399 |
| Formosulfathiazole **D | 5 | 1 | 2 | 8 | ||||||||||||
| Gentamicin *C | 1 | 1 | 1 | 1 | 6 | 10 | ||||||||||
| Kanamycin *–Isopropamide Iodide | 71 | 1 | 91 | 163 | ||||||||||||
| Lincomycin **–Spectinomycin *** | 1 | 6 | 1 | 3 | 3 | 28 | 42 | |||||||||
| Marbofloxacin *B | 325 | 105 | 35 | 416 | 31 | 45 | 9 | 48 | 1 | 6 | 48 | 4 | 1769 | 2842 | ||
| Metronidazole ***D | 3 | 104 | 1 | 2 | 1 | 11 | 209 | 331 | ||||||||
| Metronidazole ***–Spiramycin * | 1421 | 247 | 5128 | 179 | 16 | 104 | 65 | 388 | 17 | 108 | 14 | 8 | 13 | 169 | 10,822 | 18,699 |
| OxytetraciclinD | 1 | 4 | 3 | 6 | 2 | 1 | 39 | 56 | ||||||||
| Pradofloxacin *B | 262 | 55 | 6 | 48 | 2 | 11 | 1 | 13 | 7 | 1 | 364 | 770 | ||||
| Sulphadiazine/Sulphadimethoxaxole–Trimetoprim | 2 | 2 | ||||||||||||||
| Sulfametopyrazine D | 2 | 4 | 166 | 1 | 2 | 159 | 354 | 688 | ||||||||
| Tylosin *C | 2 | 1 | 13 | 16 | ||||||||||||
| Tot. | 7531 | 2928 | 6343 | 3428 | 325 | 1096 | 294 | 1397 | 48 | 381 | 43 | 436 | 112 | 619 | 37,960 | 62,941 |
| Antibiotics | Rationale for the Treatment Chosen | |||
|---|---|---|---|---|
| Skin Disease | Ear Disease | Eye Disease | Tot. | |
| Betamethasone/Clotrimazole/Gentamicin | 1182 | 1182 | ||
| Cloramphenicol/Betamethasone | 8 | 8 | ||
| Clostebol/Paromomycin/Prednisolone | 114 | 114 | ||
| Diethanolamine Fusidate/Framycetin Sulphate/Nystatin/Prednisolone | 218 | 218 | ||
| Econazole/Flumetasone/Gentamicin/Tetracaine | 104 | 237 | 341 | |
| Enrofloxacin/Silver Sulfadiazine | 797 | 797 | ||
| Fluocinolone/Neomycin | 175 | 175 | ||
| Fusidic Acid | 16 | 16 | ||
| Fusidic Acid/Betamethasone | 288 | 288 | ||
| Gentamicin | 39 | 39 | ||
| Hydrocortisone Aceponate/Miconazole Nitrate/Gentamicin Sulphate | 4263 | 4263 | ||
| Marbofloxacin/Clotrimazole/Dexamethasone | 2058 | 2058 | ||
| Marbofloxacin/Gentamicin Sulphate/Ketoconazole/Prednisolone | 810 | 810 | ||
| Marbofloxacin/Ketokonazole/Prednisolone | 11 | 11 | ||
| Miconazole Nitrate/Polymyxin B Sulphate/Prednisolone Acetate | 1321 | 2107 | 3428 | |
| Orbifloxacin/Posaconazole/Mometasone Furoate | 1322 | 1322 | ||
| Rifaximin/Colistin/Miconazole/Carbarele/Triamcinolone | 1230 | 1230 | ||
| Terbinafine/Florfenicol/Betamethasone | 3460 | 3460 | ||
| Thiabendazole/Neomycin/Dexamethasone | 633 | 633 | ||
| Tobramycin | 631 | 631 | ||
| Tot. | 1838 | 18,317 | 869 | 21,024 |
| Antibiotics | Rationale for the Treatment Chosen | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SK | RES | GI | GU | OPH | ORT | MAM | SEP | SUR | MET | CAR | NEU | ONC | PAR | Other | Tot. | |
| Amikacin *C | 1 | 1 | 2 | |||||||||||||
| Amoxicillin *D | 73 | 71 | 20 | 17 | 4 | 5 | 4 | 3 | 4 | 4 | 14 | 7 | 290 | 516 | ||
| Amoxicillin–clavulanic Acid *C | 996 | 1158 | 234 | 508 | 55 | 107 | 21 | 188 | 5 | 34 | 1 | 7 | 18 | 9 | 4703 | 8044 |
| Benzylpenicillin–Dihydrostreptomycin * | 9 | 2 | 2 | 1 | 5 | 39 | 60 | |||||||||
| Cefadroxil **C | 235 | 106 | 16 | 58 | 2 | 9 | 5 | 17 | 2 | 9 | 1 | 1 | 1 | 3 | 462 | 927 |
| Cefalexin **C | 454 | 287 | 37 | 95 | 10 | 58 | 10 | 50 | 1 | 8 | 5 | 2 | 3 | 1669 | 2689 | |
| Cefovecin **B | 2 | 1 | 1 | 1 | 1 | 21 | 27 | |||||||||
| Ceftiofur **B | 1 | 1 | ||||||||||||||
| Chlortetracycline **D | 1 | 3 | 25 | 134 | 124 | 287 | ||||||||||
| Clindamycin **C | 133 | 44 | 19 | 5 | 6 | 84 | 58 | 7 | 53 | 5 | 10 | 801 | 1225 | |||
| Doxycycline D | 90 | 1376 | 105 | 102 | 96 | 17 | 4 | 247 | 95 | 6 | 8 | 4 | 52 | 3049 | 5251 | |
| Enrofloxacin *B | 747 | 825 | 203 | 1832 | 23 | 75 | 11 | 161 | 6 | 32 | 1 | 12 | 1 | 9 | 4925 | 8863 |
| Formosulfathiazole **D | 3 | 3 | 6 | |||||||||||||
| Gentamicin *C | 2 | 2 | ||||||||||||||
| Kanamycin *–Isopropamide iodide | 95 | 1 | 1 | 88 | 185 | |||||||||||
| Lincomycin **–Spectinomycin *** | 5 | 1 | 6 | |||||||||||||
| Marbofloxacin *B | 115 | 104 | 39 | 303 | 14 | 16 | 1 | 19 | 8 | 5 | 1 | 961 | 1586 | |||
| Metronidazole ***D | 1 | 1 | 18 | 1 | 1 | 42 | 64 | |||||||||
| Metronidazole ***–Spiramycin * | 233 | 108 | 1356 | 24 | 8 | 29 | 14 | 109 | 2 | 34 | 3 | 5 | 70 | 2661 | 4656 | |
| Oxytetraciclin D | 1 | 2 | 15 | 18 | ||||||||||||
| Pradofloxacin *B | 213 | 398 | 44 | 220 | 18 | 38 | 67 | 3 | 1 | 1 | 2 | 965 | 1970 | |||
| Sulfametopyrazine | 2 | 52 | 342 | 3 | 1 | 1 | 297 | 560 | 1258 | |||||||
| Tylosin *C | 1 | 1 | ||||||||||||||
| Tot. | 3300 | 4544 | 2557 | 3174 | 238 | 438 | 71 | 929 | 16 | 236 | 9 | 99 | 53 | 597 | 21,383 | 37,644 |
| Antibiotics | Rationale for the Treatment Chosen | |||
|---|---|---|---|---|
| Skin Disease | Ear Disease | Eye Disease | Tot. | |
| Betamethasone/Clotrimazole/Gentamicin | 46 | 46 | ||
| Clostebol/Paromomycin/Prednisolone | 9 | 9 | ||
| Diethanolamine Fusidate/Framycetin Sulphate/Nystatin/Prednisolone | 33 | 33 | ||
| Econazole/Flumetasone/Gentamicin/Tetracaine | 24 | 42 | 66 | |
| Enrofloxacin/Silver Sulfadiazine | 53 | 53 | ||
| Fluocinolone/Neomycin | 58 | 58 | ||
| Fusidic Acid | 3 | 3 | ||
| Fusidic Acid/Betamethasone | 27 | 27 | ||
| Gentamicin | 19 | 19 | ||
| Hydrocortisone Aceponate/Miconazole Nitrate/Gentamicin Sulphate | 65 | 65 | ||
| Marbofloxacin/Clotrimazole/Dexamethasone | 56 | 56 | ||
| Marbofloxacin/Gentamicin Sulphate/Ketoconazole/Prednisolone | 16 | 16 | ||
| Marbofloxacin/Ketokonazole/Prednisolone | 2 | 2 | ||
| Miconazole Nitrate/Polymyxin B Sulphate/Prednisolone Acetate | 290 | 791 | 1081 | |
| Orbifloxacin/Posaconazole/Mometasone Furoate | 25 | 25 | ||
| Rifaximin/Colistin/Miconazole/Carbarele/Triamcinolone | 171 | 171 | ||
| Terbinafine/Florfenicol/Betamethasone | 40 | 40 | ||
| Thiabendazole/Neomycin/Dexamethasone | 402 | 337 | 739 | |
| Tobramycin | 324 | 324 | ||
| Tot. | 754 | 1675 | 404 | 2833 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foglia Manzillo, V.; Peruzy, M.F.; Gizzarelli, M.; Izzo, B.; Sarnelli, P.; Carrella, A.; Vinciguerra, G.; Chirollo, C.; Ben Fayala, N.E.H.; Balestrino, I.; et al. Examining the Veterinary Electronic Antimicrobial Prescriptions for Dogs and Cats in the Campania Region, Italy: Corrective Strategies Are Imperative. Animals 2023, 13, 2869. https://doi.org/10.3390/ani13182869
Foglia Manzillo V, Peruzy MF, Gizzarelli M, Izzo B, Sarnelli P, Carrella A, Vinciguerra G, Chirollo C, Ben Fayala NEH, Balestrino I, et al. Examining the Veterinary Electronic Antimicrobial Prescriptions for Dogs and Cats in the Campania Region, Italy: Corrective Strategies Are Imperative. Animals. 2023; 13(18):2869. https://doi.org/10.3390/ani13182869
Chicago/Turabian StyleFoglia Manzillo, Valentina, Maria Francesca Peruzy, Manuela Gizzarelli, Berardino Izzo, Paolo Sarnelli, Antonio Carrella, Giuseppina Vinciguerra, Claudia Chirollo, Nour El Houda Ben Fayala, Ines Balestrino, and et al. 2023. "Examining the Veterinary Electronic Antimicrobial Prescriptions for Dogs and Cats in the Campania Region, Italy: Corrective Strategies Are Imperative" Animals 13, no. 18: 2869. https://doi.org/10.3390/ani13182869
APA StyleFoglia Manzillo, V., Peruzy, M. F., Gizzarelli, M., Izzo, B., Sarnelli, P., Carrella, A., Vinciguerra, G., Chirollo, C., Ben Fayala, N. E. H., Balestrino, I., & Oliva, G. (2023). Examining the Veterinary Electronic Antimicrobial Prescriptions for Dogs and Cats in the Campania Region, Italy: Corrective Strategies Are Imperative. Animals, 13(18), 2869. https://doi.org/10.3390/ani13182869






