Key Factors behind the Dynamic Stability of Pairs of Egyptian Vultures in Continental Spain
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Analytical Procedure
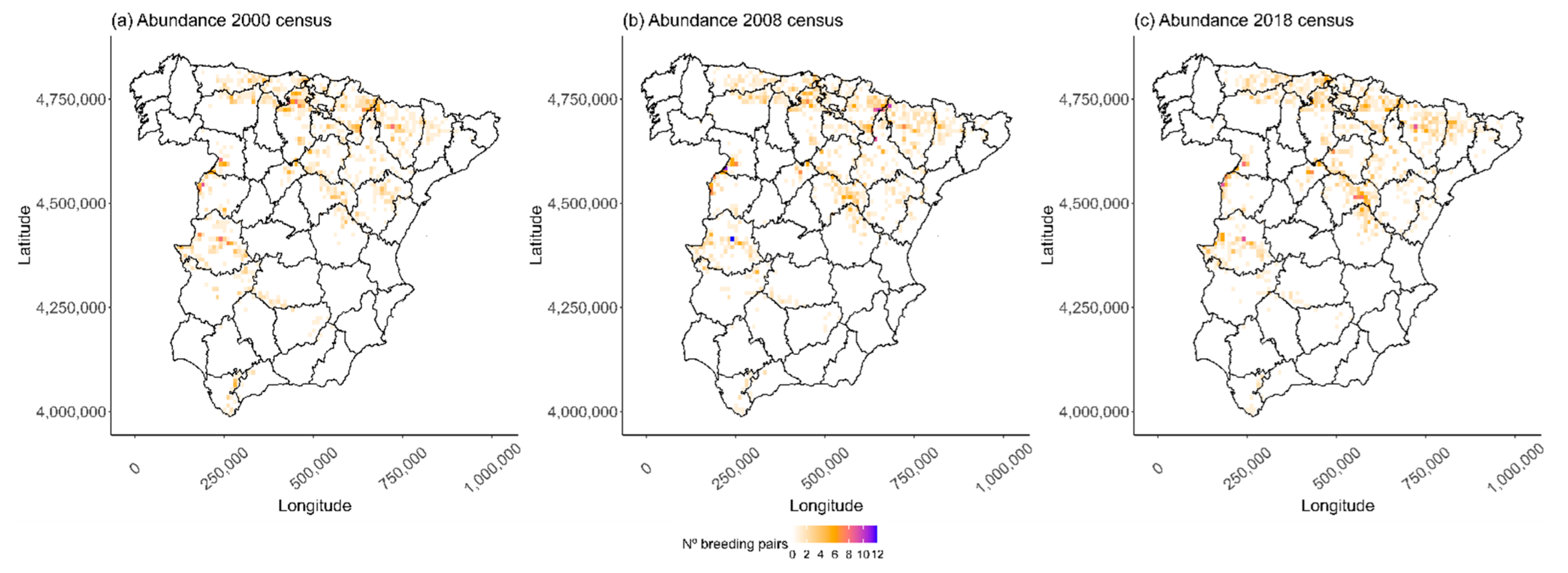
2.2.1. Analyzing the Temporal Variation in Distribution
2.2.2. Analyzing the Spatial Heterogeneity
2.2.3. Analyzing the Factors That Shape Recent Abundances at Local and Regional Scales
2.2.4. Explanatory Variables
| Acronym | Definition | Source of Information |
|---|---|---|
| (1) Habitat | ||
| ALT | Altitude (meters above sea level) | Digital Elevation Model (DEM) |
| NIC | Cover (%) of non-irrigated crops (e.g., regular annual crops, cereals, leguminous crops) | CORINE Land Cover |
| IRR | Cover (%) of irrigated crops (e.g., arable, crops, rice fields, non-permanent grass) | CORINE Land Cover |
| TREE | Cover (%) of permanent crops (e.g., olive groves, orchids, vineyards, fruit trees) | CORINE Land Cover |
| DEH | Cover (%) of agroforest systems (named dehesas in Spain) | CORINE Land Cover |
| ROC | Cover (%) of bare rocks (e.g., stable rocks with limestone pavements) | CORINE Land Cover |
| FOR | Cover (%) of forests (e.g., broad-leaved, coniferous, and mixed forests) | CORINE Land Cover |
| PAS | Cover (%) of pasturelands (e.g., permanent grasslands) | CORINE Land Cover |
| (2) Food availability | ||
| COW | Number of cows surveyed on national census | National Institute of Statistics (INE) |
| SHEEP | Number of sheep surveyed on national census | National Institute of Statistics (INE) |
| LAND | Number of landfills | MAPAMA |
| SFS | Number of supplementary feeding stations | MAPAMA |
| (3) Human pressure | ||
| URB | Cover (%) of urban areas (e.g., residential and commercial/industrial buildings, parking lots, small squares) | CORINE Land Cover |
| WTG | Number of wind turbines | Asociación Empresarial Eólica (AEE) |
| POIS | Number of poison-related mortality events of wild fauna | WWF and SEO/Birdlife [55] |
| (4) Heterospecific relationship | ||
| GF | Number of breeding pairs of griffon vultures | SEO/Birdlife [59] |
3. Results
3.1. Temporal Variation on Distribution
3.2. Spatial Variation on Distribution
3.3. Local Drivers of Abundance Patterns at Different Spatial Scales
4. Discussion
Conservation Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Digital Elevation Model
Appendix A.2. CORINE Land Cover Maps
| CLC Level 1 | CLC Level 2 | CLC Level 3 | New Code |
|---|---|---|---|
| Artificial surfaces | Urban fabric | Continuous urban fabric | URB |
| Discontinuous urban fabric | URB | ||
| Industrial, commercial, and transport units | Industrial or commercial units | URB | |
| Road and rail networks and associated land | URB | ||
| Port area | URB | ||
| Airports | URB | ||
| Mine, dump, and construction sites | Mineral extraction sites | URB | |
| Dump sites | URB | ||
| Construction sites | URB | ||
| Artificial, non-agricultural vegetated areas | Green urban areas | URB | |
| Sport and leisure facilities | URB | ||
| Agricultural areas | Arable land | Non-irrigated arable land | NIC |
| Permanently irrigated land | IRR | ||
| Rice fields | IRR | ||
| Permanent crops | Vineyards | TREE | |
| Fruit trees and berry plantations | TREE | ||
| Olive groves | TREE | ||
| Pastures | Pastures | PAS | |
| Heterogeneous agricultural areas | Annual crops associated with permanent crops | NIC | |
| Complex cultivation patterns | NIC | ||
| Land principally occupied by agriculture, with significant areas of natural vegetation | NIC | ||
| Agroforestal areas | DEH | ||
| Forest and semi natural areas | Forest | Broad-leaf forest | FOR |
| Coniferous forest | FOR | ||
| Mixed forest | FOR | ||
| Scrub and/or herbaceous vegetation associations | Natural grasslands | PAS | |
| Moors and heathland | - | ||
| Sclerophyllous vegetation | - | ||
| Transitional woodland–shrub | - | ||
| Open spaces with little or no vegetation | Beaches, dunes, sands | ROC | |
| Bare rocks | ROC | ||
| Sparsely vegetated areas | ROC | ||
| Burnt areas | - | ||
| Glaciers and perpetual snow | - | ||
| Wetlands | Inland wetlands | Inland marshes | - |
| Peat bogs | - | ||
| Maritime wetlands | Salt marshes | - | |
| Salines | - | ||
| Intertidal flats | - | ||
| Water bodies | Inland waters | Water courses | - |
| Water bodies | - | ||
| Marine waters | Coastal lagoons | - | |
| Estuaries | - | ||
| Sea and ocean | - |
Appendix A.3. INE
Appendix A.4. MAPAMA
Appendix A.5. Asociación Empresarial Eólica (AEE)
Appendix A.6. The Poison-Related Mortality Event Database from SEO/Birdlife and WWF
Appendix A.7. Griffon Vulture National Census
Appendix B
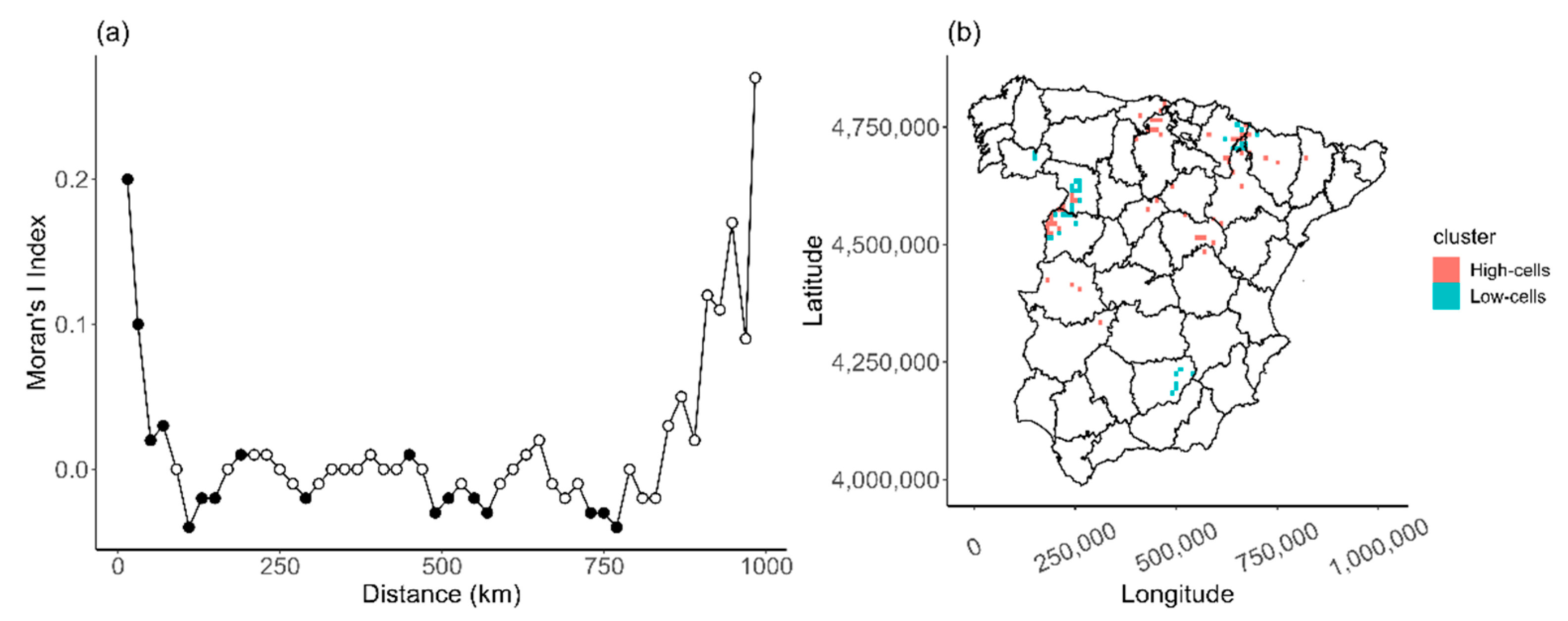
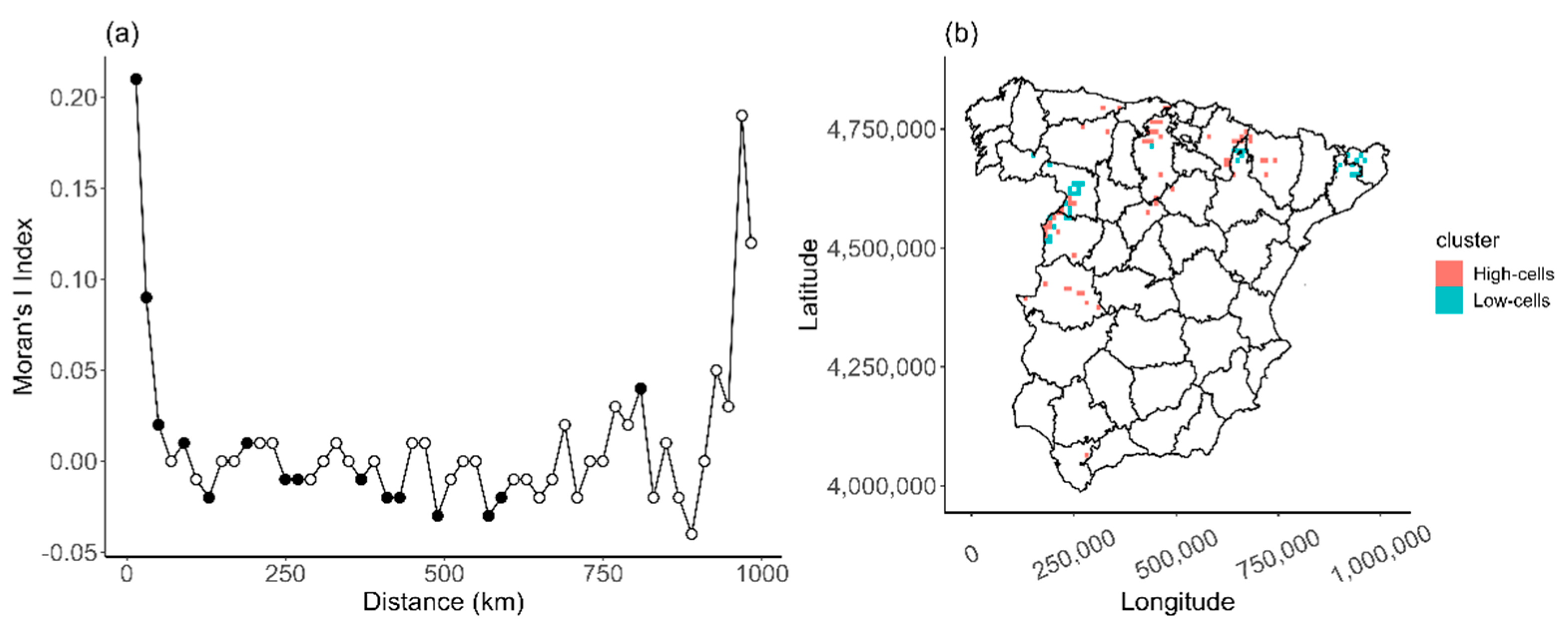
Appendix C
| Variables | H | df | P | P adj |
|---|---|---|---|---|
| (1) Habitat | ||||
| ALT | 0.069 | 1 | 0.792 | 0.3958 |
| NIC | 16.748 | 1 | 0.650 | 0.325 |
| IRR | 2.654 | 1 | 0.103 | 0.052 |
| TREE | 0.842 | 1 | 0.359 | 0.179 |
| ROC | 62.978 | 1 | 0.903 | 0.451 |
| FOR | 93.876 | 1 | 0.761 | 0.381 |
| PAS | 0.400 | 1 | 0.527 | 0.264 |
| (2) Food availability | ||||
| SHEEP | 1.444 | 1 | 0.230 | 0.115 |
| LAND | 3.648 | 1 | 0.581 | 0.291 |
| (3) Human pressure | ||||
| URB | 2.018 | 1 | 0.155 | 0.078 |
| POIS | 2.449 | 1 | 0.118 | 0.059 |
Appendix D
| Variables | E | SE | Adj SE | Z | P | |
|---|---|---|---|---|---|---|
| Model-1 | Intercept | −36.790 | 74.020 | 74.080 | 0.497 | 0.619 |
| NP08 | 0.196 | 0.012 | 0.012 | 16.185 | <0.001 | |
| COW | 1.647 × 10−5 | 6.569 × 10−6 | 1.647 × 10−5 | 2.504 | 0.012 | |
| GF | 0.141 | 0.020 | 0.020 | 6.931 | <0.001 | |
| MUL | −0.042 | 0.037 | 0.037 | 1.116 | 0.264 | |
| DEH | −0.003 | 0.003 | 0.003 | 0.981 | 0.327 | |
| WTG | −0.002 | 0.002 | 0.002 | 1.059 | 0.290 | |
| y | 2.86 × 10−5 | 6.46 × 10−5 | 6.47 × 10−5 | 0.442 | 0.658 | |
| y2 | −1.91 × 10−12 | 1.48 × 10−11 | 1.48 × 10−11 | 0.129 | 0.898 | |
| y3 | −1.96 × 10−19 | 1.19 × 10−18 | 1.19 × 10−18 | 0.164 | 0.869 | |
| x | −2.19 × 10−4 | 5.90 × 10−5 | 5.91 × 10−5 | 3.707 | <0.001 | |
| x2y | −2.76 × 10−19 | 1.85 × 10−19 | 1.86 × 10−19 | 1.488 | 0.137 | |
| xy | 9.56 × 10−11 | 2.55 × 10−11 | 2.56 × 10−11 | 3.741 | <0.001 | |
| xy2 | −1.04 × 10−17 | 2.76 × 10−18 | 2.76 × 10−18 | 3.762 | <0.001 | |
| x2 | −1.30 × 10−12 | 8.80 × 10−13 | 8.81 × 10−13 | 1.472 | 0.141 | |
| x3 | −4.23 × 10−19 | 5.32 × 10−19 | 5.33 × 10−19 | 0.795 | 0.427 | |
| Model-2 | Intercept | −68.530 | 56.810 | 56.850 | 1.205 | 0.228 |
| NP00 | 0.166 | 0.015 | 0.015 | 10.936 | <0.001 | |
| COW | 0.000 | 0.000 | 0.000 | 2.492 | 0.013 | |
| GF | 0.182 | 0.020 | 0.020 | 9.097 | <0.001 | |
| MUL | −0.026 | 0.035 | 0.035 | 0.726 | 0.468 | |
| DEH | −0.004 | 0.003 | 0.003 | 1.348 | 0.178 | |
| WTG | −0.003 | 0.002 | 0.002 | 1.214 | 0.225 | |
| y | 3.46 × 10−5 | 4.03 × 10−5 | 4.03 × 10−5 | 0.859 | 0.390 | |
| y2 | −7.19 × 10−13 | 9.99 × 10−12 | 1.00 × 10−11 | 0.072 | 0.943 | |
| y3 | −4.71 × 10−19 | 7.20 × 10−19 | 7.21 × 10−19 | 0.653 | 0.514 | |
| x | −4.36 × 10−5 | 1.06 × 10−4 | 1.06 × 10−4 | 0.412 | 0.681 | |
| x2y | 1.89 × 10−11 | 4.61 × 10−11 | 4.61 × 10−11 | 0.410 | 0.682 | |
| xy | −2.07 × 10−18 | 5.00 × 10−18 | 5.00 × 10−18 | 0.414 | 0.679 | |
| xy2 | −1.50 × 10−13 | 1.67 × 10−13 | 1.67 × 10−13 | 0.896 | 0.371 | |
| x2 | −3.10 × 10−20 | 3.58 × 10−20 | 3.58 × 10−20 | 0.867 | 0.386 | |
| x3 | −1.34 × 10−19 | 1.85 × 10−19 | 1.85 × 10−19 | 0.726 | 0.468 |
References
- Groom, M.J.; Meffe, G.K.; Carroll, C.R. Principles of Conservation Biology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2006; pp. 432–444. [Google Scholar]
- Lindenmayer, D.; Burgman, M. Practical Conservation Biology, 1st ed.; Cromwell Press: Trowbridge, UK, 2006; pp. 73–100. [Google Scholar]
- Sutherland, W.J.; Pullin, A.S.; Dolman, P.M.; Knight, T.M. The need for evidence-based conservation. Tree 2004, 19, 305–308. [Google Scholar] [CrossRef]
- Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef]
- Pearman, P.B.; Guisan, A.; Broennimann, O.; Randin, C.F. Niche dynamics in space and time. Tree 2008, 23, 149–158. [Google Scholar] [CrossRef]
- Sax, D.F.; Gaines, S.D. Species diversity: From global decreases to local increases. Tree 2003, 18, 561–566. [Google Scholar] [CrossRef]
- Whittingham, M.J.; Krebs, J.R.; Swetnam, R.D.; Vickery, J.A.; Wilson, J.D.; Freckleton, R.P. Should conservation strategies consider spatial generality? Farmland birds show regional not national patterns of habitat association. Ecol. Lett. 2007, 10, 25–35. [Google Scholar]
- Boyd, C.; Brooks, T.M.; Butchart, S.H.; Edgar, G.J.; Da Fonseca, G.A.; Hawkins, F.; Hoffmann, M.; Sechrest, W.; Stuart, S.N.; Van Dijk, P.P. Spatial scale and the conservation of threatened species. Conserv. Lett. 2008, 1, 37–43. [Google Scholar] [CrossRef]
- Lee-Yaw, J.A.; McCune, J.L.; Pironon, S.; Sheth, S.N. Species distribution models rarely predict the biology of real populations. Ecography 2022, 2022, e05877. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Margules, C.R.; Pressey, R.L. Systematic conservation planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef]
- El-Gabbas, A.; Van Opzeeland, I.; Burkhardt, E.; Boebel, O. Static species distribution models in the marine realm: The case of baleen whales in the Southern Ocean. Divers. Distrib. 2021, 27, 1536–1552. [Google Scholar] [CrossRef]
- Bateman, B.L.; VanDerWal, J.; Johnson, C.N. Nice weather for bettongs: Using weather events, not climate means, in species distribution models. Ecography 2012, 35, 306–314. [Google Scholar] [CrossRef]
- Fernandez, M.; Yesson, C.; Gannier, A.; Miller, P.I.; Azevedo, J.M. The importance of temporal resolution for niche modelling in dynamic marine environments. J. Biogeogr. 2017, 44, 2816–2827. [Google Scholar] [CrossRef]
- Milanesi, P.; Della Rocca, F.; Robinson, R.A. Integrating dynamic environmental predictors and species occurrences: Toward true dynamic species distribution models. Ecol. Evol. 2020, 10, 1087–1092. [Google Scholar] [CrossRef]
- Willis, K.J.; Whittaker, R.J. Species diversity—Scale matters. Science 2002, 295, 1245–1248. [Google Scholar] [CrossRef]
- Pearson, S.M.; Turner, M.G.; Gardner, R.H.; O’Neill, R.V. An organism-based perspective of habitat fragmentation. In Biodiversity in Managed Landscapes: Theory and Practice, 1st ed.; Szaro, R.C., Ed.; Oxford University Press: New York, NY, USA, 1996; pp. 77–95. [Google Scholar]
- Condit, R.; Ashton, P.S.; Baker, P.; Bunyavejchewin, S.; Gunatilleke, S.; Gunatilleke, N.; Hubbell, S.P.; Foster, R.B.; Itoh, A.; Lafrankie, J.V.; et al. Spatial patterns in the distribution of tropical tree species. Science 2000, 288, 1414–1418. [Google Scholar] [CrossRef]
- Lichstein, J.W.; Simons, T.R.; Shriner, S.A.; Franzreb, K.E. Spatial autocorrelation and autoregressive models in ecology. Ecol. Monogr. 2002, 72, 445–463. [Google Scholar] [CrossRef]
- Gibb, R.; Shoji, A.; Fayet, A.L.; Perrins, C.M.; Guilford, T.; Freeman, R. Remotely sensed wind speed predicts soaring behaviour in a wide-ranging pelagic seabird. J. R. Soc. Interface 2017, 14, 20170262. [Google Scholar] [CrossRef]
- Buechley, E.R.; Oppel, S.; Efrat, R.; Phipps, W.L.; Carbonell Alanis, I.; Álvarez, E.; Andreotti, A.; Arkumarev, V.; Berger-Tal, O.; Bermejo Bermejo, A.; et al. Differential survival throughout the full annual cycle of a migratory bird presents a life-history trade-off. J. Anim. Ecol. 2021, 90, 1228–1238. [Google Scholar] [CrossRef]
- Gantchoff, M.G.; Beyer, D.E., Jr.; Erb, J.D.; MacFarland, D.M.; Norton, D.C.; Roell, B.J.; Belant, J.L. Distribution model transferability for a wide-ranging species, the Gray Wolf. Sci. Rep. 2022, 12, 13556. [Google Scholar] [CrossRef]
- Oppel, S.; Dobrev, V.; Arkumarev, V.; Saravia, V.; Bounas, A.; Manolopoulos, A.; Kret, E.; Velevski, M.; Popgeorgiev, G.S.; Nikolov, S.C. Landscape factors affecting territory occupancy and breeding success of Egyptian Vultures on the Balkan Peninsula. J. Ornithol. 2017, 158, 443–457. [Google Scholar] [CrossRef]
- Zuberogoitia, I.; Zabala, J.; Martínez, J.E.; González-Oreja, J.A.; López-López, P. Effective conservation measures to mitigate the impact of human disturbances on the endangered Egyptian vulture. Anim. Conserv. 2014, 17, 410–418. [Google Scholar] [CrossRef]
- Oliva-Vidal, P.; Hernández-Matías, A.; García, D.; Colomer, M.À.; Real, J.; Margalida, A. Griffon vultures, livestock and farmers: Unraveling a complex socio-economic ecological conflict from a conservation perspective. Biol. Conserv. 2022, 272, 109664. [Google Scholar] [CrossRef]
- Donázar, J.A. Los Buitres Ibéricos: Biología y Conservación, 1st ed.; Reyero, J.M., Ed.; Librería: Madrid, Spain, 1993. [Google Scholar]
- BirdLife International. Neophron percnopterus. The IUCN Red List of Threatened Species 2021: e.T22695180A205187871.Lo. 2021. Available online: https://www.iucnredlist.org/species/22695180/205187871 (accessed on 10 February 2020).
- Carrete, M.; Grande, J.M.; Tella, J.L.; Sánchez-Zapata, J.A.; Donázar, J.A.; Díaz-Delgado, R.; Romo, A. Habitat, human pressure, and social behavior: Partialling out factors affecting large-scale territory extinction in an endangered vulture. Biol. Conserv. 2007, 136, 143–154. [Google Scholar] [CrossRef]
- Tauler, H.; Real, J.; Hernandez-Matias, A.; Aymerich, P.; Baucells, J.; Martorell, C.; Santandreu, J. Identifying key demographic parameters for the viability of a growing population of the endangered Egyptian Vulture Neophron percnopterus. Bird Conserv. Int. 2015, 25, 426–439. [Google Scholar] [CrossRef]
- Franch, M.; Hernando, S.; Anton, M.; Brotons, L. Atles dels Ocells Nidificans de Catalunya. In Distribució i Abundancia 2015–2017 i Canvis des de 1980, 1st ed.; Institut Català d’Ornitologia i Cossetània Edicions: Barcelona, Spain, 2021; pp. 132–135. [Google Scholar]
- Donázar, J.A. Alimoche común, Neophron percnopterus. In Libro Rojo de las Aves de España, 1st ed.; Madroño, A., González, C., Atienza, J.C., Eds.; Dirección General para la Biodiversidad-SEO/BirdLife: Madrid, Spain, 2004; pp. 129–130. [Google Scholar]
- Hernández, M.; Margalida, A. Poison-related mortality effects in the endangered Egyptian vulture (Neophron percnopterus) population in Spain. Eur. J. Wildl. Res. 2009, 55, 415–423. [Google Scholar] [CrossRef]
- Donázar, J.A.; Palacios, C.J.; Gangoso, L.; Ceballos, O.; González, M.J.; Hiraldo, F. Conservation status and limiting factors in the endangered population of Egyptian vulture (Neophron percnopterus) in the Canary Islands. Biol. Conserv. 2002, 107, 89–97. [Google Scholar] [CrossRef]
- Carrete, M.; Sánchez-Zapata, J.A.; Benítez, J.R.; Lobón, M.; Donázar, J.A. Large scale risk-assessment of wind-farms on population viability of a globally endangered long-lived raptor. Biol. Conserv. 2009, 142, 2954–2961. [Google Scholar] [CrossRef]
- Donázar, J.A.; Cortés-Avizanda, A.; Carrete, M. Dietary shifts in two vultures after the demise of supplementary feeding stations: Consequences of the EU sanitary legislation. Eur. J. Wildl. Res. 2010, 56, 613–621. [Google Scholar] [CrossRef]
- Del Moral, J.C.; Martí, R. El Alimoche Común en España y Portugal (I Censo Coordinado). Año 2000; SEO/BirdLife: Madrid, Spain, 2002; Volume 8, pp. 44–146. [Google Scholar]
- Del Moral, J.C. El alimoche común en España. In Población Reproductora en 2008 y Método de Censo, 1st ed.; SEO/BirdLife: Madrid, Spain, 2009; pp. 1–136. [Google Scholar]
- Del Moral, J.C.; Molina, B. El Alimoche Común en España. In Población Reproductora en 2018 y Método de Censo, 1st ed.; SEO/BirdLife: Madrid, Spain, 2018; pp. 1–149. [Google Scholar]
- Collins, C.D.; Holt, R.D.; Foster, B.L. Patch size effects on plant species decline in an experimentally fragmented landscape. Ecology 2009, 90, 2577–2588. [Google Scholar] [CrossRef]
- Moran, P.A.P. Notes on continuous stochastic phenomena. Biometrika 1950, 37, 17–37. [Google Scholar] [CrossRef]
- Anselin, L. Local indicators of spatial association—LISA. Geogr. Anal. 1995, 27, 93–115. [Google Scholar] [CrossRef]
- Nelson, T.A.; Boots, B. Detecting spatial hot spots in landscape ecology. Ecography 2008, 31, 556–566. [Google Scholar] [CrossRef]
- Martínez Batlle, J.R.; Van der Hoek, Y. Clusters of high abundance of plants detected from local indicators of spatial association (LISA) in a semi-deciduous tropical forest. PLoS ONE 2018, 13, e0208780. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R, 1st ed.; Springer: New York, NY, USA, 2009; pp. 209–237. [Google Scholar]
- Serrano, D.; Cortés-Avizanda, A.; Zuberogoitia, I.; Blanco, G.; Benítez, J.R.; Ponchon, C.; Donázar, J.A. Phenotypic and environmental correlates of natal dispersal in a long-lived territorial vulture. Sci. Rep. 2021, 11, 5424. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Fortin, M.J. Spatial pattern and ecological analysis. Vegetatio 1989, 80, 107–138. [Google Scholar] [CrossRef]
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 10 February 2020).
- Dray, S.; Bauman, D.; Blanchet, G.; Borcard, D.; Clappe, S.; Guénard, G.; Jombart, T.; Larocque, G.; Legendre, P.; Madi, N.; et al. Adespatial: Multivariate Multiscale Spatial Analysis. 2023. R Package Version 0.3-21. Available online: https://CRAN.R-project.org/package=adespatial (accessed on 10 February 2020).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002; pp. 1–498. [Google Scholar]
- Barton, K. MuMIn: Multi-Model Inference. 2009. Available online: http://r-forge.r-project.org/projects/mumin/ (accessed on 10 February 2020).
- Sugiura, N. Further analysis of the data by Akaike’s information criterion and the finite corrections: Further analysis of the data by akaike’s. Commun. Stat. Theory Methods 1978, 7, 13–26. [Google Scholar] [CrossRef]
- Margalida, A.; García, D.; Cortés-Avizanda, A. Factors influencing the breeding density of Bearded vultures, Egyptian vultures and Eurasian griffon vultures in Catalonia (NE Spain): Management implications. Anim. Biodivers. Conserv. 2007, 20, 189–200. [Google Scholar] [CrossRef]
- Cortés-Avizanda, A.; Blanco, G.; DeVault, T.L.; Markandya, A.; Virani, M.Z.; Brandt, J.; Donázar, J.A. Supplementary feeding and endangered avian scavengers: Benefits, caveats, and controversies. Front. Ecol. Evol. 2016, 14, 191–199. [Google Scholar] [CrossRef]
- Tauler-Ametller, H.; Hernández-Matías, A.; Pretus, J.L.; Real, J. Landfills determine the distribution of an expanding breeding population of the endangered Egyptian Vulture Neophron percnopterus. Ibis 2017, 159, 757–768. [Google Scholar] [CrossRef]
- De la Bodega, D.; Cano, C.; Mínguez, E. El veneno en España. Evolución del Envenenamiento de Fauna Silvestre (1992–2017), 1st ed.; WWF y SEO/BirdLife: Madrid, Spain, 2020; pp. 1–54. [Google Scholar]
- Sarà, M.; Di Vittorio, M. Factors influencing the distribution, abundance and nest-site selection of an endangered Egyptian vulture (Neophron percnopterus) population in Sicily. Anim. Conserv. 2003, 6, 317–328. [Google Scholar] [CrossRef]
- Cortés-Avizanda, A.; Carrete, M.; Donázar, J.A. Managing supplementary feeding for avian scavengers: Guidelines for optimal design using ecological criteria. Biol. Conserv. 2010, 143, 1707–1715. [Google Scholar] [CrossRef]
- Cortés-Avizanda, A.; Jovani, R.; Carrete, M.; Donázar, J.A. Resource unpredictability promotes species diversity and coexistence in an avian scavenger guild: A field experiment. Ecology 2012, 93, 2570–2579. [Google Scholar] [CrossRef]
- Del Moral, J.C.; Molina, B. El Buitre Leonado en España. In Población Reproductora en 2018 y Método de Censo, 1st ed.; SEO/BirdLife: Madrid, Spain, 2018; pp. 1–189. [Google Scholar]
- López-López, P.; García-Ripollés, C.; Urios, V. Food predictability determines space use of endangered vultures: Implications for management of supplementary feeding. Ecol. Appl. 2014, 24, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Real, J.; Tinto, A.; Zozaya, E.L.; Castell, C. Home-ranges and patterns of spatial use in territorial Bonelli’s Eagles Aquila fasciata. Ibis 2010, 152, 105–117. [Google Scholar] [CrossRef]
- Margalida, A.; Donázar, J.A.; Bustamante, J.; Hernández, F.J.; Romero-Pujante, M. Application of a predictive model to detect long-term changes in nest-site selection in the Bearded Vulture Gypaetus barbatus: Conservation in relation to territory shrinkage. Ibis 2008, 150, 242–249. [Google Scholar] [CrossRef]
- Danchin, E.; Giraldeau, L.A.; Valone, T.J.; Wagner, R.H. Public information: From nosy neighbors to cultural evolution. Science 2004, 305, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Grande, J.M.; Serrano, D.; Tavecchia, G.; Carrete, M.; Ceballos, O.; Díaz-Delgado, R.; Tella, J.L.; Donázar, J.A. Survival in a long-lived territorial migrant: Effects of life-history traits and ecological conditions in wintering and breeding areas. Oikos 2009, 118, 580–590. [Google Scholar] [CrossRef]
- Katzenberger, J.; Tabur, E.; ŞEN, B.; İsfendiyaroğlu, S.; Erkol, I.L.; Oppel, S. No short-term effect of closing a rubbish dump on reproductive parameters of an Egyptian Vulture population in Turkey. Bird Conserv. Int. 2019, 29, 71–82. [Google Scholar] [CrossRef]
- De Frutos, A.; Olea, P.P.; Vera, R. Analyzing and modelling spatial distribution of summering lesser kestrel: The role of spatial autocorrelation. Ecol. Modell. 2007, 200, 33–44. [Google Scholar] [CrossRef]
- Sanz-Aguilar, A.; Sánchez-Zapata, J.A.; Carrete, M.; Benítez, J.R.; Ávila, E.; Arenas, R.; Donázar, J.A. Action on multiple fronts, illegal poisoning and wind farm planning, is required to reverse the decline of the Egyptian vulture in southern Spain. Biol. Conserv. 2015, 187, 10–18. [Google Scholar] [CrossRef]
- Carrete, M.; Donázar, J.A. Application of central-place foraging theory shows the importance of Mediterranean dehesas for the conservation of the cinereous vulture, Aegypius monachus. Biol. Consev. 2005, 126, 582–590. [Google Scholar] [CrossRef]
- Martin-Díaz, P.; Cortés-Avizanda, A.; Serrano, D.; Arrondo, E.; Sánchez-Zapata, J.A.; Donázar, J.A. Rewilding processes shape the use of Mediterranean landscapes by an avian top scavenger. Sci. Rep. 2020, 10, 2853. [Google Scholar] [CrossRef]
- Grande, J.M. Factores Limitantes, Antrópicos y Naturales, de Poblaciones de aves Carroñeras: El caso del Alimoche (Neophron percnopterus) en el valle del Ebro. Ph.D. Thesis, University of Sevilla, Seville, Spain, 2006. [Google Scholar]
- Carrete, M.; Donazar, J.A.; Margalida, A. Density-dependent productivity depression in Pyrenean Bearded Vultures: Implications for conservation. Ecol. Appl. 2006, 16, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Tomás, P.; Olea, P.P. Livestock-driven land use change to model species distributions: Egyptian vulture as a case study. Ecol. Indic. 2015, 57, 331–340. [Google Scholar] [CrossRef]
- Negro, J.J.; Galván, I. Behavioural ecology of raptors. In Birds of Prey: Biology and Conservation in the XXI Century, 1st ed.; Sarasola, J.H., Grande, J.M., Negro, J.J., Eds.; Springer International: Cham, Switzerland, 2018; pp. 33–62. [Google Scholar]
- Tauler-Ametlller, H.; Pretus, J.L.; Hernández-Matías, A.; Ortiz-Santaliestra, M.E.; Mateo, R.; Real, J. Domestic waste disposal sites secure food availability but diminish plasma antioxidants in Egyptian vulture. Sci. Total Environ. 2019, 650, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Van Beest, F.; Van Den Bremer, L.; De Boer, W.F.; Heitkönig, I.M.; Monteiro, A.E. Population dynamics and spatial distribution of Griffon Vultures (Gyps fulvus) in Portugal. Bird Conserv. Int. 2008, 18, 102–117. [Google Scholar] [CrossRef][Green Version]
- Carlon, J. Resurgence of Egyptian Vultures in western Pyrenees, and relationship with Griffon Vultures. Br. Birds 1998, 91, 409–441. [Google Scholar]
- Bakka, H.; Rue, H.; Fuglstad, G.A.; Riebler, A.; Bolin, D.; Illian, J.; Krainski, E.; Simpson, D.; Lindgren, F. Spatial modeling with R-INLA: A review. Wiley Interdiscip. Rev. Comput. Stat. 2018, 10, e1443. [Google Scholar] [CrossRef]
- McAlpine, C.A.; Rhodes, J.R.; Bowen, M.E.; Lunney, D.; Callaghan, J.G.; Mitchell, D.L.; Possingham, H.P. Can multiscale models of species’ distribution be generalized from region to region? A case study of the koala. J. Appl. Ecol. 2008, 45, 558–567. [Google Scholar] [CrossRef]
- Blanco, G.; Cortés-Avizanda, A.; Frías, Ó.; Arrondo, E.; Donázar, J.A. Livestock farming practices modulate vulture diet-disease interactions. Glob. Ecol. Conserv. 2019, 17, e00518. [Google Scholar] [CrossRef]
- Reyes, Z.M.; García, J.M.P.; Paiz, M.M.; Zapata, J.A.S. El papel sanitario de los carroñeros en la ganadería extensiva. Quercus 2018, 394, 26–34. [Google Scholar]
- Marques, A.T.; Batalha, H.; Rodrigues, S.; Costa, H.; Pereira, M.J.R.; Fonseca, C.; Mascarenhas, M.; Bernardino, J. Understanding bird collisions at wind farms: An updated review on the causes and possible mitigation strategies. Biol. Conserv. 2014, 179, 40–52. [Google Scholar] [CrossRef]
- Carrete, M.; Sánchez-Zapata, J.A.; Benítez, J.R.; Lobón, M.; Montoya, F.; Donázar, J.A. Mortality at wind-farms is positively related to large-scale distribution and aggregation in griffon vultures. Biol. Conserv. 2012, 145, 102–108. [Google Scholar] [CrossRef]
- Palacín, C.; Farias, I.; Alonso, J.C. Detailed mapping of protected species distribution, an essential tool for renewable energy planning in agroecosystems. Biol. Conserv. 2023, 277, 109857. [Google Scholar] [CrossRef]
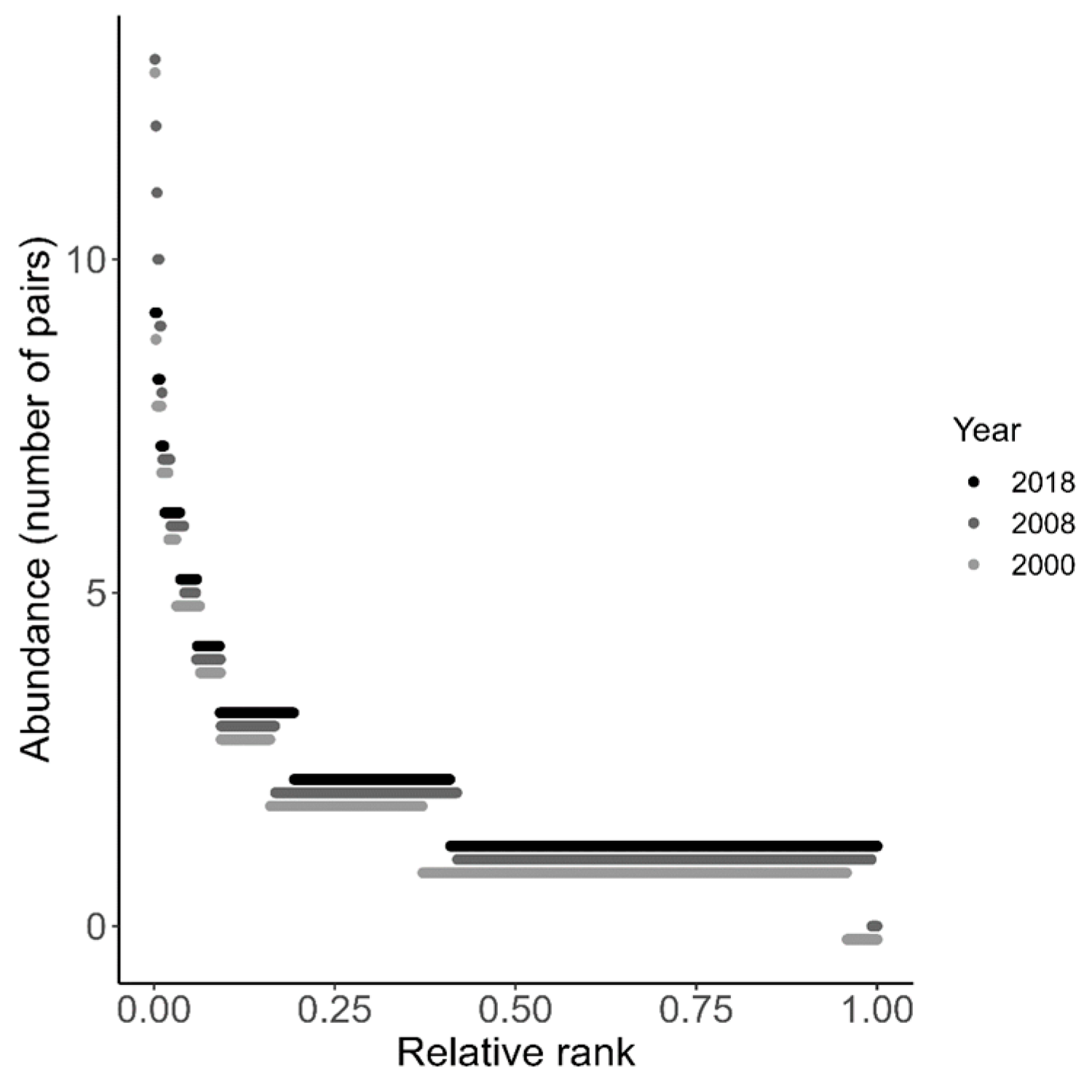
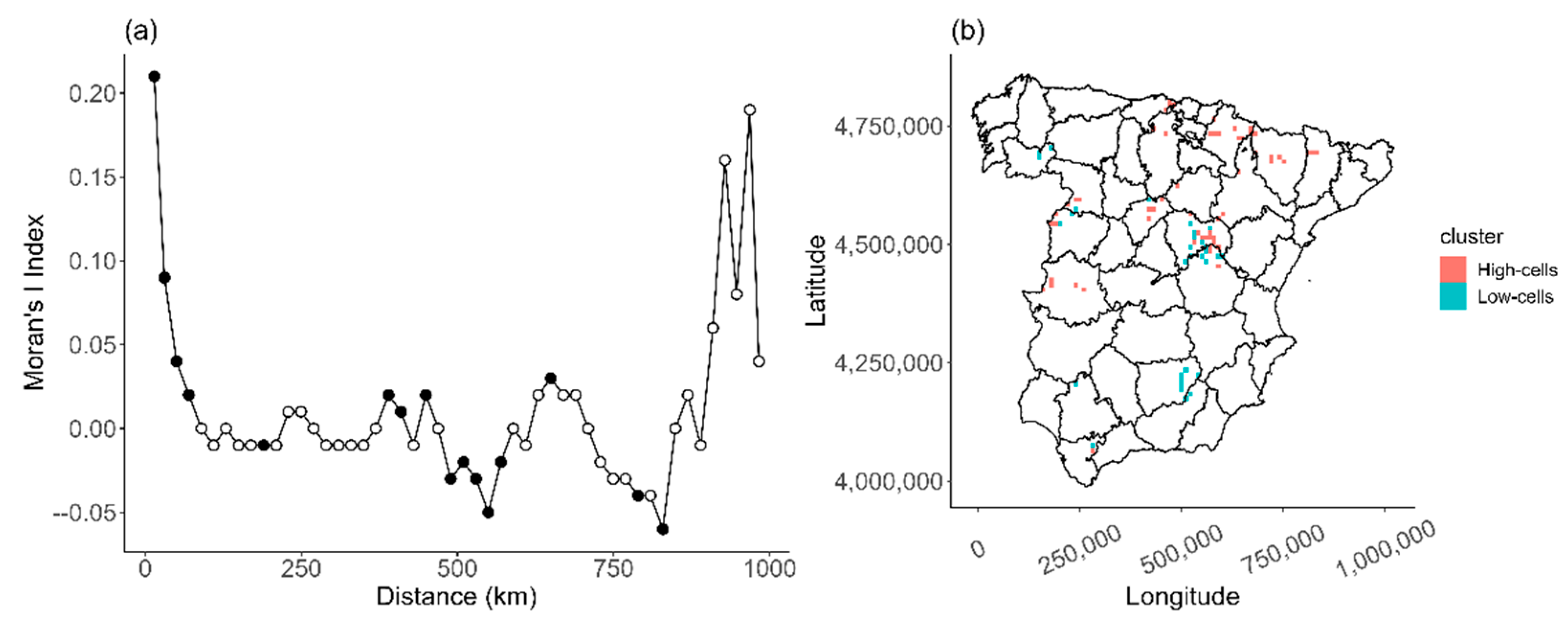
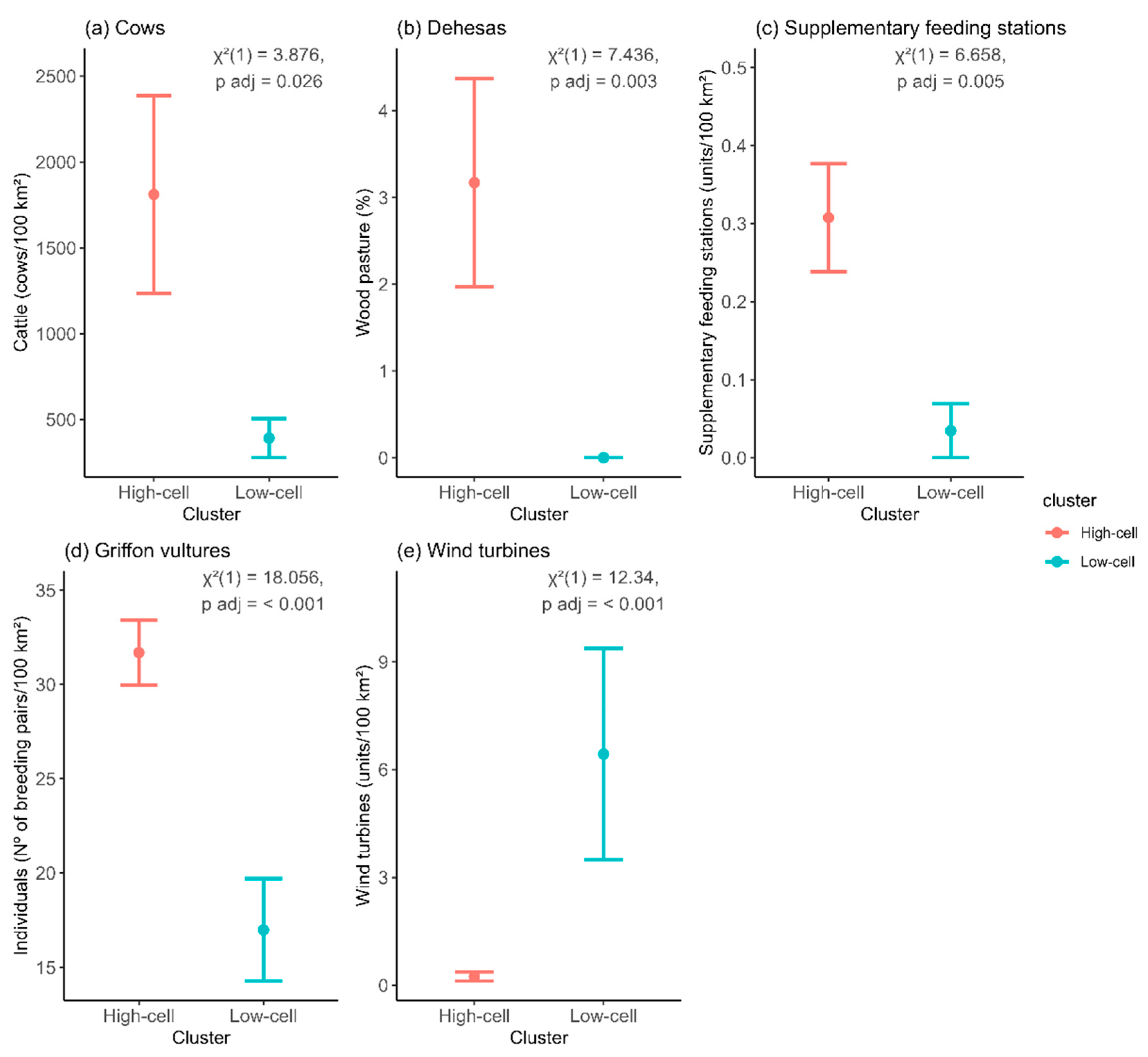
| Years | D* | P | Abundance Change | Occupancy Change |
|---|---|---|---|---|
| 2018–2008 | 0.992 | 0.648 | 8 | 6 |
| 2008–2000 | 0.970 | 0.615 | 94 | 25 |
| 2018–2000 | 0.918 | 0.640 | 102 | 31 |
| Model | Variables | df | Loglik | AICc | Delta | Weight |
|---|---|---|---|---|---|---|
| 1.1. | COW + GF + NP08 + Y + Y2 | 7 | −1365.67 | 2745.44 | 0.000 | 0.0046 |
| 1.2. | COW + GF + NP08 + Y + Y3 | 7 | −1365.69 | 2745.50 | 0.058 | 0.0045 |
| 1.3. | COW + GF + NP08 + Y2 + Y3 | 7 | −1365.73 | 2745.57 | 0.127 | 0.0043 |
| 1.4. | COW + GF + SFS + NP08 + Y + Y2 | 8 | −1365.04 | 2746.22 | 0.785 | 0.0031 |
| 1.5. | COW + GF + SFS + NP08 + Y + Y3 | 8 | −1365.07 | 2746.27 | 0.834 | 0.0030 |
| 1.6. | COW + GF + SFS + NP08 + Y2 + Y3 | 8 | −1365.10 | 2746.33 | 0.895 | 0.0029 |
| 1.7. | COW + GF + NP08 + X + X2Y + XY + XY2 | 9 | −1364.14 | 2746.46 | 1.023 | 0.0028 |
| 1.8. | COW + DEH + GF + NP08 + Y + Y2 | 8 | −1365.17 | 2746.48 | 1.039 | 0.0027 |
| 1.9. | COW + GF + NP08 + WTG + Y + Y2 | 8 | −1365.19 | 2746.52 | 1.080 | 0.0027 |
| 1.10. | COW + GF + NP08 + X + X2 + XY + XY2 | 9 | −1364.18 | 2746.54 | 1.099 | 0.0027 |
| 2.1. | COW + GF + NP00 + Y + Y2 | 7 | −1415.04 | 2844.19 | 0.000 | 0.004 |
| 2.2. | COW + GF + NP00 + Y + Y3 | 7 | −1415.08 | 2844.27 | 0.076 | 0.004 |
| 2.3. | COW + GF + NP00 + Y2 + Y3 | 7 | −1415.13 | 2844.36 | 0.168 | 0.004 |
| 2.4. | COW + GF + NP00 + WTG + Y + Y2 | 8 | −1414.27 | 2844.67 | 0.479 | 0.003 |
| 2.5. | COW + GF + NP00 + WTG + Y + Y3 | 8 | −1414.29 | 2844.72 | 0.527 | 0.003 |
| 2.6. | COW + GF + NP00 + WTG + Y2 + Y3 | 8 | −1414.32 | 2844.78 | 0.589 | 0.003 |
| 2.7. | COW + DEH + GF + NP00 + Y + Y2 | 8 | −1414.41 | 2844.97 | 0.773 | 0.003 |
| 2.8. | COW + DEH + GF + NP00 + Y + Y3 | 8 | −1414.48 | 2845.10 | 0.903 | 0.003 |
| 2.9. | COW + DEH + GF + NP00 + Y2 + Y3 | 8 | −1414.55 | 2845.25 | 1.052 | 0.002 |
| 2.10. | COW + DEH + GF + NP00 + WTG + Y + Y2 | 9 | −1413.57 | 2845.33 | 1.132 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerecedo-Iglesias, C.; Pretus, J.L.; Hernández-Matías, A.; Cortés-Avizanda, A.; Real, J. Key Factors behind the Dynamic Stability of Pairs of Egyptian Vultures in Continental Spain. Animals 2023, 13, 2775. https://doi.org/10.3390/ani13172775
Cerecedo-Iglesias C, Pretus JL, Hernández-Matías A, Cortés-Avizanda A, Real J. Key Factors behind the Dynamic Stability of Pairs of Egyptian Vultures in Continental Spain. Animals. 2023; 13(17):2775. https://doi.org/10.3390/ani13172775
Chicago/Turabian StyleCerecedo-Iglesias, Catuxa, Joan Lluís Pretus, Antonio Hernández-Matías, Ainara Cortés-Avizanda, and Joan Real. 2023. "Key Factors behind the Dynamic Stability of Pairs of Egyptian Vultures in Continental Spain" Animals 13, no. 17: 2775. https://doi.org/10.3390/ani13172775
APA StyleCerecedo-Iglesias, C., Pretus, J. L., Hernández-Matías, A., Cortés-Avizanda, A., & Real, J. (2023). Key Factors behind the Dynamic Stability of Pairs of Egyptian Vultures in Continental Spain. Animals, 13(17), 2775. https://doi.org/10.3390/ani13172775






