Is the Hippo Pathway Effector Yes-Associated Protein a Potential Key Player of Dairy Cattle Cystic Ovarian Disease Pathogenesis?
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Cystic Follicle Collection
2.2. Immunohistochemistry
2.3. Total RNA Extraction and Real-Time qPCR
2.4. Western Blotting
2.5. Hormone Assay
2.6. Statistical Analysis
3. Results
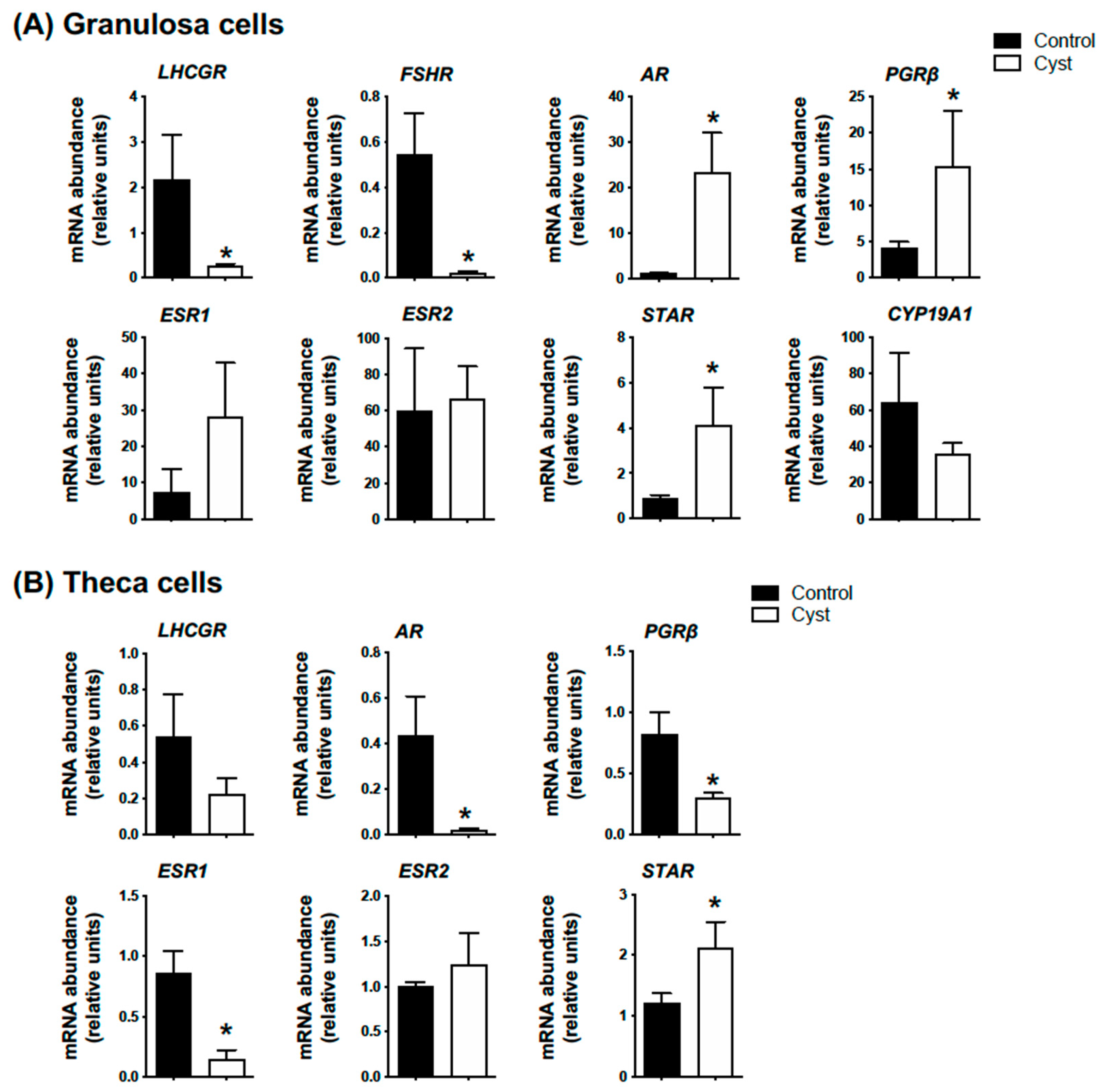
3.1. Molecular and Hormonal Validation of COD Follicle Samples
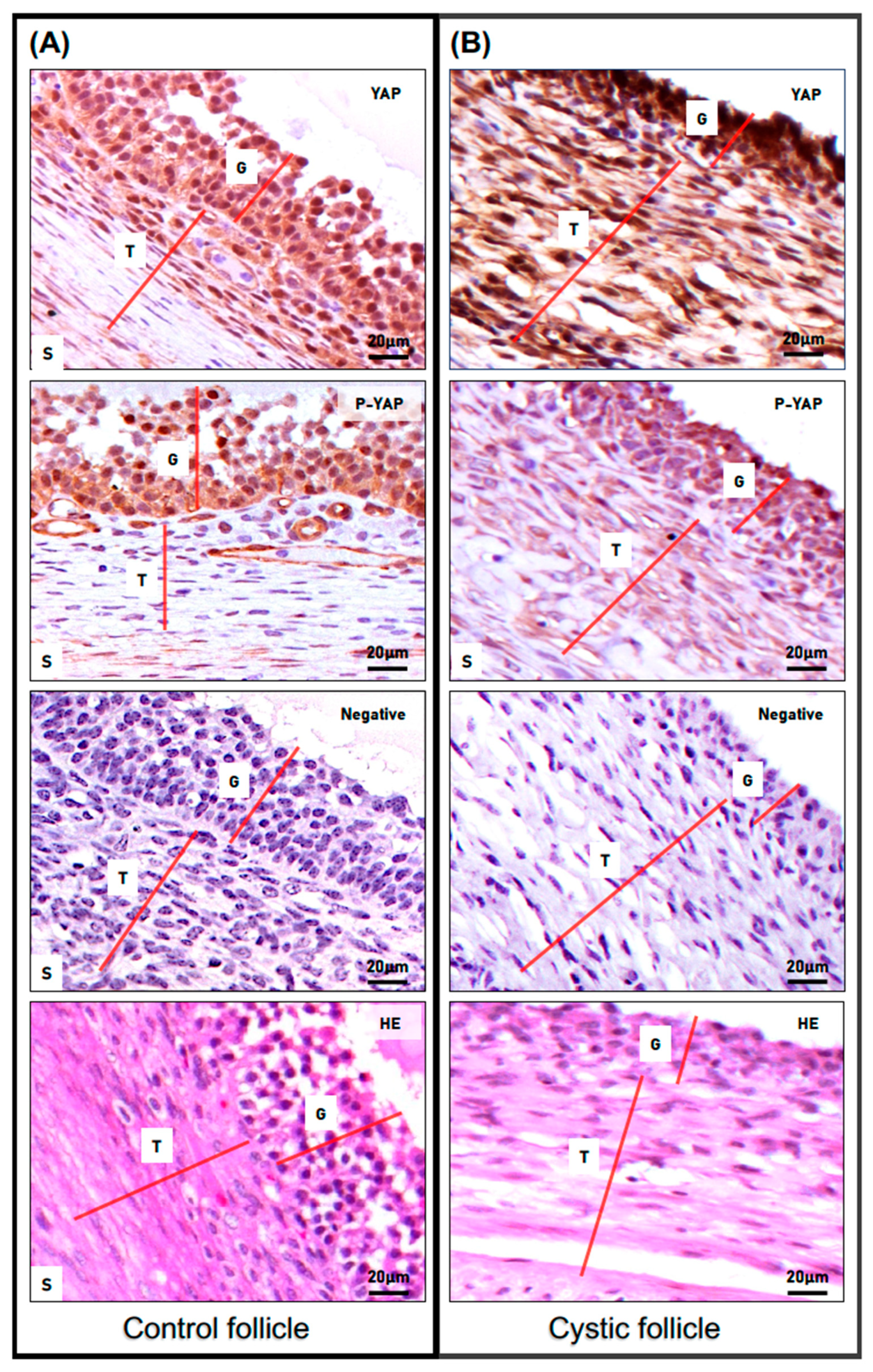
3.2. YAP and Phospho-YAP Expression Pattern in Bovine Cystic Follicles
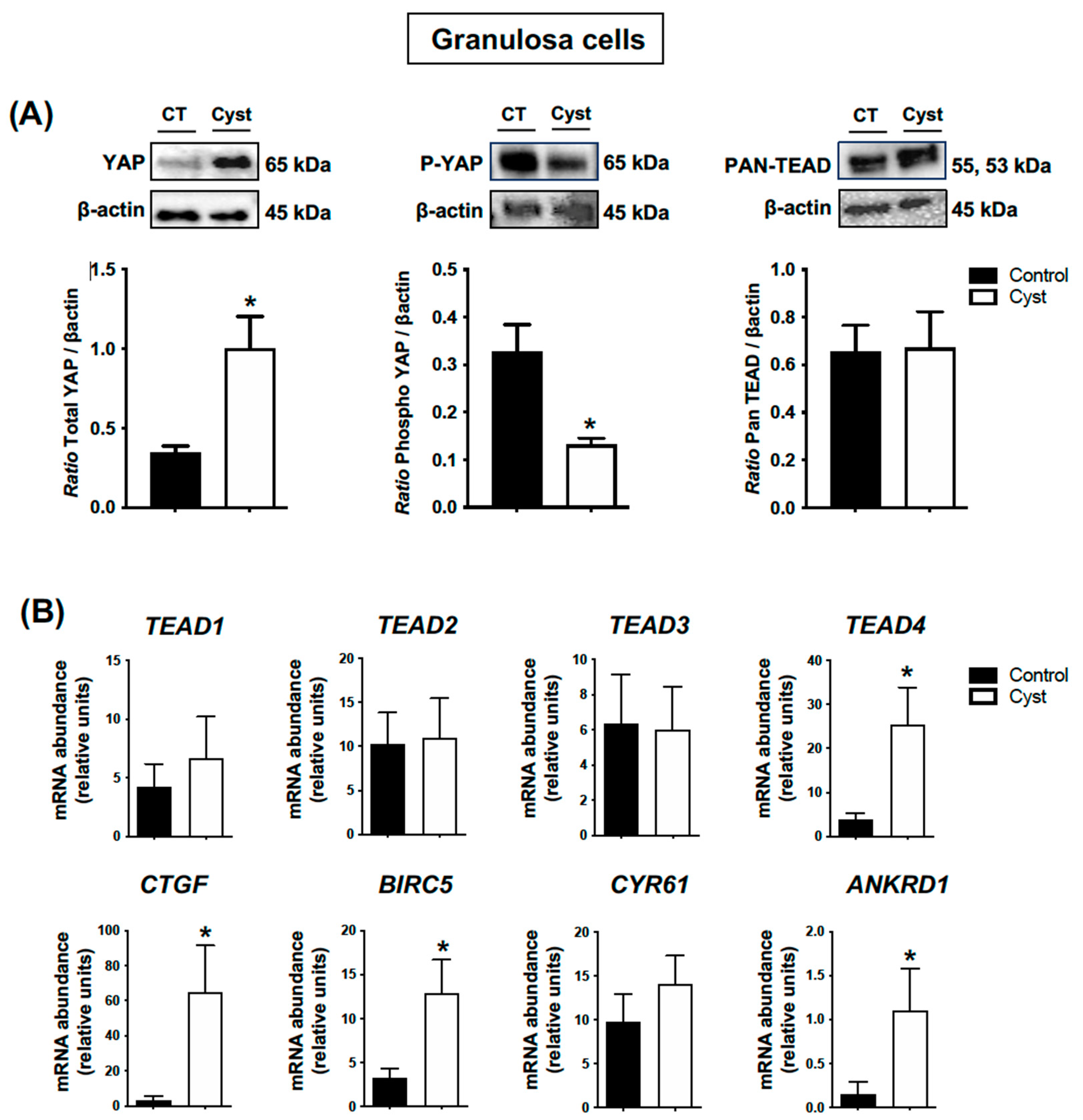
3.3. Expression Levels for YAP, TEADs and Classic YAP-TEAD Target Genes in Cystic Granulosa Cells
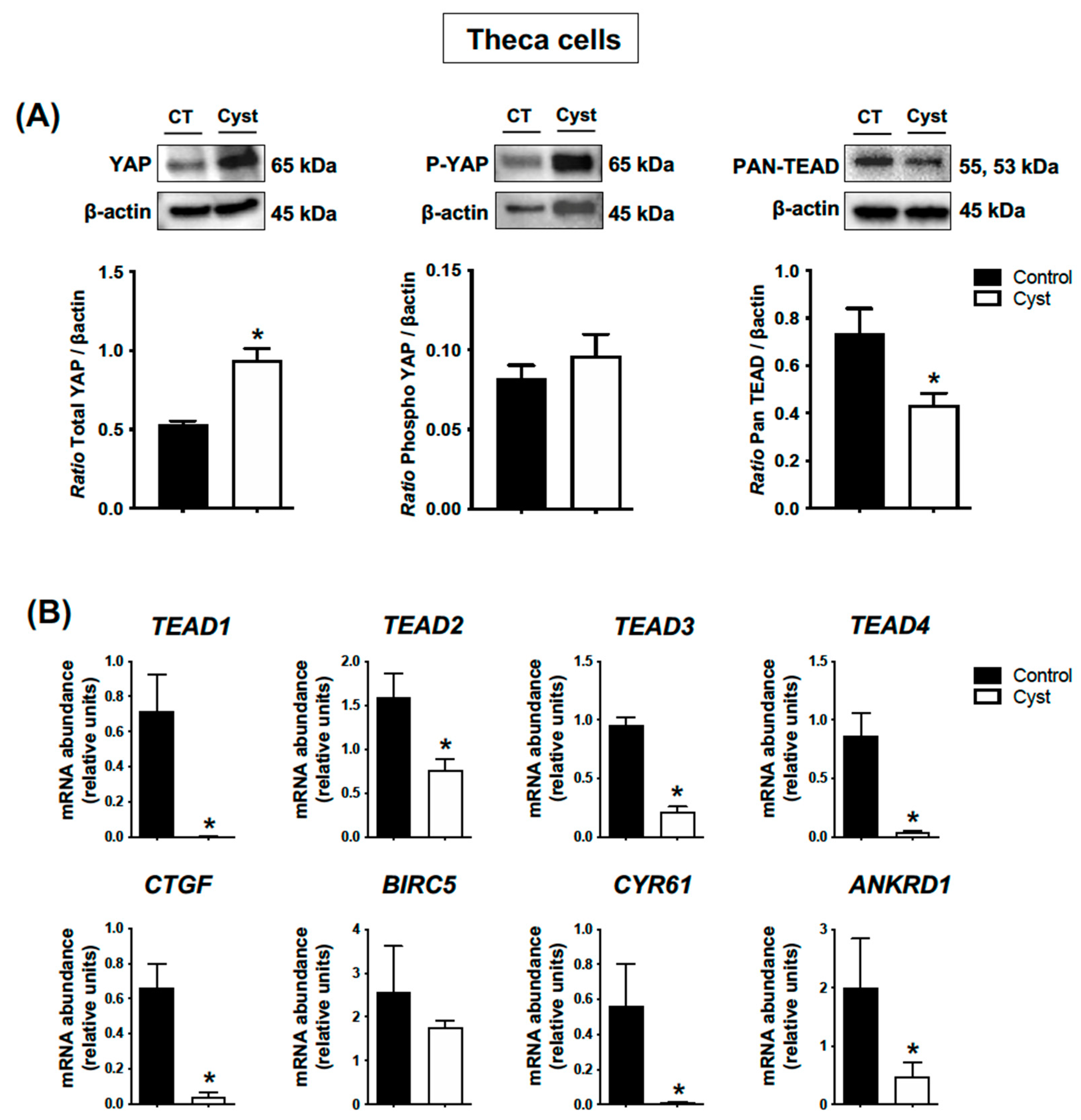
3.4. Expression Levels for YAP, TEADs and Classic YAP-TEAD Target Genes in Cystic Theca Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koeck, A.; Loker, S.; Miglior, F.; Kelton, D.F.; Jamrozik, J.; Schenkel, F.S. Genetic relationships of clinical mastitis, cystic ovaries, and lameness with milk yield and somatic cell score in first-lactation Canadian Holsteins. J. Dairy Sci. 2014, 97, 5806–5813. [Google Scholar] [CrossRef] [PubMed]
- Guarini, A.R.; Lourenco, D.A.L.; Brito, L.F.; Sargolzaei, M.; Baes, C.F.; Miglior, F.; Misztal, I.; Schenkel, F.S. Genetics and genomics of reproductive disorders in Canadian Holstein cattle. J. Dairy Sci. 2019, 102, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Silvia, W.J.; Hatler, T.B.; Nugent, A.M.; Laranja da Fonseca, L.F. Ovarian follicular cysts in dairy cows: An abnormality in folliculogenesis. Domest. Anim. Endocrinol. 2002, 23, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, L.; Signorini, M.L.; Bertoli, J.; Bartolomé, J.A.; Gareis, N.C.; Díaz, P.U.; Bó, G.A.; Ortega, H.H. Epidemiological description of cystic ovarian disease in argentine dairy herds: Risk factors and effects on the reproductive performance of lactating cows. Reprod. Domest. Anim.=Zuchthyg. 2014, 49, 1028–1033. [Google Scholar] [CrossRef]
- Vanholder, T.; Opsomer, G.; Kruif, A.D. Aetiology and pathogenesis of cystic ovarian follicles in dairy cattle: A review. Reprod. Nutr. Dev. 2006, 46, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, J.A.; Thatcher, W.W.; Melendez, P.; Risco, C.A.; Archbald, L.F. Strategies for the diagnosis and treatment of ovarian cysts in dairy cattle. J. Am. Vet. Med. Assoc. 2005, 227, 1409–1414. [Google Scholar] [CrossRef]
- Gareis, N.C.; Angeli, E.; Huber, E.; Salvetti, N.R.; Rodríguez, F.M.; Ortega, H.H.; Hein, G.J.; Rey, F. Alterations in key metabolic sensors involved in bovine cystic ovarian disease. Theriogenology 2018, 120, 138–146. [Google Scholar] [CrossRef]
- Yu, F.-X.; Guan, K.-L. The Hippo pathway: Regulators and regulations. Genes Dev. 2013, 27, 355–371. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Heath, E.; Tahri, D.; Andermarcher, E.; Schofield, P.; Fleming, S.; Boulter, C.A. Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev. Biol. 2008, 8, 18. [Google Scholar] [CrossRef]
- Lai, D.; Ho, K.C.; Hao, Y.; Yang, X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011, 71, 2728–2738. [Google Scholar] [CrossRef]
- Mauviel, A.; Nallet-Staub, F.; Varelas, X. Integrating developmental signals: A Hippo in the (path)way. Oncogene 2012, 31, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.R.; Liszewska, E.; Jaworski, J. Matricellular proteins of the Cyr61/CTGF/NOV (CCN) family and the nervous system. Front. Cell. Neurosci. 2015, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Plewes, M.R.; Hou, X.; Zhang, P.; Liang, A.; Hua, G.; Wood, J.R.; Cupp, A.S.; Lv, X.; Wang, C.; Davis, J.S. Yes-associated protein 1 is required for proliferation and function of bovine granulosa cells in vitro. Biol. Reprod. 2019, 101, 1001–1017. [Google Scholar] [CrossRef]
- Dos Santos, E.C.; Lalonde-Larue, A.; Antoniazzi, A.Q.; Barreta, M.H.; Price, C.A.; Dias Gonçalves, P.B.; Portela, V.M.; Zamberlam, G. YAP signaling in preovulatory granulosa cells is critical for the functioning of the EGF network during ovulation. Mol. Cell. Endocrinol. 2022, 541, 111524. [Google Scholar] [CrossRef]
- Koch, J.; Portela, V.M.; Dos Santos, E.C.; Missio, D.; de Andrade, L.G.; da Silva, Z.; Gasperin, B.G.; Antoniazzi, A.Q.; Gonçalves, P.B.D.; Zamberlam, G. The Hippo pathway effectors YAP and TAZ interact with EGF-like signaling to regulate expansion-related events in bovine cumulus cells in vitro. J. Assist. Reprod. Genet. 2022, 39, 481–492. [Google Scholar] [CrossRef]
- Ji, S.Y.; Liu, X.M.; Li, B.T.; Zhang, Y.L.; Liu, H.B.; Zhang, Y.C.; Chen, Z.J.; Liu, J.; Fan, H.Y. The polycystic ovary syndrome-associated gene Yap1 is regulated by gonadotropins and sex steroid hormones in hyperandrogenism-induced oligo-ovulation in mouse. Mol. Hum. Reprod. 2017, 23, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, H.; Zhao, X.; Zhang, B.; Cui, L.; Shi, Y.; Li, G.; Wang, P.; Chen, Z.J. Identification of YAP1 as a novel susceptibility gene for polycystic ovary syndrome. J. Med. Genet. 2012, 49, 254–257. [Google Scholar] [CrossRef]
- Zhang, Y.; Ho, K.; Keaton, J.M.; Hartzel, D.N.; Day, F.; Justice, A.E.; Josyula, N.S.; Pendergrass, S.A.; Actkins, K.; Davis, L.K.; et al. A genome-wide association study of polycystic ovary syndrome identified from electronic health records. Am. J. Obstet. Gynecol. 2020, 223, 559.e1–559.e21. [Google Scholar] [CrossRef]
- Braw-Tal, R.; Pen, S.; Roth, Z. Ovarian cysts in high-yielding dairy cows. Theriogenology 2009, 72, 690–698. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Alfaro, N.S.; Salvetti, N.R.; Velazquez, M.M.; Stangaferro, M.L.; Rey, F.; Ortega, H.H. Steroid receptor mRNA expression in the ovarian follicles of cows with cystic ovarian disease. Res. Vet. Sci. 2012, 92, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Portela, V.M.; Zamberlam, G.; Goncalves, P.B.; de Oliveira, J.F.; Price, C.A. Role of angiotensin II in the periovulatory epidermal growth factor-like cascade in bovine granulosa cells in vitro. Biol. Reprod. 2011, 85, 1167–1174. [Google Scholar] [CrossRef][Green Version]
- Orisaka, M.; Mizutani, T.; Tajima, K.; Orisaka, S.; Shukunami, K.; Miyamoto, K.; Kotsuji, F. Effects of ovarian theca cells on granulosa cell differentiation during gonadotropin-independent follicular growth in cattle. Mol. Reprod. Dev. 2006, 73, 737–744. [Google Scholar] [CrossRef]
- Bishop, C.V.; Reiter, T.E.; Erikson, D.W.; Hanna, C.B.; Daughtry, B.L.; Chavez, S.L.; Hennebold, J.D.; Stouffer, R.L. Chronically elevated androgen and/or consumption of a Western-style diet impairs oocyte quality and granulosa cell function in the nonhuman primate periovulatory follicle. J. Assist. Reprod. Genet. 2019, 36, 1497–1511. [Google Scholar] [CrossRef]
- Brito, C.; Palmer, C.W. Cystic Ovarian Disease in Cattle. Can. Vet.—Large Anim. Vet. Rounds 2004, 4, 1–6. [Google Scholar]
- Larsen, R.E. Veterinary obstetrics and genital diseases (Theriogenology) by S.J. Roberts (ed.); 981 pages, 1986, 3rd edition. Published by the author, Woodstock, VT 05091. Distributed by David and Charles Inc., North Pomfret, VT 05053. Theriogenology 1986, 26, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Garverick, H.A. Ovarian Follicular Cysts in Dairy Cows1. J. Dairy Sci. 1997, 80, 995–1004. [Google Scholar] [CrossRef]
- Millward, S.; Mueller, K.; Smith, R.; Higgins, H.M. A Post-mortem Survey of Bovine Female Reproductive Tracts in the UK. Front. Vet. Sci. 2019, 6, 451. [Google Scholar] [CrossRef]
- Gareis, N.C.; Huber, E.; Hein, G.J.; Rodríguez, F.M.; Salvetti, N.R.; Angeli, E.; Ortega, H.H.; Rey, F. Impaired insulin signaling pathways affect ovarian steroidogenesis in cows with COD. Anim. Reprod. Sci. 2018, 192, 298–312. [Google Scholar] [CrossRef]
- Shimizu, T.; Ishizawa, S.; Magata, F.; Kobayashi, M.; Fricke, P.M.; Miyamoto, A. Involvement of lipopolysaccharide in ovarian cystic follicles in dairy cow: Expressions of LPS receptors and steroidogenesis-related genes in follicular cells of cystic follicles. Anim. Reprod. Sci. 2018, 195, 89–95. [Google Scholar] [CrossRef]
- Kawate, N.; Inaba, T.; Mori, J. A quantitative comparison in the bovine of steroids and gonadotropin receptors in normally developing follicles and in follicular and luteinized cysts. Anim. Reprod. Sci. 1990, 23, 273–281. [Google Scholar] [CrossRef]
- Marelli, B.E.; Diaz, P.U.; Salvetti, N.R.; Rey, F.; Ortega, H.H. mRNA expression pattern of gonadotropin receptors in bovine follicular cysts. Reprod. Biol. 2014, 14, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Yoshimura, Y. Localization of apoptotic cells in the cystic ovarian follicles of cows: A DNA-end labeling histochemical study. Theriogenology 2000, 53, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.H.; Marelli, B.E.; Rey, F.; Amweg, A.N.; Díaz, P.U.; Stangaferro, M.L.; Salvetti, N.R. Molecular aspects of bovine cystic ovarian disease pathogenesis. Reproduction 2015, 149, R251–R264. [Google Scholar] [CrossRef]
- Nagashima, T.; Kim, J.; Li, Q.; Lydon, J.P.; DeMayo, F.J.; Lyons, K.M.; Matzuk, M.M. Connective Tissue Growth Factor Is Required for Normal Follicle Development and Ovulation. Mol. Endocrinol. 2011, 25, 1740–1759. [Google Scholar] [CrossRef]
- Chang, H.M.; Pan, H.H.; Cheng, J.C.; Zhu, Y.M.; Leung, P.C.K. Growth differentiation factor 8 suppresses cell proliferation by up-regulating CTGF expression in human granulosa cells. Mol. Cell. Endocrinol. 2016, 422, 9–17. [Google Scholar] [CrossRef]
- Johnson, A.L.; Langer, J.S.; Bridgham, J.T. Survivin as a Cell Cycle-Related and Antiapoptotic Protein in Granulosa Cells. Endocrinology 2002, 143, 3405–3413. [Google Scholar] [CrossRef]
- Zhang, N.; Ye, F.; Zhu, W.; Hu, D.; Xiao, C.; Nan, J.; Su, S.; Wang, Y.; Liu, M.; Gao, K.; et al. Cardiac ankyrin repeat protein attenuates cardiomyocyte apoptosis by upregulation of Bcl-2 expression. Biochim. et Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 3040–3049. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, H.; Shi, Y.; Cao, Y.; Yang, D.; Li, Z.; Zhang, B.; Liang, X.; Li, T.; Chen, J.; et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat. Genet. 2012, 44, 1020–1025. [Google Scholar] [CrossRef]
- McAllister, J.M.; Legro, R.S.; Modi, B.P.; Strauss, J.F. Functional genomics of PCOS: From GWAS to molecular mechanisms. Trends Endocrinol. Metab. 2015, 26, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Xie, J.K.; Cui, J.Q.; Wei, D.; Yin, B.L.; Zhang, Y.N.; Chen, Y.H.; Han, X.; Wang, Q.; Zhang, C.L. Promoter methylation of yes-associated protein (YAP1) gene in polycystic ovary syndrome. Medicine 2017, 96, e5768. [Google Scholar] [CrossRef] [PubMed]
- Sirois, J.; Fortune, J.E. Ovarian Follicular Dynamics during the Estrous Cycle in Heifers Monitored by Real-Time UItrasonograph1. Biol. Reprod. 1988, 39, 308–317. [Google Scholar] [CrossRef]
- Adams, G.P.; Matteri, R.L.; Kastelic, J.P.; Ko, J.C.; Ginther, O.J. Association between surges of follicle-stimulating hormone and the emergence of follicular waves in heifers. J. Reprod. Fertil. 1992, 94, 177–188. [Google Scholar] [CrossRef]
- Campbell, B.K.; Souza, C.; Gong, J.; Webb, R.; Kendall, N.; Marsters, P.; Robinson, G.; Mitchell, A.; Telfer, E.E.; Baird, D.T. Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reprod. Suppl. 2003, 61, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. A new model for ovarian follicular development during the human menstrual cycle. Fertil. Steril. 2003, 80, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Malhi, P.S.; Adams, G.P.; Singh, J. Bovine Model for the Study of Reproductive Aging in Women: Follicular, Luteal, and Endocrine Characteristics1. Biol. Reprod. 2005, 73, 45–53. [Google Scholar] [CrossRef] [PubMed]

| Name of Antibody | Manufacturer (cat. no.) | Type | Dilution WB | Dilution IHC |
|---|---|---|---|---|
| ß-actin (C4) | Santa Cruz (sc-47778 HRP) | CkM | 1:10,000 | |
| YAP (D8H1X) | Cell signaling (14074) | RbM | 1:1000 | 1:250 |
| Phospho-YAP (Ser127) (D9W2I) | Cell signaling (13008) | RbM | 1:1000 | 1:250 |
| Pan-TEAD (D3F7L) | Cell signaling (13295) | RbM | 1:1000 | |
| Anti-Rabbit IgG-HRP Conjugate | Promega (W401B) | Rb | 1:1000 |
| Gene | Sequence 5′→3′ | Accession Number |
|---|---|---|
| ANKRD1 | F: ATCAGTGCGCGGGATAAGTT | NM_001034378.2 |
| R: GGGAGTATCTCCTTCCCGGT | ||
| AR | F: CCTGGTTTTCAATGAGTACCGCATG | [22] |
| R: TTGATTTTTCAGCCCATCCACTGGA | ||
| BIRC5 | F: CTGAGAACGAGCCCGACTTG | NM_001001855.3 |
| R: ATGTTCTTCTATAGGGTCGTCATCT | ||
| CTGF | F: AGCTGAGCGAGTTGTGTACC | [15] |
| R: TCCGAAAATGTAGGGGGCAC | ||
| CYP19A1 | F: CTGAAGCAACAGGAGTCCTAAATGTACA | [23] |
| R: AATGAGGGGCCCAATTCCCAGA | ||
| CYR61 | F: GGCTCCCCGTTTTGGAATG | NM_001034340.2 |
| R: TCATTGGTAACGCGTGTGGA | ||
| ESR1 | F: AGGGAAGCTCCTATTTGCTCC | [22] |
| R: CGGTGGATGTGGTCCTTCTCT | ||
| ESR2 | F: CTTCGTGGAGCTCAGCCTGT | NM_174051 |
| R: GAGATATTCTTTGTGTTGGAGTTT | ||
| FSHR | F: AGCCCCTTGTCACAACTCTATGTC | NM_174061.1 |
| R: GTTCCTCACCGTGAGGTAGATGT | ||
| GAPDH | F: GATTGTCAGCAATGCCTCCT | NM_001034034.2 |
| R: GGTCATAAGTCCCTCCACGA | ||
| H2AFZ | F: GAGGAGCTGAACAAGCTGTTG | [23] |
| R: TTGTGGTGGCTCTCAGTCTTC | ||
| LHCGR | F: TGGCTGGGATTATGACTATGGTT | [24] |
| R: ATTTCCCGTGATGGCTAGGATA | ||
| PGRb | F: TGCGAGACCCCCAGAGAAGGA | XM_583951 |
| R: GCGCCAGCAGGGTGTCCAG | ||
| RLP19 | F: CCGGCTGCTTAGACGATACC | NM_001040516.1 |
| R: CCGCTTGTTTTTGAACACGTT | ||
| STAR | F: CCCAGCAGAAGGGTGTCATC | [24] |
| R: TGCGAGAGGACCTGGTTGAT | ||
| TEAD1 | F: CACAAGACGTCAAGCCCTTTG | [15] |
| R: CCAGGCGAAGTTTGGTTGTG | ||
| TEAD2 | F: CTGAATGTGGACCAGGTTTCCAA | [15] |
| R: GACCAAAACTGGAAAAGCTCCG | ||
| TEAD3 | F: CGAATATTCCGCCTTCATGGAG | [15] |
| R: TATCTGACGCACATCCACTGC | ||
| TEAD4 | F: ATGTCGTCCGCCCAGATCAT | [15] |
| R: AGGTTTCACATCATGGGACGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dos Santos, E.C.; Boyer, A.; St-Jean, G.; Jakuc, N.; Gévry, N.; Price, C.A.; Zamberlam, G. Is the Hippo Pathway Effector Yes-Associated Protein a Potential Key Player of Dairy Cattle Cystic Ovarian Disease Pathogenesis? Animals 2023, 13, 2851. https://doi.org/10.3390/ani13182851
Dos Santos EC, Boyer A, St-Jean G, Jakuc N, Gévry N, Price CA, Zamberlam G. Is the Hippo Pathway Effector Yes-Associated Protein a Potential Key Player of Dairy Cattle Cystic Ovarian Disease Pathogenesis? Animals. 2023; 13(18):2851. https://doi.org/10.3390/ani13182851
Chicago/Turabian StyleDos Santos, Esdras Corrêa, Alexandre Boyer, Guillaume St-Jean, Natalia Jakuc, Nicolas Gévry, Christopher A. Price, and Gustavo Zamberlam. 2023. "Is the Hippo Pathway Effector Yes-Associated Protein a Potential Key Player of Dairy Cattle Cystic Ovarian Disease Pathogenesis?" Animals 13, no. 18: 2851. https://doi.org/10.3390/ani13182851
APA StyleDos Santos, E. C., Boyer, A., St-Jean, G., Jakuc, N., Gévry, N., Price, C. A., & Zamberlam, G. (2023). Is the Hippo Pathway Effector Yes-Associated Protein a Potential Key Player of Dairy Cattle Cystic Ovarian Disease Pathogenesis? Animals, 13(18), 2851. https://doi.org/10.3390/ani13182851







