Simple Summary
Cystic ovarian disease is an important ovarian disorder that leads to anovulatory infertility in dairy cows. In the present study, we used spontaneous ovarian follicular cysts to show the first evidence that the deregulation of Hippo pathway effector yes-associated protein expression and/or activity can be a potential key to better understand cystic ovarian disease pathogenesis. This finding also allows us to point towards Hippo pathway as a potential therapeutic target for the treatment of this major ovarian disorder in cattle.
Abstract
Cystic ovarian disease (COD) in dairy cattle is characterized by preovulatory follicles that become cysts, fail to ovulate and persist in the ovary; consequently, interfering with normal ovarian cyclicity. The intraovarian key players that orchestrate the alterations occurring in the preovulatory follicle and that culminate with cyst formation and persistence, however, remain uncertain. Interestingly, the Hippo pathway effector yes-associated protein (YAP) has been described in humans and mice as a key player of anovulatory cystic disorders. To start elucidating if YAP deregulation in ovarian follicle cells can be also involved in the pathogenesis of COD, we have generated a series of novel results using spontaneously occurring cystic follicles in cattle. We found that mRNA and protein levels of YAP are significantly higher in granulosa (GCs) and theca cells (TCs) isolated from cystic follicles (follicular structures of at least 20 mm in diameter) in comparison to respective cell types isolated from non-cystic large follicles (≥12 mm). In addition, immunohistochemistry and Western blot analyses used to determine YAP phosphorylation pattern suggest that YAP transcriptional activity is augmented is cystic GCs. These results were confirmed by a significant increase in the mRNA levels encoding for the classic YAP-TEAD transcriptional target genes CTGF, BIRC5 and ANKRD1 in GCs from follicle cysts in comparison to non-cystic large follicles. Taken together, these results provide considerable insight of a completely novel signaling pathway that seems to play an important role in ovarian cystic disease pathogenesis in dairy cattle.
Keywords:
ovary; cow; Hippo; CTGF; CYR61; ANKRD1; TEAD; anovulation; cyst; COD; granulosa cells; theca cells 1. Introduction
The fertility of high-yielding dairy cows has been declining for decades due to many factors, and notably ovarian malfunctions are at the top of the list [1,2]. Among ovarian disorders observed in dairy cows, cystic ovarian disease (COD) is very common. COD has been defined by the presence (in one or both ovaries) of one or more follicular cysts larger than 20 mm in diameter and that persist for up to 10 days without luteal tissue [3]. This disorder directly causes reproductive failure and represents severe economic loss to the dairy industry because it increases both the days open in the post-partum period and replacement rates due to infertility [3,4].
Ovarian cysts normally develop from preovulatory follicles that have failed to ovulate, they persist in the ovary and thus interfere with normal ovarian cyclicity causing anovulatory infertility [5]. Although it has been accepted that the development of ovarian cysts is associated with an endocrine imbalance in the hypothalamic–pituitary–gonadal axis [6,7], the key intraovarian components involved in the mechanism of cyst formation and persistence remain uncertain. The Hippo pathway effector yes-associated protein (YAP) has emerged as a potential candidate.
The Hippo intracellular signaling pathway is evolutionarily highly conserved with well-known roles in cell differentiation, proliferation and apoptosis in several tissues, particularly during embryogenesis [8,9]. The core Hippo pathway consists of a kinase cascade that regulates the activity of the transcriptional activators YAP and transcriptional co-activator with PDZ-binding motif (TAZ). When phosphorylated, YAP/TAZ are sequestered in the cytoplasm or degraded, and are unable to alter gene transcription. Conversely, unphosphorylated YAP/TAZ accumulate in the nucleus and form complexes with distinct transcription factors, particularly those of the TEAD family, resulting in the regulation of the transcriptional activity of target genes in a cell type- and context-specific manner [9,10,11,12,13].
A number of studies indicate the importance of Hippo effectors to ovarian physiology in adult animals, including cattle [14,15,16]. Interestingly, it has been shown that overexpression of YAP negatively impacts LH action in mouse granulosa cells (GCs) [17] and that YAP is considered a susceptibility gene for polycystic ovarian syndrome in women [18,19]. It has been recently demonstrated that YAP transcriptional activity is necessary for activation of the LH-induced preovulatory cascade in bovine GCs, and that inhibiting YAP activity blocks ovulation in cattle in vivo [15]. However, it is unknown if YAP activity deregulation in the preovulatory follicle contributes to ovarian cyst formation in this species. To address this, the objectives of the present study were to measure activation of the main Hippo signaling pathway effector and the expression of classic YAP-TEAD target genes in spontaneously occurring cystic follicles.
2. Material and Methods
The reagents used in the present study were obtained from Thermo Fisher Scientific (Saint-Laurent, QC, Canada) except where otherwise stated.
2.1. Cystic Follicle Collection
Ovarian follicle cysts were selected based on macroscopic evaluation, and were identified as follicles larger than 20 mm and with a follicle wall of up to 3 mm thickness [20]; follicles with greater than 3 mm wall thickness were likely luteal cysts and were discarded. Histology was performed on a subset of the selected ovaries which were examined for the presence of classic alterations that are normally observed in cystic follicles, including partial loss of GCs with pyknotic cells, and interruption in the basement membrane with invasion of GCs into the hypertrophied theca cell layer [3,5,20]. Cysts presenting only a fibrous tissue capsule and liquid were not included in the present study. Non-cystic follicles were defined as those between 12 and 17 mm diameter.
Bovine ovaries were collected at different days from random adult cows at a local abattoir and were transported to the laboratory in PBS at 35 °C containing penicillin (100 IU/mL), streptomycin (100 μg/mL) and fungizone (1 μg/mL). For histology, ovaries were fixed in formalin (10% buffered), paraffin-embedded, stained in H&E and evaluated histologically. An approximate volume of 400 µL of follicular fluid was collected from each follicle and stored at −80 °C for hormone assay. GCs were then collected from the cystic and non-cystic follicles by aspiration and washed twice by centrifugation at 219× g for 10 min each. After assessing GC viability (with 0.4% Trypan blue stain) and counting the number of viable cells, 1 × 106 viable cells were placed in RLT buffer for RNA extraction or in M-PER® mammalian protein extraction reagent for protein extraction, and stored at −80 °C. Following GCs removal, some follicles were then used for TCs isolation. Briefly, the TCs layer was dissected from the surrounding stroma and washed several times with PBS plus heparin-enriched solution to remove all remaining attached GCs. Then, samples were aliquoted (50 mg each) for total RNA and protein extraction as described above.
2.2. Immunohistochemistry
For immunohistochemistry (IHC) analyses, bovine ovaries and sample selection for experimental or control groups were performed as described above. At the laboratory, entire ovaries (at least four per group) were fixed in 10% formaldehyde solution for 24 h, rinsed and dehydrated in alcohol until embedded in paraffin. Serial sections were prepared (at a thickness of 3 µm) followed by deparaffinization, rehydration, sodium citrate heat-mediated antigen retrieval, peroxidase block and protein blocking (10% goat for 30 min), and then slides were probed with primary antibody against total and phosphorylated forms of YAP or against Pan-TEAD (Table 1) overnight at 4 °C. Protein detection was then performed with the Vectastain Elite ABC HRP Kit (VECTPK6101; Vector Laboratories Inc., Burlingame, CA, United States) and stained with DAB substrate kit (VECTSK4100; Vector Laboratories Inc., Burlingame, CA, United States). Slides were then counterstained with hematoxylin and dehydrated with graded alcohols prior to mounting. Negative controls consisted of slides for which the primary antibody was omitted. Photomicrographs were taken using a Carl Zeiss Axio Imager M1 microscope (Carl Zeiss Canada Ltd., Toronto, ON, Canada) at ×1000 magnification and using the Zen 2012 blue edition software (Carl Zeiss, Oberkochen, Germany).

Table 1.
List of antibodies used for IHC and WB.
2.3. Total RNA Extraction and Real-Time qPCR
Total RNA from GCs and TCs from control and cystic follicles were extracted using the Total RNA Mini Kit (Blood/Cultured Cell; Geneaid Biotech Ltd., New Taipei City, Taiwan) according to the manufacturer’s protocol. Reverse transcription was performed using 50 ng of total RNA and the SuperScript Vilo cDNA synthesis kit. Real-time qPCR was performed using Advanced supergreen qPCR mastermix (WISENT Inc., Saint-Bruno, QC, Canada) and a CFX96 Touch™ instrument (Bio-Rad, Hercules, CA, USA). Each PCR reaction consisted of 7.5 μL of SsoAdvanced SYBR Green PCR Master Mix, 2.3 μL of water, 4 μL of cDNA sample and 0.6 μL (10 pmol) of gene-specific primers (Table 2). Cycling conditions were 3 min at 95 °C followed by 40 cycles of 15 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C. In each run, melting curve analysis was used to verify that a single product was amplified. Each reaction was performed in duplicate and the average threshold cycle (Ct) value was used to calculate relative mRNA abundance of target genes relative to the geometric mean of three housekeeping genes (H2AFZ, GAPDH and RPL19) with the 2−∆∆Ct method and correction for amplification efficiency [21]. Primers not published previously were designed based on sequences from GenBank, using Primer-BLAST platform and their respective amplicons were sequenced to confirm specificity.

Table 2.
Sequences of primers used in the expression analysis of target genes.
2.4. Western Blotting
Total protein from GCs and TCs were extracted using M-PER® mammalian protein extraction reagent according to the manufacturer’s instructions and protein levels were quantified using the Pierce™ BCA Protein Assay Kit. Halt™ Protease and Phosphatase Inhibitor Cocktails were added to the samples final solution to avoid protein degradation. Samples (20–40 μg) were resolved on 12% sodium dodecyl sulfate-polyacrylamide gels and transferred to Hybond-P PVDF membrane (GE Amersham, Amersham, UK). Membranes were then probed at 4 °C overnight in 5% BSA in TTBS with different primary antibodies (details and dilutions for each antibody are indicated in Table 1). After washing three times with TTBS, membranes were incubated for 1 h at room temperature with anti-rabbit HRP-conjugated IgG diluted in 5% non-fat dry milk in TTBS. Protein bands were visualized by chemiluminescence (ECL; Millipore, Burlington, MA, USA) and quantified using a ChemiDoc MP detection system (Bio-Rad) and Image Lab™ software version 6.0.1.
2.5. Hormone Assay
Follicular fluid concentrations of estradiol (E2), progesterone (P4) and testosterone (T) were measured by liquid chromatography–tandem triple quadrupole mass spectrometry (LC-MS/MS) in the Endocrine Technologies Core (ETC) at the Oregon National Primate Research Center (ONPRC) as previously described [25]. Briefly, 10 µL of follicular fluid was combined with deuterium-labeled standards for E2, P4 and T and subjected to supported liquid extraction (Biotage). Samples were then analyzed for hormone concentrations on a Shimadzu Nexera-LCMS-8050 (Shimadzu Scientific Instruments, Durham, NC, USA) LC-MS/MS instrument. Intra-assay coefficients of variation (CV) for E2, P4 and T were 2.8%, 6.0% and 1.4%, respectively, and inter-assay CVs for E2, P4 and T were 3.5%, 6.2% and 1.6%, respectively.
2.6. Statistical Analysis
All analyses were performed using four to ten independent follicle samples per group (cystic or non-cystic), each isolated from ovaries collected at different days. The statistical analyses for all assessments were performed using GraphPad Prism software version 9.3.1 (GraphPad Software Inc., La Jolla, CA, USA). Data from mRNA abundance or target protein levels that were not normally distributed (Shapiro–Wilk test) were transformed to natural logarithms. Two-tailed t-tests were used to compare the two experimental groups. All data are presented as means ± SEM and variables that were considered statistically significant at p < 0.05 are represented with an asterisk symbol (*).
3. Results
3.1. Molecular and Hormonal Validation of COD Follicle Samples
We first confirmed if the spontaneously occurring cystic follicles collected from the abattoir presented the same expression pattern expected for classic markers for COD such as progesterone receptor beta (PGRb), androgen receptor (AR), steroidogenic acute regulatory protein (STAR), luteinizing hormone (LHCGR), follicle stimulating hormone receptor (FSHR) and estrogen receptors type 1 (ESR1) and 2 (ESR2). Abundance of mRNA encoding PGRb, AR and STAR was significantly increased in GCs from cysts compared to control GCs, and mRNA levels for both LHCGR and FSHR were significantly lower in cyst CGs in comparison to controls (p < 0.05; Figure 1A). Although a clear tendency was observed, neither ESR1 nor CYP19A1 mRNA abundance was statistically altered in cysts (p > 0.05; Figure 1A). In terms of theca cells gene expression profile, while STAR mRNA abundance was significantly increased (p < 0.05; Figure 1B), PGRb, AR and ESR1 mRNA levels were significantly lower in cystic compared to control follicles (p < 0.05; Figure 1B).
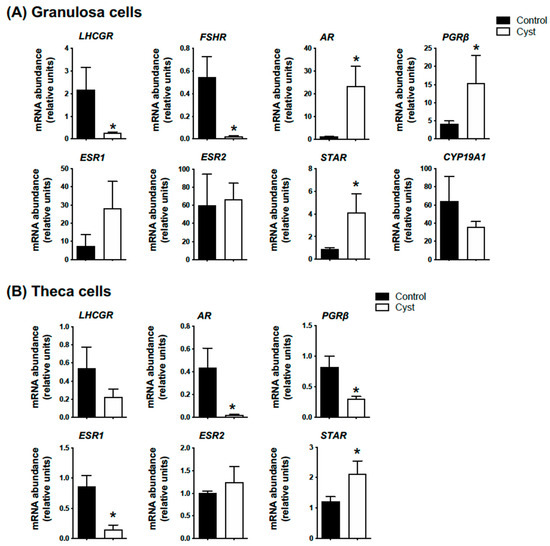
Figure 1.
Molecular validation of spontaneous cystic follicle samples. Relative messenger RNA abundance was measured by real-time qPCR and normalized to the geometric mean of three housekeeping genes H2AFZ, GAPDH and RLP19. Granulosa (A) and theca cells (B) were isolated from ovarian follicular cysts (structures of at least 20 mm in diameter; Cyst group) and from non-cystic large follicles (≥12 mm; Control group). Data represent the mean ± SEM from granulosa (n = 10) and theca cells (n = 4) independent follicle samples per group. An asterisk (*) indicates significant difference between groups (p < 0.05).
Progesterone concentrations in follicular fluid were significantly increased in cyst samples in comparison to controls (p < 0.05; Figure 2), whereas E2 and T concentrations were not statistically different between control and cyst groups (p > 0.05; Figure 2). Together, these results allowed us to confirm the COD status of the experimental samples.
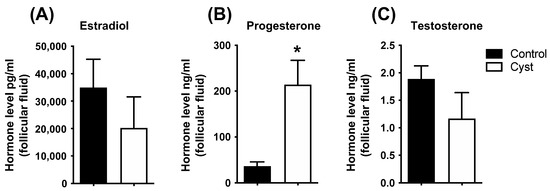
Figure 2.
Steroids concentration in follicular fluid of control and cyst groups. Follicular fluid steroid concentrations for estradiol (A), progesterone (B) and testosterone (C) were measured by liquid chromatography–tandem triple quadrupole mass spectrometry. Data represent the mean ± SEM of independent follicle samples (n = 10) per group. An asterisk (*) indicates significant difference between groups (p < 0.05).
3.2. YAP and Phospho-YAP Expression Pattern in Bovine Cystic Follicles
We next determined the cellular and subcellular localization of total and phosphorylated YAP (phospho-YAP at serine 127: pYAP; Ser 127) proteins in both non-cystic and cystic follicles (Figure 3). Immunostaining for total YAP was observed in the nuclei and cytoplasm of GCs and TCs in both cystic and non-cystic follicles. A more intense staining detected in nuclei and cytoplasm of both cell layers from the cyst group (in comparison to respective cell layers and subcellular compartments in control group) suggested a higher expression of total YAP protein in cystic follicles. Phospho-YAP staining was also observed in the cytoplasm and nuclei of granulosa cell layers in both cyst and control follicles.
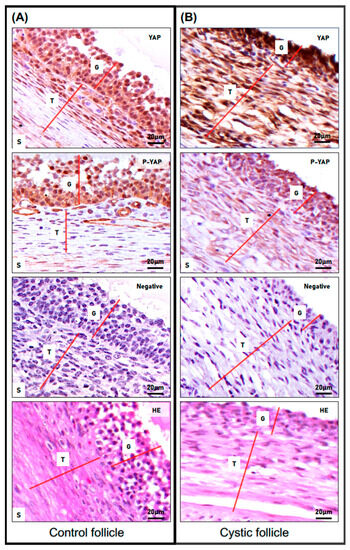
Figure 3.
Expression pattern of total and phosphorylated YAP proteins in spontaneous cystic follicles in cows. Immunohistochemistry (IHC) analysis was used to compare the cellular and subcellular localization of total and phospho-YAP (P-YAP; Ser 127) proteins in control non-cystic large follicles (A) and in spontaneous cystic follicles (B). The red lines differentiate the distinct cell layers observed in the images (G: granulosa cells; T: theca cells; S: stromal cells). Representative IHC images of staining for total and phospho-YAP (objective 63×) and their antibody control (NEG) showing negative staining of total YAP and phospho-YAP (objective 63×). Structure and cellular architecture of both groups can be observed in the H&E representative images.
Interestingly, GCs from cystic follicles seemed to present not only a lower number of nuclei positive for phospho-YAP, but also the intensity of such a signal in the nucleus and in the cytoplasm of these cells was weaker when compared to respective cells from the control group. Most interestingly, while the theca cell layer from cyst follicles presented some extent of positive signal for phospho-YAP (predominantly in their cytoplasm), a poor to absent signal for phospho-YAP was detected in theca cells from non-cystic control follicles.
3.3. Expression Levels for YAP, TEADs and Classic YAP-TEAD Target Genes in Cystic Granulosa Cells
To complement our IHC analyses and to better assess if YAP total protein levels were indeed augmented in GCs isolated from cyst group samples, we then employed Western blot analyses. The results demonstrated that total YAP protein levels were significantly higher in GCs from cystic follicles in comparison to GCs from control follicles, but also that phospho-YAP abundance was decreased (p < 0.05; Figure 4A) whereas total TEAD protein levels (Pan-TEAD) were not different.
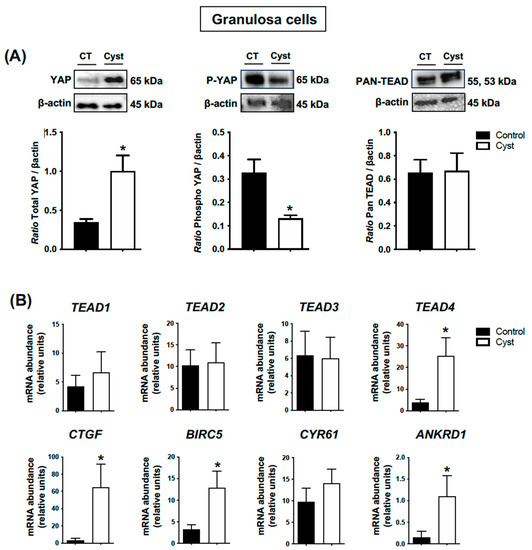
Figure 4.
Expression levels for YAP (total and phospho forms), TEADs and classic YAP-TEAD target genes in cystic granulosa cells. Granulosa cells were isolated from ovarian follicular cysts and from non-cystic large control follicles. Total and phospho-YAP (pYAP; Ser 127) and Pan-TEAD protein levels (A) were measured by Western blot (WB) and normalized to β-actin (n = 5 for each group). Relative messenger RNA abundance (B) was measured by real-time qPCR and normalized to the geometric mean of three housekeeping genes H2AFZ, GAPDH and RLP19 (n = 10 for each group). Data represent the group mean ± SEM. An asterisk (*) indicates difference between groups (p < 0.05).
We then measured the abundance of mRNA encoding for TEAD 1–4 and the classic YAP-TEAD target genes connective tissue growth factor (CTGF), baculoviral IAP repeat-containing protein 5 (BIRC5), cysteine-rich angiogenic inducer 61 (CYR61) and ankyrin repeat domain 1 (ANKRD1) as a measure of Hippo pathway activity. The results showed that the mRNA abundance for TEAD4 was significantly increased in the cyst group (p < 0.05; Figure 4B) while the other TEAD family members (TEAD1, TEAD 2 and TEAD3) were not statistically different between the control and cyst group (p > 0.05; Figure 4B), and that CTGF, BIRC5 and ANKRD1 mRNA abundance was significantly higher in cyst group in comparison to controls (p < 0.05; Figure 4B). Together, these findings showed that YAP total expression is augmented in cystic GCs as phospho-YAP protein levels decrease and, consequently, its TEAD-related transcriptional activity increases.
3.4. Expression Levels for YAP, TEADs and Classic YAP-TEAD Target Genes in Cystic Theca Cells
In contrast to the results suggested by the IHC analyses, the results obtained with Western blot assessment did not confirm a significant increase in phospho-YAP protein levels in theca cells from cystic follicles when compared to controls (p > 0.05; Figure 5A). On the other hand, total YAP protein levels were significantly increased in cystic follicles (p < 0.05; Figure 5A). Pan-TEAD immunoreactive bands indicated lower TEAD protein expression levels in theca cells from cystic follicles compared with control follicles (p < 0.05; Figure 5A), and correspondingly the abundance of mRNA encoding TEAD1, TEAD2, TEAD3 and TEAD4 were all significantly lower in TCs from cysts in comparison with TCs from control follicles (p < 0.05; Figure 5B). The abundance of mRNA encoding classic YAP-TEAD target genes CTGF, CYR61 and ANKRD1 were also significantly lower in TCs from cysts in comparison to TCs from control follicles (p < 0.05; Figure 5B). Together, these results indicated that the YAP-TEAD-related transcriptional activity in TCs from cysts differs to what was observed in granulosa cells.
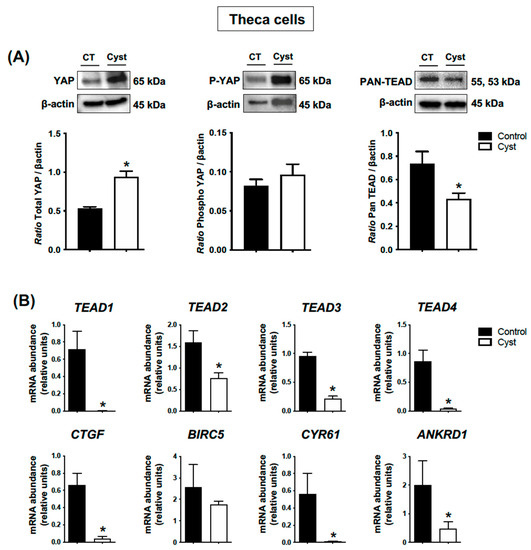
Figure 5.
Expression levels for YAP (total and phospho forms), TEADs and classic YAP-TEAD target genes in cystic theca cells. Theca cells were isolated from ovarian follicular cysts and from non-cystic large control follicles. Total and phospho-YAP (pYAP; Ser 127) and Pan-TEAD protein levels (A) were measured by Western blot (WB) and normalized to β-actin (n = 4 for each group). Relative messenger RNA abundance (B) was measured by real-time qPCR and normalized to the geometric mean of three housekeeping genes H2AFZ, GAPDH and RLP19 (n = 4 for each group). Data represent the group mean ± SEM. An asterisk (*) indicates difference between groups (p < 0.05).
4. Discussion
In the past 50 years, many studies have clarified important aspects of cystic ovarian disease (COD) characterization in cattle; nevertheless, the core players that orchestrate the alterations occurring in the large antral follicle and that culminate with cyst formation and persistence remain uncertain. In the present study, we used spontaneous ovarian follicular cysts to show the first evidence that the deregulation of Hippo effector YAP expression and/or activity can be a potential key to better understand COD pathogenesis. We show herein that YAP expression and transcriptional activity is significantly increased in granulosa cells of cystic follicles in comparison to that observed in non-cystic large preovulatory follicles. Conversely, YAP-TEAD-related transcriptional activity in theca cells from cysts is highly compromised, most likely due to lower expression levels for TEADs observed in these cells.
Phenotypically, bovine ovarian cysts are normally defined as anovulatory ovarian structures with a cavity greater than 20 mm in diameter and that persist for at least 10 days in the absence of a functional corpus luteum. The main macroscopic difference between follicular cysts and luteinized follicular cysts is based on their follicle wall thickness which is less than 3 mm in follicular cysts and greater than 3 mm in luteinized follicular cysts [26,27,28]. The diagnosis of COD due to the presence of a follicular anovulatory cyst is, however, significantly more frequent in dairy farms [29]. In addition to macroscopic and histological evaluations, we also validated the follicular cyst-like status of our samples performing molecular and hormone analyses which were compatible with several previous reports. As expected for GCs [22,30,31], the mRNA abundance for the classic overexpressed COD markers PGRb, AR and STAR was significantly increased in our cyst groups in comparison to controls. In addition, a known characteristics of COD follicles is their low responsiveness to gonadotropins due to decreased gonadotropin receptors in GCs [30,31,32,33]. Indeed, mRNA levels for both LHCGR and FSHR were significantly lower in our cyst group samples in comparison to controls.
Although the experimental samples analyzed herein do not allow us to state conclusively that YAP expression and transcriptional activity has a causal effect on COD pathogenesis, the potential functions exerted by CTGF, BIRC5 and ANKRD1 (YAP-TEAD-target genes that were increased in cystic GCs herein) may represent strong evidence of its potential key role in cyst formation and persistence. In addition to the lower expression of gonadotropin receptors described previously, GCs in bovine ovarian cysts generally have low rates of proliferation and of apoptosis [34,35]. Interestingly, the conditional deletion of Ctgf in mouse GCs increases Lhcgr mRNA levels and concomitantly increases GCs apoptosis [36]. In addition, recombinant CTGF protein suppresses proliferation of the human SVOG granulosa cell line [37]. BIRC5 protein is associated with both inhibition of apoptosis and regulation of the cell cycle in chicken granulosa cells. It can interact with components of the G2/M checkpoint and preserve the integrity of the cellular mitotic-related apparatus as it may act as an anti-apoptotic protein by attenuating caspase-3 activity [38]. Finally, ANKRD1 has been shown to induce gene expression of the well-known anti-apoptotic marker BCL2 [39]. Taken together, the functions normally exerted by these altered classic YAP-TEAD genes can be directly related with the main pillars established so far for COD functional pathogenesis, which include altered patterns of differentiation, proliferation and apoptosis in cystic GCs.
The main GCs-related results showed herein are compatible with important findings in humans. First, YAP was identified as a susceptibility gene for polycystic ovarian syndrome (PCOS) in humans [18,40,41], and second it has been suggested that increased YAP mRNA and protein levels in GCs from PCOS patients accelerate the evolution of PCOS [39,42]. Both of the latter studies employed associative approaches (methylation status of YAP promoter activity and genome-wide associations with PCOS, respectively) and therefore did not test experimentally a causal effect of YAP deregulation on the formation of the ovarian cysts in women. Although we still need to perform further studies to confirm such causal effect in cattle too, the results obtained with the spontaneously occurring cystic follicles employed herein show us strong evidence that YAP-TEAD-dependent transcriptional activity can be indeed directly related to the functional and morphological changes normally observed in bovine follicular cysts. Therefore, as for humans, YAP-TEAD signaling components may represent an important potential therapeutic target for the treatment of COD in cattle. The potential success of this type of treatment will not only be extremely important for the dairy industry, as it may represent an alternative model to translate treatments into women, but bovine models of ovarian cyst studies could also be used to test drugs and protocols that may be useful for humans affected by PCOS. Women and cows actually present common aspects at the ovarian physiology level. The sizes of follicles at distinct stages of development and the dynamics of follicle wave emergence and growth are similar in both species [42,43,44,45,46]. In addition, cattle and women share many features related to reproductive aging [47].
A puzzling finding of our study is the paradox between YAP expression vs. YAP-TEAD transcriptional activity in theca cells from cystic follicles. In these cells, total YAP protein abundance increased with no change in phospho-YAP, suggesting an accumulation of non-phosphorylated YAP, and it is this unphosphorylated YAP that can accumulate in the nucleus and activate TEAD-dependent transcription [9]. However, abundance of classic TEAD-response genes (CTGF, CYR61 and ANKRD1) decreased in TCs from cystic follicles. One explanation is the decrease in TEAD expression observed in these cells, such that even in the presence of increased YAP the availability of TEAD proteins became a limiting factor and abrogated expression of the Hippo target genes. However, the mechanism behind such a decrease and its relevance for COD pathogenesis remains to be elucidated.
5. Conclusions
We provide a novel insight that the Hippo pathway effector YAP may play a key role in the pathogenesis of cystic ovarian disease in dairy cattle. Further studies are still required to establish its causal effect in the etiology of COD, and to determine the factors/conditions leading to YAP deregulation in ovarian follicle cells. Nonetheless, this first evidence points to YAP as a potential therapeutic target for the treatment of this major ovarian disorder in cattle.
Author Contributions
E.C.D.S., N.G., C.A.P. and G.Z. were involved in the conceptualization and design of the study; E.C.D.S., A.B., G.S.-J. and N.J. performed experiments and/or were involved in the acquisition, analyses and interpretation of data; A.B., N.G., C.A.P. and G.Z. contributed with resources and/or for funding acquisition; E.C.D.S. and G.Z wrote the original draft; E.C.D.S., C.A.P. and G.Z. reviewed and edited the manuscript for publication. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Discovery Grant RGPIN-2018-06470 from Natural Sciences and Engineering Research Council of Canada (NSERC) to Zamberlam and by a New Collaboration Grant from the Réseau Québécois en Reproduction (RQR)/Fonds de Recherche Québec-Nature et Technologies (FQRNT) to Zamberlam, Price and Gévry. Dos Santos Ph.D. program was also funded by FRQNT (Bourse de doctorat en recherche B2X). The Endocrine Technologies Core (ETC) is supported (in part) by NIH grant P51 OD011092 for operation of the Oregon National Primate Research Center.
Institutional Review Board Statement
Not applicable because all analyses were performed using abattoir-derived bovine ovaries.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koeck, A.; Loker, S.; Miglior, F.; Kelton, D.F.; Jamrozik, J.; Schenkel, F.S. Genetic relationships of clinical mastitis, cystic ovaries, and lameness with milk yield and somatic cell score in first-lactation Canadian Holsteins. J. Dairy Sci. 2014, 97, 5806–5813. [Google Scholar] [CrossRef] [PubMed]
- Guarini, A.R.; Lourenco, D.A.L.; Brito, L.F.; Sargolzaei, M.; Baes, C.F.; Miglior, F.; Misztal, I.; Schenkel, F.S. Genetics and genomics of reproductive disorders in Canadian Holstein cattle. J. Dairy Sci. 2019, 102, 1341–1353. [Google Scholar] [CrossRef] [PubMed]
- Silvia, W.J.; Hatler, T.B.; Nugent, A.M.; Laranja da Fonseca, L.F. Ovarian follicular cysts in dairy cows: An abnormality in folliculogenesis. Domest. Anim. Endocrinol. 2002, 23, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, L.; Signorini, M.L.; Bertoli, J.; Bartolomé, J.A.; Gareis, N.C.; Díaz, P.U.; Bó, G.A.; Ortega, H.H. Epidemiological description of cystic ovarian disease in argentine dairy herds: Risk factors and effects on the reproductive performance of lactating cows. Reprod. Domest. Anim.=Zuchthyg. 2014, 49, 1028–1033. [Google Scholar] [CrossRef]
- Vanholder, T.; Opsomer, G.; Kruif, A.D. Aetiology and pathogenesis of cystic ovarian follicles in dairy cattle: A review. Reprod. Nutr. Dev. 2006, 46, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, J.A.; Thatcher, W.W.; Melendez, P.; Risco, C.A.; Archbald, L.F. Strategies for the diagnosis and treatment of ovarian cysts in dairy cattle. J. Am. Vet. Med. Assoc. 2005, 227, 1409–1414. [Google Scholar] [CrossRef]
- Gareis, N.C.; Angeli, E.; Huber, E.; Salvetti, N.R.; Rodríguez, F.M.; Ortega, H.H.; Hein, G.J.; Rey, F. Alterations in key metabolic sensors involved in bovine cystic ovarian disease. Theriogenology 2018, 120, 138–146. [Google Scholar] [CrossRef]
- Yu, F.-X.; Guan, K.-L. The Hippo pathway: Regulators and regulations. Genes Dev. 2013, 27, 355–371. [Google Scholar] [CrossRef]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes Dev. 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Heath, E.; Tahri, D.; Andermarcher, E.; Schofield, P.; Fleming, S.; Boulter, C.A. Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev. Biol. 2008, 8, 18. [Google Scholar] [CrossRef]
- Lai, D.; Ho, K.C.; Hao, Y.; Yang, X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011, 71, 2728–2738. [Google Scholar] [CrossRef]
- Mauviel, A.; Nallet-Staub, F.; Varelas, X. Integrating developmental signals: A Hippo in the (path)way. Oncogene 2012, 31, 1743–1756. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.R.; Liszewska, E.; Jaworski, J. Matricellular proteins of the Cyr61/CTGF/NOV (CCN) family and the nervous system. Front. Cell. Neurosci. 2015, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Plewes, M.R.; Hou, X.; Zhang, P.; Liang, A.; Hua, G.; Wood, J.R.; Cupp, A.S.; Lv, X.; Wang, C.; Davis, J.S. Yes-associated protein 1 is required for proliferation and function of bovine granulosa cells in vitro. Biol. Reprod. 2019, 101, 1001–1017. [Google Scholar] [CrossRef]
- Dos Santos, E.C.; Lalonde-Larue, A.; Antoniazzi, A.Q.; Barreta, M.H.; Price, C.A.; Dias Gonçalves, P.B.; Portela, V.M.; Zamberlam, G. YAP signaling in preovulatory granulosa cells is critical for the functioning of the EGF network during ovulation. Mol. Cell. Endocrinol. 2022, 541, 111524. [Google Scholar] [CrossRef]
- Koch, J.; Portela, V.M.; Dos Santos, E.C.; Missio, D.; de Andrade, L.G.; da Silva, Z.; Gasperin, B.G.; Antoniazzi, A.Q.; Gonçalves, P.B.D.; Zamberlam, G. The Hippo pathway effectors YAP and TAZ interact with EGF-like signaling to regulate expansion-related events in bovine cumulus cells in vitro. J. Assist. Reprod. Genet. 2022, 39, 481–492. [Google Scholar] [CrossRef]
- Ji, S.Y.; Liu, X.M.; Li, B.T.; Zhang, Y.L.; Liu, H.B.; Zhang, Y.C.; Chen, Z.J.; Liu, J.; Fan, H.Y. The polycystic ovary syndrome-associated gene Yap1 is regulated by gonadotropins and sex steroid hormones in hyperandrogenism-induced oligo-ovulation in mouse. Mol. Hum. Reprod. 2017, 23, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhao, H.; Zhao, X.; Zhang, B.; Cui, L.; Shi, Y.; Li, G.; Wang, P.; Chen, Z.J. Identification of YAP1 as a novel susceptibility gene for polycystic ovary syndrome. J. Med. Genet. 2012, 49, 254–257. [Google Scholar] [CrossRef]
- Zhang, Y.; Ho, K.; Keaton, J.M.; Hartzel, D.N.; Day, F.; Justice, A.E.; Josyula, N.S.; Pendergrass, S.A.; Actkins, K.; Davis, L.K.; et al. A genome-wide association study of polycystic ovary syndrome identified from electronic health records. Am. J. Obstet. Gynecol. 2020, 223, 559.e1–559.e21. [Google Scholar] [CrossRef]
- Braw-Tal, R.; Pen, S.; Roth, Z. Ovarian cysts in high-yielding dairy cows. Theriogenology 2009, 72, 690–698. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Alfaro, N.S.; Salvetti, N.R.; Velazquez, M.M.; Stangaferro, M.L.; Rey, F.; Ortega, H.H. Steroid receptor mRNA expression in the ovarian follicles of cows with cystic ovarian disease. Res. Vet. Sci. 2012, 92, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Portela, V.M.; Zamberlam, G.; Goncalves, P.B.; de Oliveira, J.F.; Price, C.A. Role of angiotensin II in the periovulatory epidermal growth factor-like cascade in bovine granulosa cells in vitro. Biol. Reprod. 2011, 85, 1167–1174. [Google Scholar] [CrossRef][Green Version]
- Orisaka, M.; Mizutani, T.; Tajima, K.; Orisaka, S.; Shukunami, K.; Miyamoto, K.; Kotsuji, F. Effects of ovarian theca cells on granulosa cell differentiation during gonadotropin-independent follicular growth in cattle. Mol. Reprod. Dev. 2006, 73, 737–744. [Google Scholar] [CrossRef]
- Bishop, C.V.; Reiter, T.E.; Erikson, D.W.; Hanna, C.B.; Daughtry, B.L.; Chavez, S.L.; Hennebold, J.D.; Stouffer, R.L. Chronically elevated androgen and/or consumption of a Western-style diet impairs oocyte quality and granulosa cell function in the nonhuman primate periovulatory follicle. J. Assist. Reprod. Genet. 2019, 36, 1497–1511. [Google Scholar] [CrossRef]
- Brito, C.; Palmer, C.W. Cystic Ovarian Disease in Cattle. Can. Vet.—Large Anim. Vet. Rounds 2004, 4, 1–6. [Google Scholar]
- Larsen, R.E. Veterinary obstetrics and genital diseases (Theriogenology) by S.J. Roberts (ed.); 981 pages, 1986, 3rd edition. Published by the author, Woodstock, VT 05091. Distributed by David and Charles Inc., North Pomfret, VT 05053. Theriogenology 1986, 26, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Garverick, H.A. Ovarian Follicular Cysts in Dairy Cows1. J. Dairy Sci. 1997, 80, 995–1004. [Google Scholar] [CrossRef]
- Millward, S.; Mueller, K.; Smith, R.; Higgins, H.M. A Post-mortem Survey of Bovine Female Reproductive Tracts in the UK. Front. Vet. Sci. 2019, 6, 451. [Google Scholar] [CrossRef]
- Gareis, N.C.; Huber, E.; Hein, G.J.; Rodríguez, F.M.; Salvetti, N.R.; Angeli, E.; Ortega, H.H.; Rey, F. Impaired insulin signaling pathways affect ovarian steroidogenesis in cows with COD. Anim. Reprod. Sci. 2018, 192, 298–312. [Google Scholar] [CrossRef]
- Shimizu, T.; Ishizawa, S.; Magata, F.; Kobayashi, M.; Fricke, P.M.; Miyamoto, A. Involvement of lipopolysaccharide in ovarian cystic follicles in dairy cow: Expressions of LPS receptors and steroidogenesis-related genes in follicular cells of cystic follicles. Anim. Reprod. Sci. 2018, 195, 89–95. [Google Scholar] [CrossRef]
- Kawate, N.; Inaba, T.; Mori, J. A quantitative comparison in the bovine of steroids and gonadotropin receptors in normally developing follicles and in follicular and luteinized cysts. Anim. Reprod. Sci. 1990, 23, 273–281. [Google Scholar] [CrossRef]
- Marelli, B.E.; Diaz, P.U.; Salvetti, N.R.; Rey, F.; Ortega, H.H. mRNA expression pattern of gonadotropin receptors in bovine follicular cysts. Reprod. Biol. 2014, 14, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Yoshimura, Y. Localization of apoptotic cells in the cystic ovarian follicles of cows: A DNA-end labeling histochemical study. Theriogenology 2000, 53, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Ortega, H.H.; Marelli, B.E.; Rey, F.; Amweg, A.N.; Díaz, P.U.; Stangaferro, M.L.; Salvetti, N.R. Molecular aspects of bovine cystic ovarian disease pathogenesis. Reproduction 2015, 149, R251–R264. [Google Scholar] [CrossRef]
- Nagashima, T.; Kim, J.; Li, Q.; Lydon, J.P.; DeMayo, F.J.; Lyons, K.M.; Matzuk, M.M. Connective Tissue Growth Factor Is Required for Normal Follicle Development and Ovulation. Mol. Endocrinol. 2011, 25, 1740–1759. [Google Scholar] [CrossRef]
- Chang, H.M.; Pan, H.H.; Cheng, J.C.; Zhu, Y.M.; Leung, P.C.K. Growth differentiation factor 8 suppresses cell proliferation by up-regulating CTGF expression in human granulosa cells. Mol. Cell. Endocrinol. 2016, 422, 9–17. [Google Scholar] [CrossRef]
- Johnson, A.L.; Langer, J.S.; Bridgham, J.T. Survivin as a Cell Cycle-Related and Antiapoptotic Protein in Granulosa Cells. Endocrinology 2002, 143, 3405–3413. [Google Scholar] [CrossRef]
- Zhang, N.; Ye, F.; Zhu, W.; Hu, D.; Xiao, C.; Nan, J.; Su, S.; Wang, Y.; Liu, M.; Gao, K.; et al. Cardiac ankyrin repeat protein attenuates cardiomyocyte apoptosis by upregulation of Bcl-2 expression. Biochim. et Biophys. Acta (BBA)—Mol. Cell Res. 2016, 1863, 3040–3049. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, H.; Shi, Y.; Cao, Y.; Yang, D.; Li, Z.; Zhang, B.; Liang, X.; Li, T.; Chen, J.; et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat. Genet. 2012, 44, 1020–1025. [Google Scholar] [CrossRef]
- McAllister, J.M.; Legro, R.S.; Modi, B.P.; Strauss, J.F. Functional genomics of PCOS: From GWAS to molecular mechanisms. Trends Endocrinol. Metab. 2015, 26, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Xie, J.K.; Cui, J.Q.; Wei, D.; Yin, B.L.; Zhang, Y.N.; Chen, Y.H.; Han, X.; Wang, Q.; Zhang, C.L. Promoter methylation of yes-associated protein (YAP1) gene in polycystic ovary syndrome. Medicine 2017, 96, e5768. [Google Scholar] [CrossRef] [PubMed]
- Sirois, J.; Fortune, J.E. Ovarian Follicular Dynamics during the Estrous Cycle in Heifers Monitored by Real-Time UItrasonograph1. Biol. Reprod. 1988, 39, 308–317. [Google Scholar] [CrossRef]
- Adams, G.P.; Matteri, R.L.; Kastelic, J.P.; Ko, J.C.; Ginther, O.J. Association between surges of follicle-stimulating hormone and the emergence of follicular waves in heifers. J. Reprod. Fertil. 1992, 94, 177–188. [Google Scholar] [CrossRef]
- Campbell, B.K.; Souza, C.; Gong, J.; Webb, R.; Kendall, N.; Marsters, P.; Robinson, G.; Mitchell, A.; Telfer, E.E.; Baird, D.T. Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reprod. Suppl. 2003, 61, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Baerwald, A.R.; Adams, G.P.; Pierson, R.A. A new model for ovarian follicular development during the human menstrual cycle. Fertil. Steril. 2003, 80, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Malhi, P.S.; Adams, G.P.; Singh, J. Bovine Model for the Study of Reproductive Aging in Women: Follicular, Luteal, and Endocrine Characteristics1. Biol. Reprod. 2005, 73, 45–53. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).