An Overview of the Health Effects of Bisphenol A from a One Health Perspective
Abstract
Simple Summary
Abstract
1. Introduction
2. Health Effects of Bisphenol A
2.1. Effects on the Reproductive System
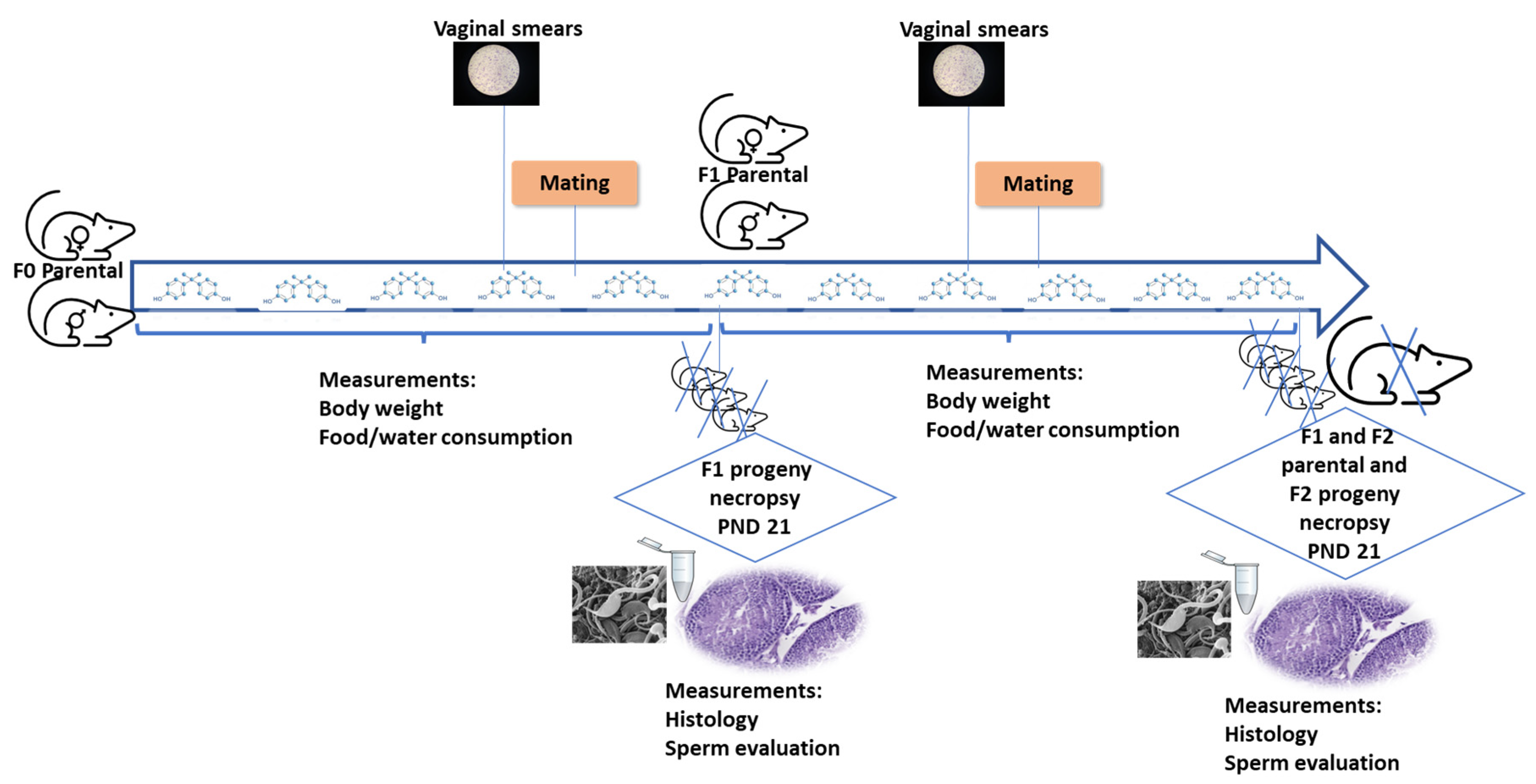
2.1.1. Effects on the Ovary
2.1.2. Effects on the Uterus
2.1.3. Effects on the Placenta
2.1.4. Effects on the Fetus
Birth Weight
Premature Labor
Fetal Malformation
2.1.5. Effects on Male Reproduction
2.2. Developmental and Neurobehavioral Effects
2.3. Transgenerational and Multigenerational Effects
2.4. Metabolic Effects
2.5. Immunological Effects on Oxidative Stress and Inflammation
2.6. Effects on Thyroid Function
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32011R0010 (accessed on 12 July 2023).
- Commission Regulation (EU) 2018/213 of 12 February 2018 on the Use of Bisphenol A in Varnishes and Coatings Intended to Come into Contact with Food and Amending Regulation (EU) No 10/2011 as Regards the Use of that Substance in Plastic Food Contact Materials. Available online: https://eur-lex.europa.eu/eli/reg/2018/213/oj (accessed on 12 July 2023).
- Commission Regulation (EU) 2016/2235 of 12 December 2016 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Bisphenol A. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32016R2235 (accessed on 12 July 2023).
- Commission Directive (EU) 2017/898 of 24 May 2017 Amending, for the Purpose of Adopting Specific Limit Values for Chemicals Used in Toys, Appendix C to Annex II to Directive 2009/48/EC of the European Parliament and of the Council on the Safety of Toys, as Regards Bisphenol A. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017L0898 (accessed on 12 July 2023).
- Scientific Committee on Consumer Safety (SCCS). 2020. Available online: https://health.ec.europa.eu/other-pages/health-sc-basic-page/final-opinion-bpa-clothing-articles_en (accessed on 12 July 2023).
- EFSA CEP Panel (EFSA Panel on Food Contact Materials, Enzymes and Processing Aids). Scientific Opinion on the re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2023, 21, e6857. [Google Scholar] [CrossRef]
- Fu, P.; Kawamura, K. Ubiquity of bisphenol A in the atmosphere. Environ. Pollut. 2010, 158, 3138–3143. [Google Scholar] [CrossRef]
- Rocha, S.; Domingues, V.F.; Pinho, C.; Fernandes, V.C.; Delerue-Matos, C.; Gameiro, P.; Mansilha, C. Occurrence of bisphenol A, estrone, 17β-estradiol and 17α-ethinylestradiol in Portuguese rivers. Bull. Environ. Contam. Toxicol. 2013, 90, 73–78. [Google Scholar] [CrossRef]
- Lee, C.C.; Jiang, L.Y.; Kuo, Y.; Hsieh, C.Y.; Chen, C.S.; Tien, C.J. The potential role of water quality parameters on occurrence of nonylphenol and bisphenol A and identification of their discharge sources in the river ecosystems. Chemosphere 2013, 91, 904–911. [Google Scholar] [CrossRef]
- Vom Saal, F.S.; Vandenberg, L.N. Update on the Health Effects of Bisphenol A: Overwhelming Evidence of Harm. Endocrinology 2021, 162, bqaa171. [Google Scholar] [CrossRef]
- Bujalance-Reyes, F.; Molina-López, A.M.; Ayala-Soldado, N.; Lora-Benitez, A.; Mora-Medina, R.; Moyano-Salvago, R. Analysis of Indirect Biomarkers of Effect after Exposure to Low Doses of Bisphenol A in a Study of Successive Generations of Mice. Animals 2022, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Trullemans, L.; Koelewijn, S.F.; Scodeller, I.; Hendrickx, T.; Van Puyvelde, P.; Sels, B. A guide towards safe, functional and renewable BPA alternatives by rational molecular design: Structure-property and structure-toxicity relationships. Polym. Chem. 2021, 12, 5870–5901. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kumar, S.; Kumar, V.; Lee, Y.-M.; Kim, Y.-S. Bisphenols as a legacy pollutant, and their effects on organ vulnerability. Int. J. Environ. Res. Public Health 2020, 17, 112. [Google Scholar] [CrossRef]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.; Feng, Y.; Wu, Y.; Widelka, M. Bisphenol Analogues Other Than BPA: Environmental Occurrence, Human Exposure, and Toxicity—A Review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef]
- Cho, Y.J.; Park, S.B.; Park, J.W.; Oh, S.R.; Han, M. Bisphenol A modulates inflammation and proliferation pathway in human endometrial stromal cells by inducing oxidative stress. Reprod. Toxicol. 2018, 81, 41–49. [Google Scholar] [CrossRef]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Combined Toxicity of Xenobiotics Bisphenol A and Heavy Metals on Zebrafish Embryos (Danio rerio). Toxics 2021, 9, 344. [Google Scholar] [CrossRef]
- Fernandez, M.O.; Bourguignon, N.S.; Arocena, P.; Rosa, M.; Libertun, C.; Lux-Lantos, V. Neonatal exposure to bisphenol A alters the hypothalamic-pituitary-thyroid axis in female rats. Toxicol. Lett. 2018, 285, 81–86. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, P.; Romano, R.M.; Kizys, M.M.; Oliveira, K.C.; Kasamatsu, T.; Giannocco, G.; Chiamolera, M.I.; Dias-da-Silva, M.R.; Romano, M.A. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic-pituitary-testicular axis. Toxicology 2015, 329, 1–9. [Google Scholar] [CrossRef]
- Lora, A.J.; Molina, A.M.; Bellido, C.; Blanco, A.; Monterde, J.G.; Moyano, M.R. Adverse effects of bisphenol A on the testicular parenchyma of zebrafish revealed using histomorphological methods. Vet. Med. 2016, 61, 577–589. [Google Scholar] [CrossRef]
- Santangeli, S.; Consales, C.; Pacchierotti, F.; Habibi, H.R.; Carnevali, O. Transgenerational effects of BPA on female reproduction. Sci. Total. Environ. 2019, 685, 1294–1305. [Google Scholar] [CrossRef]
- Molina, A.M.; Abril, N.; Morales-Prieto, N.; Monterde, J.G.; Lora, A.J.; Ayala, N.; Moyano, R. Evaluation of toxicological endpoints in female zebrafish after bisphenol A exposure. Food Chem. Toxicol. 2018, 112, 19–25. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Chen, P.P.; Liu, C.; Deng, Y.; Miao, Y.; Zhang, M.; Cui, F.P.; Lu, T.T.; Shi, T.; Yang, K.D.; et al. Bisphenol A analogues in associations with serum hormone levels among reproductive-aged Chinese men. Environ. Int. 2022, 167, 107446. [Google Scholar] [CrossRef]
- Di Nardo, G.; Zhang, C.; Marcelli, A.G.; Gilardi, G. Molecular and Structural Evolution of Cytochrome P450 Aromatase. Int. J. Mol. Sci. 2021, 22, 631. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Iyengar, N.M.; Morrow, M.; Elemento, O.; Zhou, X.K.; Dannenberg, A.J. Prostaglandin E2 down-regulates sirtuin 1 (SIRT1), leading to elevated levels of aromatase, providing insights into the obesity-breast cancer connection. J. Biol. Chem. 2019, 294, 361–371. [Google Scholar] [CrossRef]
- Donnini, S.; Bazzani, L.; Ziche, M.; Terzuoli, E. Nitric Oxide and PGE-2 Cross-Talk in EGFR-Driven Epithelial Tumor Cells. Crit. Rev. Oncog. 2016, 21, 325–331. [Google Scholar] [CrossRef]
- Duliban, M.; Gorowska-Wojtowicz, E.; Tworzydlo, W.; Rak, A.; Brzoskwinia, M.; Krakowska, I.; Wolski, J.K.; Kotula-Balak, M.; Płachno, B.J.; Bilinska, B. Interstitial Leydig Cell Tumorigenesis-Leptin and Adiponectin Signaling in Relation to Aromatase Expression in the Human Testis. Int. J. Mol. Sci. 2020, 21, 3649. [Google Scholar] [CrossRef]
- OECD. Test No. 416: Two-Generation Reproduction Toxicity. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2001. [Google Scholar] [CrossRef]
- Berger, A.; Ziv-Gal, A.; Cudiamat, J.; Wang, W.; Zhou, C.; Flaws, J.A. The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice. Reprod. Toxicol. 2016, 60, 39–52. [Google Scholar] [CrossRef]
- Liang, M.; Zhou, J.; Sun, X.; He, C.; Zhang, K.; Hu, K. Effects of bisphenol A on apoptosis of ovarian preantral follicular granulosa cells and ovarian development in mice. J. South. Med. Univ. 2021, 41, 93–99. [Google Scholar] [CrossRef]
- Wang, W.; Hafner, K.S.; Flaws, J.A. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol. Appl. Pharmacol. 2014, 276, 157–164. [Google Scholar] [CrossRef]
- Meng, Y.; Lin, R.; Wu, F.; Sun, Q.; Jia, L. Decreased Capacity for Sperm Production Induced by Perinatal Bisphenol A Exposure Is Associated with an Increased Inflammatory Response in the Offspring of C57BL/6 Male Mice. Int. J. Environ. Res. Public Health 2018, 15, 2158. [Google Scholar] [CrossRef]
- Ma, S.; Shi, W.; Wang, X.; Song, P.; Zhong, X. Bisphenol A Exposure during Pregnancy Alters the Mortality and Levels of Reproductive Hormones and Genes in Offspring Mice. Biomed Res. Int. 2017, 2017, 3585809. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, G.G.; Yu, B.; Yang, Y.; Wu, J. Effects of bisphenol A on ovarian follicular development and female germline stem cells. Arch. Toxicol. 2018, 92, 1581–1591. [Google Scholar] [CrossRef]
- Hu, Y.; Wen, S.; Yuan, D.; Peng, L.; Zeng, R.; Yang, Z.; Liu, Q.; Xu, L.; Kang, D. The association between the environmental endocrine disruptor bisphenol A and polycystic ovary syndrome: A systematic review and meta-analysis. Gynecol. Endocrinol. 2018, 34, 370–377. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Liu, F. Chronic exposure of BPA impairs male germ cell proliferation and induces lower sperm quality in male mice. Chemosphere 2021, 262, 127880. [Google Scholar] [CrossRef]
- Nguyen, J.L.; Ricke, E.A.; Liu, T.T.; Gerona, R.; MacGillivray, L.; Wang, Z.; Timms, B.G.; Bjorling, D.E.; Vom Saal, F.S.; Ricke, W.A. Bisphenol-A analogs induce lower urinary tract dysfunction in male mice. Biochem. Pharmacol. 2022, 197, 114889. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Liu, J.; Wang, W.; Li, H.; Zhu, J.; Weng, S.; Xiao, S.; Wu, T. Prepubertal bisphenol A exposure interferes with ovarian follicle development and its relevant gene expression. Reprod. Toxicol. 2014, 44, 33–40. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Mabrey, N.; Adewale, H.B.; Sullivan, A.W. Soy but not bisphenol A (BPA) induces hallmarks of polycystic ovary syndrome (PCOS) and related metabolic co-morbidities in rats. Reprod. Toxicol. 2014, 49, 209–218. [Google Scholar] [CrossRef]
- Santamaría, C.; Durando, M.; Muñoz de Toro, M.; Luque, E.H.; Rodriguez, H.A. Ovarian dysfunctions in adult female rat offspring born to mothers perinatally exposed to low doses of bisphenol A. J. Steroid. Biochem. Mol. Biol. 2016, 158, 220–230. [Google Scholar] [CrossRef]
- Delclos, K.B.; Camacho, L.; Lewis, S.M.; Vanlandingham, M.M.; Latendresse, J.R.; Olson, G.R.; Davis, K.J.; Patton, R.E.; Gamboa da Costa, G.; Woodling, K.A.; et al. Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90. Toxicol. Sci. 2014, 139, 174–197, Erratum in: Toxicol. Sci. 2016, 153, 212. [Google Scholar] [CrossRef]
- Ganesan, S.; Keating, A.F. Bisphenol A-Induced Ovotoxicity Involves DNA Damage Induction to Which the Ovary Mounts a Protective Response Indicated by Increased Expression of Proteins Involved in DNA Repair and Xenobiotic Biotransformation. Toxicol. Sci. 2016, 152, 169–180. [Google Scholar] [CrossRef][Green Version]
- Migliaccio, M.; Chioccarelli, T.; Ambrosino, C.; Suglia, A.; Manfrevola, F.; Carnevali, O.; Fasano, S.; Pierantoni, R.; Cobellis, G. Characterization of Follicular Atresia Responsive to BPA in Zebrafish by Morphometric Analysis of Follicular Stage Progression. Int. J. Endocrinol. 2018, 2018, 4298195. [Google Scholar] [CrossRef]
- Ruiz, T.F.R.; Grigio, V.; Ferrato, L.J.; de Souza, L.G.; Colleta, S.J.; Amaro, G.M.; Góes, R.M.; Vilamaior, P.S.L.; Leonel, E.C.R.; Taboga, S.R. Impairment of steroidogenesis and follicle development after bisphenol A exposure during pregnancy and lactation in the ovaries of Mongolian gerbils aged females. Mol. Cell. Endocrinol. 2023, 566–567, 111892. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Li, Q.; Zhang, T.; Deng, Z.; Lian, J.; Jia, D.; Li, R.; Zheng, T.; Ding, X.; et al. Low concentration of BPA induces mice spermatocytes apoptosis via GPR30. Oncotarget 2017, 8, 49005–49015. [Google Scholar] [CrossRef]
- Vahedi, M.; Saeedi, A.; Poorbaghi, S.L.; Sepehrimanesh, M.; Fattahi, M. Metabolic and endocrine effects of bisphenol A exposure in market seller women with polycystic ovary syndrome. Environ. Sci. Pollut. Res. Int. 2016, 23, 23546–23550. [Google Scholar] [CrossRef]
- Acuña-Hernández, D.G.; Arreola-Mendoza, L.; Santacruz-Márquez, R.; García-Zepeda, S.P.; Parra-Forero, L.Y.; Olivares-Reyes, J.A.; Hernández-Ochoa, I. Bisphenol A alters oocyte maturation by prematurely closing gap junctions in the cumulus cell-oocyte complex. Toxicol. Appl. Pharmacol. 2018, 344, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Nishio, M.; Kobayashi, N.; Hiradate, Y.; Hoshino, Y.; Sato, E.; Tanemura, K. Comparison of the effects of BPA and BPAF on oocyte spindle assembly and polar body release in mice. Zygote 2016, 24, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Orvieto, R. Bisphenol A, oocyte maturation, implantation, and IVF outcome: Review of animal and human data. Reprod. Biomed. Online 2014, 29, 404–410. [Google Scholar] [CrossRef]
- Loup, B.; Poumerol, E.; Jouneau, L.; Fowler, P.A.; Cotinot, C.; Mandon-Pépin, B. BPA disrupts meiosis I in oogonia by acting on pathways including cell cycle regulation, meiosis initiation and spindle assembly. Reprod. Toxicol. 2022, 111, 166–177. [Google Scholar] [CrossRef]
- Neff, A.M.; Blanco, S.C.; Flaws, J.A.; Bagchi, I.C.; Bagchi, M.K. Chronic Exposure of Mice to Bisphenol-A Alters Uterine Fibroblast Growth Factor Signaling and Leads to Aberrant Epithelial Proliferation. Endocrinology 2019, 160, 1234–1246. [Google Scholar] [CrossRef]
- Kendziorski, J.A.; Belcher, S.M. Strain-specific induction of endometrial periglandular fibrosis in mice exposed during adulthood to the endocrine disrupting chemical bisphenol A. Reprod. Toxicol. 2015, 58, 119–130. [Google Scholar] [CrossRef]
- Li, Q.; Davila, J.; Kannan, A.; Flaws, J.A.; Bagchi, M.K.; Bagchi, I.C. Chronic Exposure to Bisphenol A Affects Uterine Function During Early Pregnancy in Mice. Endocrinology 2016, 157, 1764–1774. [Google Scholar] [CrossRef]
- Mallozzi, M.; Leone, C.; Manurita, F.; Bellati, F.; Caserta, D. Endocrine Disrupting Chemicals and Endometrial Cancer: An Overview of Recent Laboratory Evidence and Epidemiological Studies. Int. J. Environ. Res. Public Health 2017, 14, 334. [Google Scholar] [CrossRef]
- Adu-Gyamfi, E.A.; Rosenfeld, C.S.; Tuteja, G. The impact of bisphenol A on the placenta. Biol. Reprod. 2022, 106, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.; Tassinari, R.; Maranghi, F.; Mantovani, A. Bisphenol A affects placental layers morphology and angiogenesis during early pregnancy phase in mice. J. Appl. Toxicol. 2015, 35, 1278–1291. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, C.; Kang, H.Y.; Hong, E.J.; Hyun, S.H.; Choi, K.C.; Jeung, E.B. Effects of Octylphenol and Bisphenol A on the Metal Cation Transporter Channels of Mouse Placentas. Int. J. Environ. Res. Public Health 2016, 13, 965. [Google Scholar] [CrossRef]
- Lan, X.; Fu, L.J.; Zhang, J.; Liu, X.Q.; Zhang, H.J.; Zhang, X.; Ma, M.F.; Chen, X.M.; He, J.L.; Li, L.B.; et al. Bisphenol A exposure promotes HTR-8/SVneo cell migration and impairs mouse placentation involving upregulation of integrin-β1 and MMP-9 and stimulation of MAPK and PI3K signaling pathways. Oncotarget 2017, 8, 51507–51521. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Kinkade, J.A.; Bivens, N.J.; Rosenfeld, C.S. miRNA changes in the mouse placenta due to bisphenol A exposure. Epigenomics 2021, 13, 1909–1919. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, S.; Lu, J.; Zhang, M.; Shi, L.; Qin, J.; Lv, J.; Li, D.; Ma, L.; Zhang, Y. BPA induces placental trophoblast proliferation inhibition and fetal growth restriction by inhibiting the expression of SRB1. Environ. Sci. Pollut. Res. Int. 2023, 30, 60805–60819. [Google Scholar] [CrossRef] [PubMed]
- Strakovsky, R.S.; Wang, H.; Engeseth, N.J.; Flaws, J.A.; Helferich, W.G.; Pan, Y.X.; Lezmi, S. Developmental bisphenol A (BPA) exposure leads to sex-specific modification of hepatic gene expression and epigenome at birth that may exacerbate high-fat diet-induced hepatic steatosis. Toxicol. Appl. Pharmacol. 2015, 284, 101–112. [Google Scholar] [CrossRef]
- Shi, M.; Whorton, A.E.; Sekulovski, N.; MacLean, J.A.; Hayashi, K. Prenatal Exposure to Bisphenol A, E, and S Induces Transgenerational Effects on Female Reproductive Functions in Mice. Toxicol. Sci. 2019, 170, 320–329. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, J.; Zhang, E.; Wu, Q.; Wu, X.; Zhang, D.; Liu, Y.; Wang, R.; Li, W. Bisphenol A affects ovarian development in adolescent mice caused by genes expression change. Gene 2020, 740, 144535. [Google Scholar] [CrossRef]
- Snijder, C.A.; Heederik, D.; Pierik, F.H.; Hofman, A.; Jaddoe, V.W.; Koch, H.M.; Longnecker, M.P.; Burdorf, A. Fetal growth and prenatal exposure to bisphenol A: The generation R study. Environ. Health Perspect. 2013, 121, 393–398. [Google Scholar] [CrossRef]
- Pinney, S.E.; Mesaros, C.A.; Snyder, N.W.; Busch, C.M.; Xiao, R.; Aijaz, S.; Ijaz, N.; Blair, I.A.; Manson, J.M. Second trimester amniotic fluid bisphenol A concentration is associated with decreased birth weight in term infants. Reprod. Toxicol. 2017, 67, 1–9. [Google Scholar] [CrossRef]
- Burstyn, I.; Martin, J.W.; Beesoon, S.; Bamforth, F.; Li, Q.; Yasui, Y.; Cherry, N.M. Maternal exposure to bisphenol-A and fetal growth restriction: A case-referent study. Int. J. Environ. Res. Public Health 2013, 10, 7001–7014. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chiung, Y.M.; Lu, F.; Qiu, S.; Ji, M.; Huo, X. Associations of cadmium, bisphenol A and polychlorinated biphenyl co-exposure in utero with placental gene expression and neonatal outcomes. Reprod. Toxicol. 2015, 52, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Li, F.L.; Hua, X.G.; Jiang, W.; Mao, C.; Zhang, X.J. The association between prenatal bisphenol A exposure and birth weight: A meta-analysis. Reprod. Toxicol. 2018, 79, 21–31. [Google Scholar] [CrossRef]
- Troisi, J.; Mikelson, C.; Richards, S.; Symes, S.; Adair, D.; Zullo, F.; Guida, M. Placental concentrations of bisphenol A and birth weight from births in the Southeastern U.S. Placenta 2014, 35, 947–952. [Google Scholar] [CrossRef]
- Susiarjo, M.; Xin, F.; Bansal, A.; Stefaniak, M.; Li, C.; Simmons, R.A.; Bartolomei, M.S. Bisphenol a exposure disrupts metabolic health across multiple generations in the mouse. Endocrinology 2015, 156, 2049–2058. [Google Scholar] [CrossRef]
- Cantonwine, D.E.; Ferguson, K.K.; Mukherjee, B.; McElrath, T.F.; Meeker, J.D. Urinary Bisphenol A Levels during Pregnancy and Risk of Preterm Birth. Environ. Health Perspect. 2015, 123, 895–901. [Google Scholar] [CrossRef]
- Weinberger, B.; Vetrano, A.M.; Archer, F.E.; Marcella, S.W.; Buckley, B.; Wartenberg, D.; Robson, M.G.; Klim, J.; Azhar, S.; Cavin, S.; et al. Effects of maternal exposure to phthalates and bisphenol A during pregnancy on gestational age. J. Matern.-Fetal Neonatal Med. 2014, 27, 323–327. [Google Scholar] [CrossRef]
- Smarr, M.M.; Grantz, K.L.; Sundaram, R.; Maisog, J.M.; Kannan, K.; Louis, G.M. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environ. Health 2015, 14, 73. [Google Scholar] [CrossRef]
- Behnia, F.; Peltier, M.; Getahun, D.; Watson, C.; Saade, G.; Menon, R. High bisphenol A (BPA) concentration in the maternal, but not fetal, compartment increases the risk of spontaneous preterm delivery. J. Matern.-Fetal Neonatal Med. 2016, 29, 3583–3589. [Google Scholar] [CrossRef]
- Barberio, L.; Paulesu, L.; Canesi, L.; Grasselli, E.; Mandalà, M. Bisphenol a Interferes with Uterine Artery Features and Impairs Rat Feto-Placental Growth. Int. J. Mol. Sci. 2021, 22, 6912. [Google Scholar] [CrossRef]
- Guida, M.; Troisi, J.; Ciccone, C.; Granozio, G.; Cosimato, C.; Di SpiezioSardo, A.; Ferrara, C.; Guida, M.; Nappi, C.; Zullo, F.; et al. Bisphenol A and congenital developmental defects in humans. Mutat. Res. 2015, 774, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.; Axelstad, M.; Boberg, J.; Vinggaard, A.M.; Pedersen, G.A.; Hass, U. Low-dose effects of bisphenol A on early sexual development in male and female rats. Reproduction 2014, 147, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Turi, N.; Ullah, W.; Siddiqui, M.F.; Zakria, M.; Lodhi, K.Z.; Khan, M.M. Impact of low-dose chronic exposure to bisphenol A and its analogue bisphenol B, bisphenol F and bisphenol S on hypothalamo-pituitary-testicular activities in adult rats: A focus on the possible hormonal mode of action. Food Chem. Toxicol. 2018, 121, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.F.; Arrebola, J.P.; Jiménez-Díaz, I.; Sáenz, J.M.; Molina-Molina, J.M.; Ballesteros, O.; Kortenkamp, A.; Olea, N. Bisphenol A and other phenols in human placenta from children with cryptorchidism or hypospadias. Reprod. Toxicol. 2016, 59, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Mammadov, E.; Uncu, M.; Dalkan, C. High Prenatal Exposure to Bisphenol A Reduces Anogenital Distance in Healthy Male Newborns. J. Clin. Res. Pediatr. Endocrinol. 2018, 10, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyńska, M.M.; Gajowik, A.; Jankowska-Steifer, E.A.; Radzikowska, J.; Tyrkiel, E.J. Reproductive and developmental F1 toxicity following exposure of pubescent F0 male mice to bisphenol A alone and in a combination with X-rays irradiation. Toxicology 2018, 410, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Chianese, R.; Troisi, J.; Richards, S.; Scafuro, M.; Fasano, S.; Guida, M.; Pierantoni, R.; Meccariello, R. Bisphenol A in Reproduction: Epigenetic Effects. Curr. Med. Chem. 2018, 25, 748–770. [Google Scholar] [CrossRef]
- Zhang, G.L.; Zhang, X.F.; Feng, Y.M.; Li, L.; Huynh, E.; Sun, X.F.; Sun, Z.Y.; Shen, W. Exposure to bisphenol A results in a decline in mouse spermatogenesis. Reprod. Fertil. Dev. 2013, 25, 847–859. [Google Scholar] [CrossRef]
- Lan, H.C.; Wu, K.Y.; Lin, I.W.; Yang, Z.J.; Chang, A.A.; Hu, M.C. Bisphenol A disrupts steroidogenesis and induces a sex hormone imbalance through c-Jun phosphorylation in Leydig cells. Chemosphere 2017, 185, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Mruk, D.D.; Lee, W.M.; Wong, C.K.; Cheng, C.Y. Is toxicant-induced Sertoli cell injury in vitro a useful model to study molecular mechanisms in spermatogenesis? Semin. Cell. Dev. Biol. 2016, 59, 141–156. [Google Scholar] [CrossRef]
- Kotwicka, M.; Skibinska, I.; Jendraszak, M.; Jedrzejczak, P. 17β-estradiol modifies human spermatozoa mitochondrial function in vitro. Reprod. Biol. Endocrinol. 2016, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Miao, M.; Liang, H.; Shi, H.; Ruan, D.; Li, Y.; Wang, J.; Yuan, W. Exposure of environmental Bisphenol A in relation to routine sperm parameters and sperm movement characteristics among fertile men. Sci. Rep. 2018, 8, 17548. [Google Scholar] [CrossRef] [PubMed]
- Vitku, J.; Chlupacova, T.; Sosvorova, L.; Hampl, R.; Hill, M.; Heracek, J.; Bicikova, M.; Starka, L. Development and validation of LC-MS/MS method for quantification of bisphenol A and estrogens in human plasma and seminal fluid. Talanta 2015, 140, 62–67. [Google Scholar] [CrossRef]
- Manfo, F.P.; Jubendradass, R.; Nantia, E.A.; Moundipa, P.F.; Mathur, P.P. Adverse effects of bisphenol A on male reproductive function. Rev. Environ. Contam. Toxicol. 2014, 228, 57–82. [Google Scholar] [CrossRef]
- Sun, X.; Li, D.; Liang, H.; Miao, M.; Song, X.; Wang, Z.; Zhou, Z.; Yuan, W. Maternal exposure to bisphenol A and anogenital distance throughout infancy: A longitudinal study from Shanghai, China. Environ. Int. 2018, 121, 269–275. [Google Scholar] [CrossRef]
- Barrett, E.S.; Sathyanarayana, S.; Mbowe, O.; Thurston, S.W.; Redmon, J.B.; Nguyen, R.H.N.; Swan, S.H. First-Trimester Urinary Bisphenol A Concentration in Relation to Anogenital Distance, an Androgen-Sensitive Measure of Reproductive Development, in Infant Girls. Environ. Health Perspect. 2017, 125, 077008. [Google Scholar] [CrossRef]
- Liu, D.; Shen, L.; Tao, Y.; Kuang, Y.; Cai, L.; Wang, D.; He, M.; Tong, X.; Zhou, S.; Sun, J.; et al. Alterations in gene expression during sexual differentiation in androgen receptor knockout mice induced by environmental endocrine disruptors. Int. J. Mol. Med. 2019, 44, 1183, Erratum for: Int. J. Mol. Med. 2015, 35, 399–404. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.S.; Guo, T.L. Modulation of cytokine/chemokine production in human macrophages by bisphenol A: A comparison to analogues and interactions with genistein. J. Immunotoxicol. 2018, 15, 96–103. [Google Scholar] [CrossRef]
- Berger, K.; Eskenazi, B.; Kogut, K.; Parra, K.; Lustig, R.H.; Greenspan, L.C.; Holland, N.; Calafat, A.M.; Ye, X.; Harley, K.G. Association of Prenatal Urinary Concentrations of Phthalates and Bisphenol A and Pubertal Timing in Boys and Girls. Environ. Health Perspect. 2018, 126, 97004. [Google Scholar] [CrossRef]
- Perera, F.; Nolte, E.L.R.; Wang, Y.; Margolis, A.E.; Calafat, A.M.; Wang, S.; Garcia, W.; Hoepner, L.A.; Peterson, B.S.; Rauh, V.; et al. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environ. Res. 2016, 151, 195–202. [Google Scholar] [CrossRef]
- Jensen, T.K.; Mustieles, V.; Bleses, D.; Frederiksen, H.; Trecca, F.; Schoeters, G.; Andersen, H.R.; Grandjean, P.; Kyhl, H.B.; Juul, A.; et al. Prenatal bisphenol A exposure is associated with language development but not with ADHD-related behavior in toddlers from the Odense Child Cohort. Environ. Res. 2019, 170, 398–405. [Google Scholar] [CrossRef]
- Kanlayaprasit, S.; Thongkorn, S.; Panjabud, P.; Jindatip, D.; Hu, V.W.; Kikkawa, T.; Osumi, N.; Sarachana, T. Autism-Related Transcription Factors Underlying the Sex-Specific Effects of Prenatal Bisphenol A Exposure on Transcriptome-Interactome Profiles in the Offspring Prefrontal Cortex. Int. J. Mol. Sci. 2021, 22, 13201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Meng, L.; Kuang, H.; Liu, J.; Lv, X.; Pang, Q.; Fan, R. Maternal exposure to environmental bisphenol A impairs the neurons in hippocampus across generations. Toxicology 2020, 432, 152393. [Google Scholar] [CrossRef] [PubMed]
- Bi, N.; Ding, J.; Zou, R.; Gu, X.; Liu, Z.H.; Wang, H.L. Developmental exposure of bisphenol A induces spatial memory deficits by weakening the excitatory neural circuits of CA3-CA1 and EC-CA1 in mice. Toxicol. Appl. Pharmacol. 2021, 426, 115641. [Google Scholar] [CrossRef]
- Wang, Z.; Alderman, M.H.; Asgari, C.; Taylor, H.S. Fetal Bisphenol-A Induced Changes in Murine Behavior and Brain Gene Expression Persisted in Adult-aged Offspring. Endocrinology 2020, 161, bqaa164. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Hu, L.; Yang, S.; Ni, L.; Ma, L.; Zhao, Y.; Zheng, A.; Jin, Y.; Fu, Z. Bisphenol A impairs cognitive function and 5-HT metabolism in adult male mice by modulating the microbiota-gut-brain axis. Chemosphere 2021, 282, 130952. [Google Scholar] [CrossRef]
- Heredia-García, G.; Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M.; Islas-Flores, H.; García-Medina, S.; Galar-Martínez, M.; Dublán-García, O. Realistic concentrations of Bisphenol-A trigger a neurotoxic response in the brain of zebrafish: Oxidative stress, behavioral impairment, acetylcholinesterase inhibition, and gene expression disruption. Chemosphere 2023, 330, 138729. [Google Scholar] [CrossRef]
- Schirmer, E.; Schuster, S.; Machnik, P. Bisphenols exert detrimental effects on neuronal signaling in mature vertebrate brains. Commun. Biol. 2021, 4, 465. [Google Scholar] [CrossRef]
- Kochmanski, J.J.; Marchlewicz, E.H.; Cavalcante, R.G.; Perera, B.P.U.; Sartor, M.A.; Dolinoy, D.C. Longitudinal Effects of Developmental Bisphenol A Exposure on Epigenome-Wide DNA Hydroxymethylation at Imprinted Loci in Mouse Blood. Environ. Health Perspect. 2018, 126, 077006. [Google Scholar] [CrossRef]
- Perrot-Applanat, M.; Kolf-Clauw, M.; Michel, C.; Beausoleil, C. Alteration of mammary gland development by bisphenol and evidence of a mode of action mediated through endocrine disruption. Mol. Cell. Endocrinol. 2018, 475, 29–53. [Google Scholar] [CrossRef]
- Wolstenholme, J.T.; Goldsby, J.A.; Rissman, E.F. Transgenerational effects of prenatal bisphenol A on social recognition. Horm. Behav. 2013, 64, 833–839. [Google Scholar] [CrossRef]
- Bhandari, R.K.; Deem, S.L.; Holliday, D.K.; Jandegian, C.M.; Kassotis, C.D.; Nagel, S.C.; Tillitt, D.E.; Vom Saal, F.S.; Rosenfeld, C.S. Effects of the environmental estrogenic contaminants bisphenol A and 17α-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen. Comp. Endocrinol. 2015, 214, 195–219. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Rivera, F.J.; Guerrero-Bosagna, C. Bisphenol-A and metabolic diseases: Epigenetic, developmental, and transgenerational basis. Environ. Epigenet. 2016, 2, dvw022. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Li, C.; Xin, F.; Duemler, A.; Li, W.; Rashid, C.; Bartolomei, M.S.; Simmons, R.A. Transgenerational effects of maternal bisphenol: An exposure on offspring metabolic health. J. Dev. Orig. Health Dis. 2019, 10, 164–175, Erratum in: J. Dev. Orig. Health Dis. 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.; Ther, L.; Gao, L.; Wang, W.; Ziv-Gal, A.; Flaws, J.A. The effects of in utero bisphenol A exposure on ovarian follicle numbers and steroidogenesis in the F1 and F2 generations of mice. Reprod. Toxicol. 2017, 74, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Pang, W.K.; Ryu, D.Y.; Park, Y.J.; Pang, M.G. Multigenerational and transgenerational impact of paternal bisphenol A exposure on male fertility in a mouse model. Hum. Reprod. 2020, 35, 1740–1752. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.; Abril, N.; Morales-Prieto, N.; Monterde, J.; Ayala, N.; Lora, A.; Moyano, R. Hypothalamic-pituitary-ovarian axis perturbation in the basis of bisphenol A (BPA) reproductive toxicity in female zebrafish (Danio rerio). Ecotox. Environ. Safe. 2018, 156, 116–124. [Google Scholar] [CrossRef]
- Sharma, S.; Ahmad, S.; Afjal, M.A.; Habib, H.; Parvez, S.; Raisuddin, S. Dichotomy of bisphenol A-induced expression of peroxisome proliferator-activated receptors in hepatic and testicular tissues in mice. Chemosphere 2019, 236, 124264, Erratum in Chemosphere 2020, 249, 126601. [Google Scholar] [CrossRef]
- Shi, M.; Whorton, A.E.; Sekulovski, N.; MacLean, J.A.; Hayashi, K. Prenatal Exposure to Bisphenol A, E, and S Induces Transgenerational Effects on Male Reproductive Functions in Mice. Toxicol. Sci. 2019, 172, 303–315. [Google Scholar] [CrossRef]
- Wolstenholme, J.T.; Drobná, Z.; Henriksen, A.D.; Goldsby, J.A.; Stevenson, R.; Irvin, J.W.; Flaws, J.A.; Rissman, E.F. Transgenerational Bisphenol A Causes Deficits in Social Recognition and Alters Postsynaptic Density Genes in Mice. Endocrinology 2019, 160, 1854–1867. [Google Scholar] [CrossRef]
- García-Arevalo, M.; Alonso-Magdalena, P.; Rebelo Dos Santos, J.; Quesada, I.; Carneiro, E.M.; Nadal, A. Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS ONE 2014, 9, e100214. [Google Scholar] [CrossRef] [PubMed]
- Marmugi, A.; Lasserre, F.; Beuzelin, D.; Ducheix, S.; Huc, L.; Polizzi, A.; Chetivaux, M.; Pineau, T.; Martin, P.; Guillou, H.; et al. Adverse effects of long-term exposure to bisphenol A during adulthood leading to hyperglycaemia and hypercholesterolemia in mice. Toxicology 2014, 325, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Lejonklou, M.H.; Dunder, L.; Bladin, E.; Pettersson, V.; Rönn, M.; Lind, L.; Waldén, T.B.; Lind, P.M. Effects of Low-Dose Developmental Bisphenol A Exposure on Metabolic Parameters and Gene Expression in Male and Female Fischer 344 Rat Offspring. Environ. Health Perspect. 2017, 125, 067018. [Google Scholar] [CrossRef]
- Moon, M.K.; Jeong, I.K.; Jung, O.T.; Ahn, H.Y.; Kim, H.H.; Park, Y.J.; Jang, H.C.; Park, K.S. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance. J. Endocrinol. 2015, 226, 35–42. [Google Scholar] [CrossRef]
- Oliveira, K.M.; Figueiredo, L.S.; Araujo, T.R.; Freitas, I.N.; Silva, J.N.; Boschero, A.C.; Ribeiro, R.A. Prolonged bisphenol-A exposure decreases endocrine pancreatic proliferation in response to obesogenic diet in ovariectomized mice. Steroids 2020, 160, 108658. [Google Scholar] [CrossRef]
- Legeay, S.; Faure, S. Is bisphenol A an environmental obesogen? Fundam. Clin. Pharmacol. 2017, 31, 594–609. [Google Scholar] [CrossRef]
- Errico, S.; Portaccio, M.; Nicolucci, C.; Meccariello, R.; Chianese, R.; Scafuro, M.; Lepore, M.; Diano, N. A novel experimental approach for liver analysis in rats exposed to Bisphenol A by means of LC-mass spectrometry and infrared spectroscopy. J. Pharm. Biomed. Anal. 2019, 165, 207–212. [Google Scholar] [CrossRef]
- Roepke, T.A.; Yang, J.A.; Yasrebi, A.; Mamounis, K.J.; Oruc, E.; Zama, A.M.; Uzumcu, M. Regulation of arcuate genes by developmental exposures to endocrine-disrupting compounds in female rats. Reprod. Toxicol. 2016, 62, 18–26. [Google Scholar] [CrossRef]
- Desai, M.; Ferrini, M.G.; Jellyman, J.K.; Han, G.; Ross, M.G. In vivo and in vitro bisphenol A exposure effects on adiposity. J. Dev. Orig. Health Dis. 2018, 9, 678–687. [Google Scholar] [CrossRef]
- Aktağ, E.; Yurdakök, K.; Yalçın, S.S.; Kandemir, N. Urinary bisphenol A levels in prepubertal children with exogenous obesity according to presence of metabolic syndrome. J. Pediatr. Endocrinol. Metab. 2021, 34, 495–502. [Google Scholar] [CrossRef]
- Shu, X.; Tang, S.; Peng, C.; Gao, R.; Yang, S.; Luo, T.; Cheng, Q.; Wang, Y.; Wang, Z.; Zhen, Q.; et al. Bisphenol A is not associated with a 5-year incidence of type 2 diabetes: A prospective nested case-control study. Acta Diabetol. 2018, 55, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Hong, Y.P.; Chae, S.A. Reduction in semen quality after mixed exposure to bisphenol A and isobutylparaben in utero and during lactation periods. Hum. Exp. Toxicol. 2016, 35, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Meng, Q.; Diamante, G.; Tsai, B.; Chen, Y.W.; Mikhail, A.; Luk, H.; Ritz, B.; Allard, P.; Yang, X. Prenatal Bisphenol A Exposure in Mice Induces Multi tissue Multiomics Disruptions Linking to Cardiometabolic Disorders. Endocrinology 2019, 160, 409–429. [Google Scholar] [CrossRef]
- Wehbe, Z.; Nasser, S.A.; El-Yazbi, A.; Nasreddine, S.; Eid, A.H. Estrogen and Bisphenol A in Hypertension. Curr. Hypertens. Rep. 2020, 22, 23. [Google Scholar] [CrossRef]
- Moon, S.; Yu, S.H.; Lee, C.B.; Park, Y.J.; Yoo, H.J.; Kim, D.S. Effects of bisphenol A on cardiovascular disease: An epidemiological study using National Health and Nutrition Examination Survey 2003–2016 and meta-analysis. Sci. Total Environ. 2021, 763, 142941. [Google Scholar] [CrossRef]
- Agas, D.; Lacava, G.; Sabbieti, M.G. Bone and bone marrow disruption by endocrine-active substances. J. Cell. Physiol. 2018, 234, 192–213. [Google Scholar] [CrossRef]
- Thent, Z.C.; Froemming, G.R.A.; Muid, S. Bisphenol A exposure disturbs the bone metabolism: An evolving interest towards an old culprit. Life Sci. 2018, 198, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Aimuzi, R.; Nian, M.; Zhang, Y.; Luo, K.; Zhang, J. Bisphenol A substitutes and sex hormones in children and adolescents. Chemosphere 2021, 278, 130396. [Google Scholar] [CrossRef]
- Molina, A.M.; Abril, N.; Lora, A.J.; Huertas-Abril, P.V.; Ayala, N.; Blanco, C.; Moyano, M.R. Proteomic profile of the effects of low-dose bisphenol A on zebrafish ovaries. Food Chem. Toxicol. 2021, 156, 112435. [Google Scholar] [CrossRef]
- Park, J.C.; Lee, M.C.; Yoon, D.S.; Han, J.; Kim, M.; Hwang, U.K.; Jung, J.H.; Lee, J.S. Effects of bisphenol A and its analogs bisphenol F and S on life parameters, antioxidant system, and response of defensome in the marine rotifer Brachionuskoreanus. Aquat. Toxicol. 2018, 199, 21–29. [Google Scholar] [CrossRef]
- Xu, J.; Huang, G.; Guo, T.L. Developmental Bisphenol A Exposure Modulates Immune-Related Diseases. Toxics 2016, 4, 23. [Google Scholar] [CrossRef]
- Xu, J.; Huang, G.; Nagy, T.; Teng, Q.; Guo, T.L. Sex-dependent effects of bisphenol A on type 1 diabetes development in non-obese diabetic (NOD) mice. Arch. Toxicol. 2019, 93, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Olukole, S.G.; Lanipekun, D.O.; Ola-Davies, E.O.; Oke, B.O. Maternal exposure to environmentally relevant doses of bisphenol A causes reproductive dysfunction in F1 adult male rats: Protective role of melatonin. Environ. Sci. Pollut. Res Int. 2019, 26, 28940–28950. [Google Scholar] [CrossRef]
- Kaur, K.; Chauhan, V.; Gu, F.; Chauhan, A. Bisphenol A induces oxidative stress and mitochondrial dysfunction in lymphoblasts from children with autism and unaffected siblings. Free Radic. Biol. Med. 2014, 76, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.; Mousavi, S.N.; Aghapour, F.; Rezaee, B.; Sadeghi, F.; Moghadamnia, A.A. Induction Effect of Bisphenol A on Gene Expression Involving Hepatic Oxidative Stress in Rat. Oxid. Med. Cell. Longev. 2016, 2016, 6298515. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Kong, Y.; Ommati, M.M.; Tang, Z.; Li, H.; Li, L.; Zhao, C.; Shi, Z.; Wang, J. Bisphenol A-induced apoptosis, oxidative stress and DNA damage in cultured rhesus monkey embryo renal epithelial Marc-145 cells. Chemosphere 2019, 234, 682–689. [Google Scholar] [CrossRef]
- Abedelhaffez, A.S.; El-Aziz, E.A.A.; Aziz, M.A.A.; Ahmed, A.M. Lung injury induced by Bisphenol A: A food contaminant, is ameliorated by selenium supplementation. Pathophysiology 2017, 24, 81–89. [Google Scholar] [CrossRef]
- Shirani, M.; Alizadeh, S.; Mahdavinia, M.; Dehghani, M.A. The ameliorative effect of quercetin on bisphenol A-induced toxicity in mitochondria isolated from rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 7688–7696. [Google Scholar] [CrossRef]
- Silva, M.M.D.; Xavier, L.L.F.; Gonçalves, C.F.L.; Santos-Silva, A.P.; Paiva-Melo, F.D.; Freitas, M.L.; Fortunato, R.S.; Alves, L.M.; Ferreira, A.C.F. Bisphenol A increases hydrogen peroxide generation by thyrocytes both in vivo and in vitro. Endocr. Connect. 2018, 7, 1196–1207. [Google Scholar] [CrossRef]
- Da Silva, M.M.; Gonçalves, C.F.L.; Miranda-Alves, L.; Fortunato, R.S.; Carvalho, D.P.; Ferreira, A.C.F. Inhibition of Type 1 Iodothyronine Deiodinase by Bisphenol A. Horm. Metab. Res. 2019, 51, 671–677. [Google Scholar] [CrossRef]
- Silva, B.S.; Bertasso, I.M.; Pietrobon, C.B.; Lopes, B.P.; Santos, T.R.; Peixoto-Silva, N.; Carvalho, J.C.; Claudio-Neto, S.; Manhães, A.C.; Cabral, S.S.; et al. Effects of maternal bisphenol A on behavior, sex steroid and thyroid hormones levels in the adult rat offspring. Life Sci. 2019, 218, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lu, Y.; Zhang, G.; Chen, L.; Tian, D.; Shen, X.; Yang, Y.; Dong, F. Bisphenol A promotes dendritic morphogenesis of hippocampal neurons through estrogen receptor-mediated ERK1/2 signal pathway. Chemosphere 2014, 96, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, R.N.; Park, P.; Neese, S.L.; Ferguson, D.C.; Schantz, S.L.; Juraska, J.M. Effects of perinatal bisphenol A exposure during early development on radial arm maze behavior in adult male and female rats. Neurotoxicol. Teratol. 2014, 42, 17–24. [Google Scholar] [CrossRef]
- Sanlidag, B.; Dalkan, C.; Yetkin, O.; Bahçeciler, N.N. Evaluation of Dose Dependent Maternal Exposure to Bisphenol A on Thyroid Functions in Newborns. J. Clin. Med. 2018, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, Y.; Zhao, H.; Jiang, Y.; Cai, Z. Evaluation of bisphenol A exposure induced oxidative RNA damage by liquid chromatography-mass spectrometry. Chemosphere 2019, 222, 235–242. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-López, A.M.; Bujalance-Reyes, F.; Ayala-Soldado, N.; Mora-Medina, R.; Lora-Benítez, A.; Moyano-Salvago, R. An Overview of the Health Effects of Bisphenol A from a One Health Perspective. Animals 2023, 13, 2439. https://doi.org/10.3390/ani13152439
Molina-López AM, Bujalance-Reyes F, Ayala-Soldado N, Mora-Medina R, Lora-Benítez A, Moyano-Salvago R. An Overview of the Health Effects of Bisphenol A from a One Health Perspective. Animals. 2023; 13(15):2439. https://doi.org/10.3390/ani13152439
Chicago/Turabian StyleMolina-López, Ana M., Francisca Bujalance-Reyes, Nahúm Ayala-Soldado, Rafael Mora-Medina, Antonio Lora-Benítez, and Rosario Moyano-Salvago. 2023. "An Overview of the Health Effects of Bisphenol A from a One Health Perspective" Animals 13, no. 15: 2439. https://doi.org/10.3390/ani13152439
APA StyleMolina-López, A. M., Bujalance-Reyes, F., Ayala-Soldado, N., Mora-Medina, R., Lora-Benítez, A., & Moyano-Salvago, R. (2023). An Overview of the Health Effects of Bisphenol A from a One Health Perspective. Animals, 13(15), 2439. https://doi.org/10.3390/ani13152439








