The Chicken cGAS–STING Pathway Exerts Interferon-Independent Antiviral Function via Cell Apoptosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Viruses
2.2. Antibodies
2.3. Cells and Cell Transfections
2.4. Molecular Cloning and Gene Mutations
2.5. Promoter-Driven Luciferase Reporter Gene Assay
2.6. RNA and DNA Extraction, Reverse Transcription and Quantitative PCR (qPCR)
2.7. Western Blotting Analysis
2.8. Plaque Assay
2.9. Viral Fluorescence Imaging
2.10. Flow Cytometry for Apoptosis Detection
2.11. Statistical Analysis
3. Results
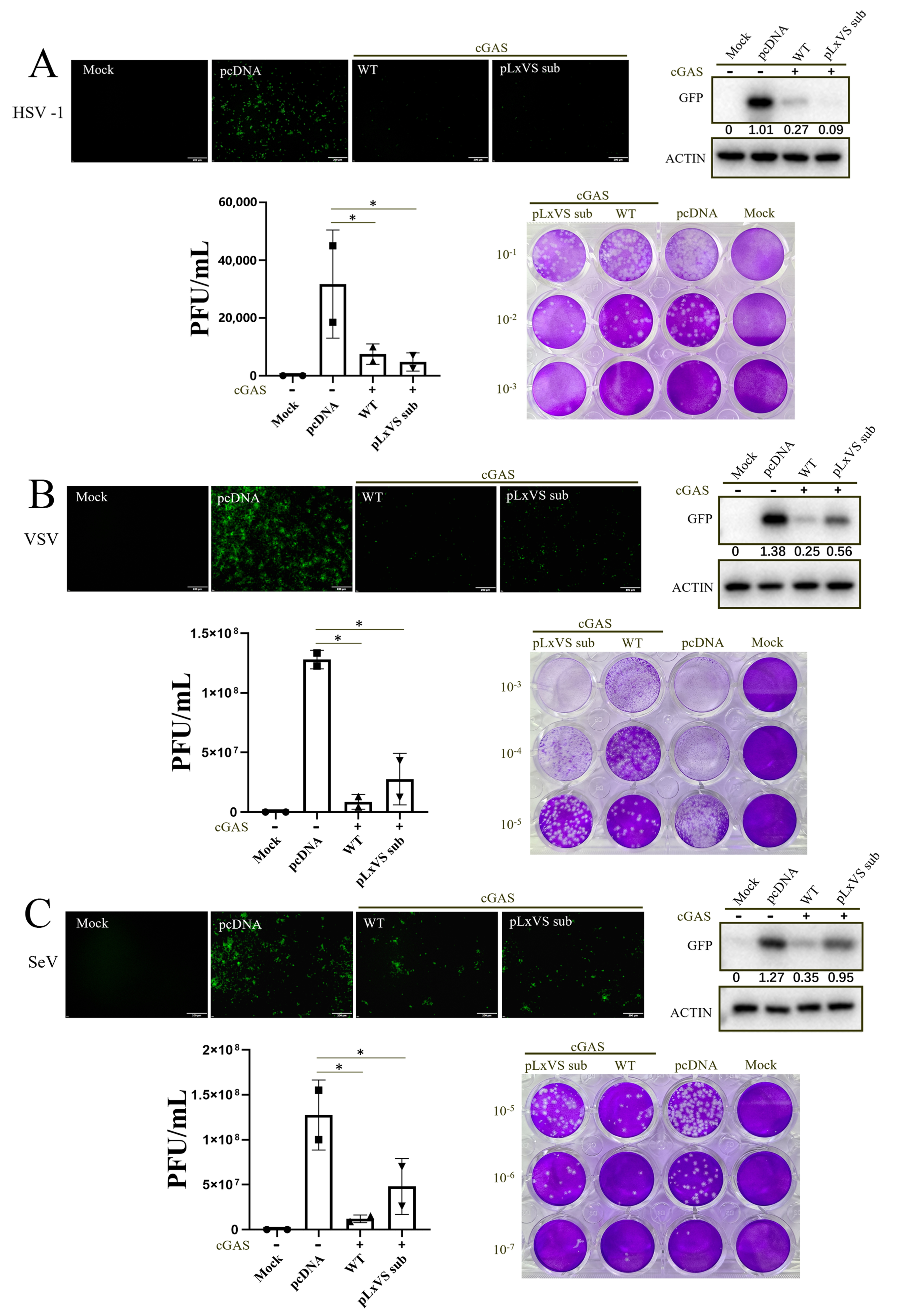
3.1. chSTING Exerts an IFN-Independent Antiviral Activity in Mammalian 293T Cells
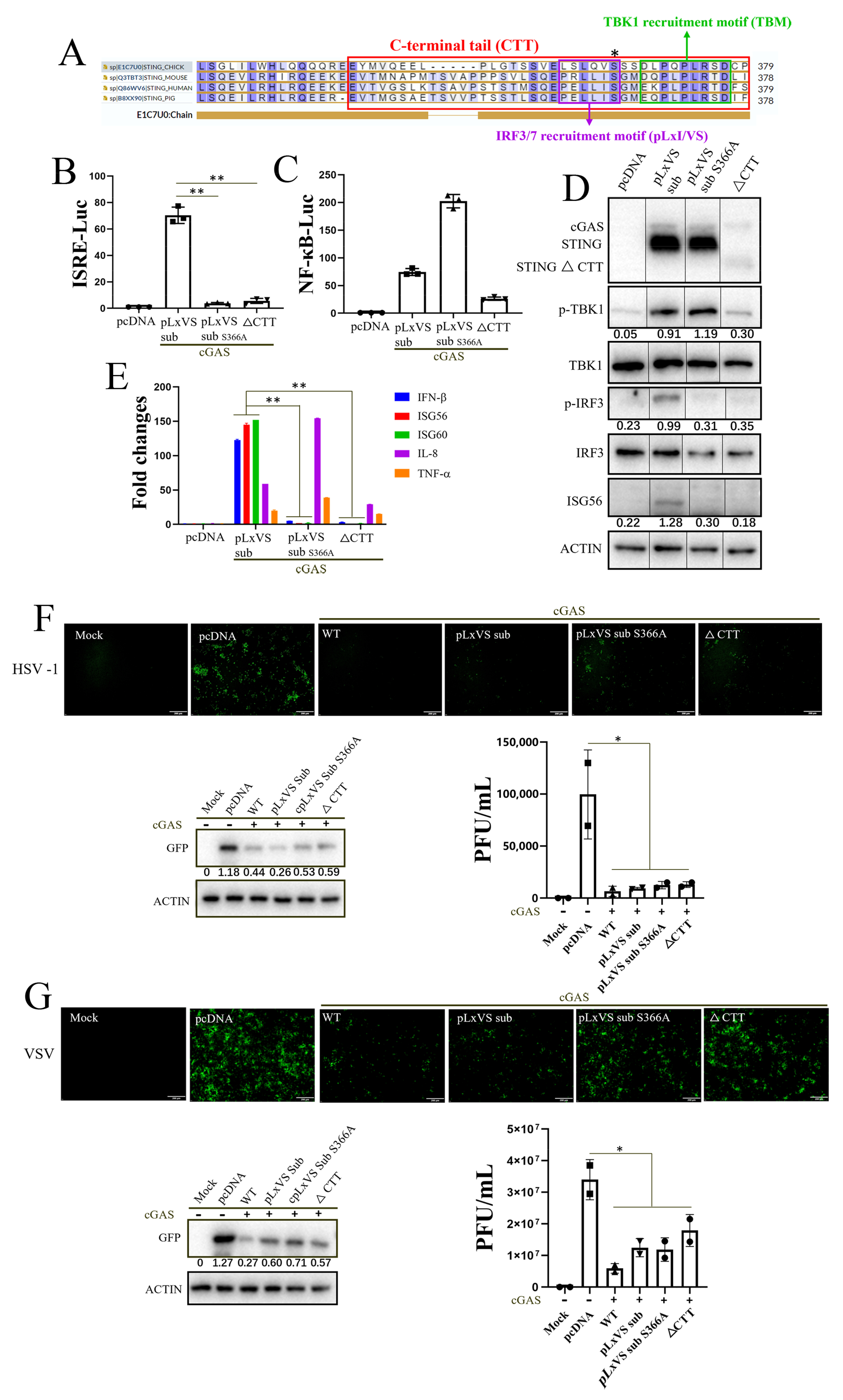
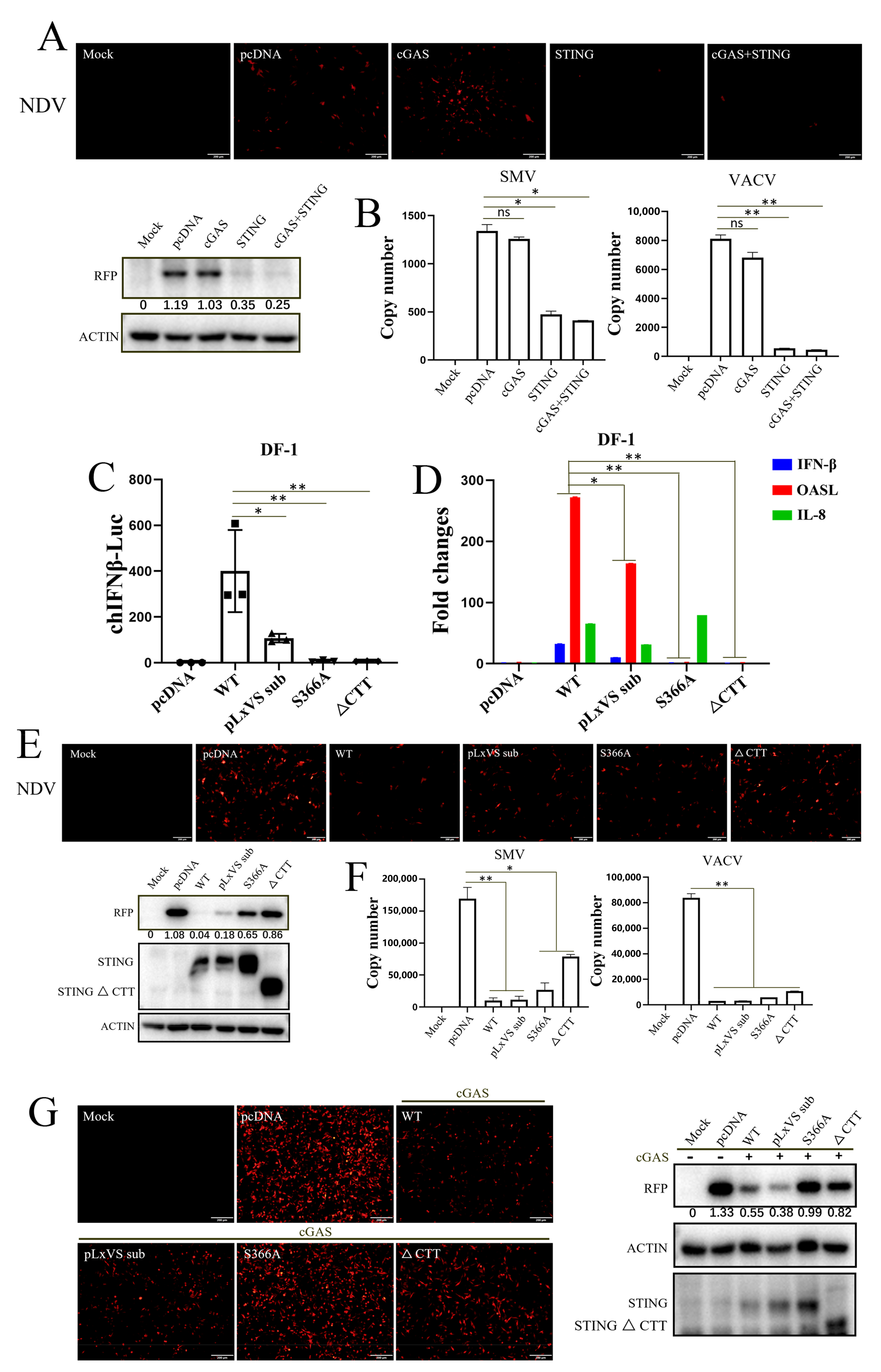
3.2. chSTING Exhibits a Robust IFN-Independent Antiviral Function in Chicken Cells
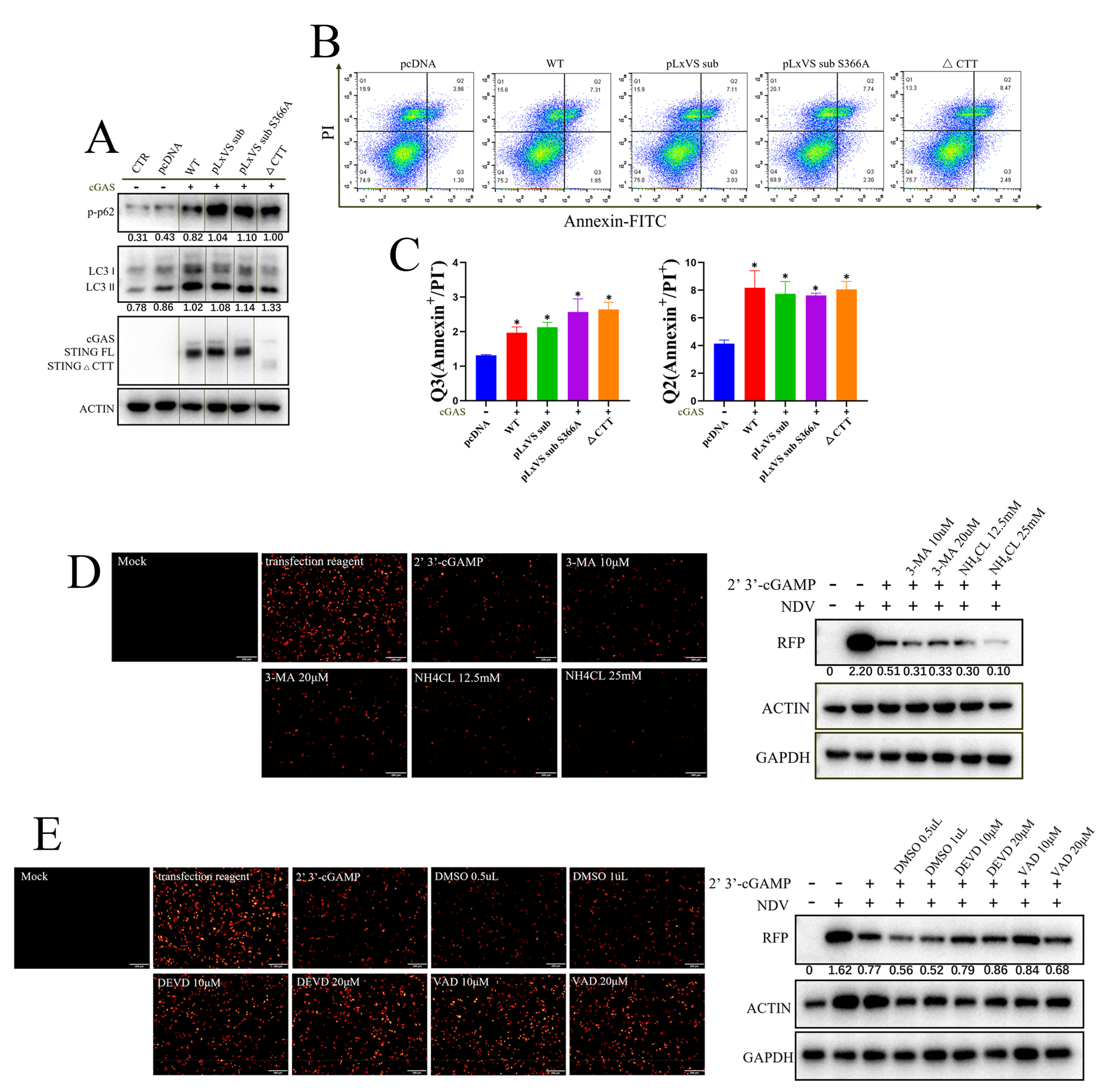
3.3. Apoptosis Rather Than Autophagy Is Involved in the Antiviral Function of chSTING
3.4. IRF7 Is Required for STING Antiviral Function in Chicken HD11 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Chen, Y.; Wang, H.Y.; Wang, R.F. Mechanisms and pathways of innate immune activation and regulation in health and cancer. Hum. Vaccin Immunother. 2014, 10, 3270–3285. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, A.; Bowie, A.G. Innate immune recognition of DNA: A recent history. Virology 2015, 479–480, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Hopfner, K.P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Burdette, D.L.; Monroe, K.M.; Sotelo-Troha, K.; Iwig, J.S.; Eckert, B.; Hyodo, M.; Hayakawa, Y.; Vance, R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011, 478, 515–518. [Google Scholar] [CrossRef]
- Zhang, C.; Shang, G.; Gui, X.; Zhang, X.; Bai, X.C.; Chen, Z.J. Structural basis of STING binding with and phosphorylation by TBK1. Nature 2019, 567, 394–398. [Google Scholar] [CrossRef]
- Zhao, B.; Shu, C.; Gao, X.; Sankaran, B.; Du, F.; Shelton, C.L.; Herr, A.B.; Ji, J.Y.; Li, P. Structural basis for concerted recruitment and activation of IRF-3 by innate immune adaptor proteins. Proc. Natl. Acad. Sci. USA 2016, 113, E3403-12. [Google Scholar] [CrossRef]
- Li, X.D.; Wu, J.; Gao, D.; Wang, H.; Sun, L.; Chen, Z.J. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 2013, 341, 1390–1394. [Google Scholar] [CrossRef]
- Schoggins, J.W.; MacDuff, D.A.; Imanaka, N.; Gainey, M.D.; Shrestha, B.; Eitson, J.L.; Mar, K.B.; Richardson, R.B.; Ratushny, A.V.; Litvak, V.; et al. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 2014, 505, 691–695. [Google Scholar] [CrossRef]
- Balka, K.R.; Louis, C.; Saunders, T.L.; Smith, A.M.; Calleja, D.J.; D’Silva, D.B.; Moghaddas, F.; Tailler, M.; Lawlor, K.E.; Zhan, Y.; et al. TBK1 and IKKepsilon Act Redundantly to Mediate STING-Induced NF-kappaB Responses in Myeloid Cells. Cell Rep. 2020, 31, 107492. [Google Scholar] [CrossRef]
- Zheng, W.; Xia, N.; Zhang, J.; Chen, N.; Meurens, F.; Liu, Z.; Zhu, J. How the Innate Immune DNA Sensing cGAS-STING Pathway Is Involved in Autophagy. Int. J. Mol. Sci. 2021, 22, 13232. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, A.; Xia, N.; Chen, N.; Meurens, F.; Zhu, J. How the Innate Immune DNA Sensing cGAS-STING Pathway Is Involved in Apoptosis. Int. J. Mol. Sci. 2023, 24, 3029. [Google Scholar] [CrossRef]
- Moretti, J.; Roy, S.; Bozec, D.; Martinez, J.; Chapman, J.R.; Ueberheide, B.; Lamming, D.W.; Chen, Z.J.; Horng, T.; Yeretssian, G.; et al. STING Senses Microbial Viability to Orchestrate Stress-Mediated Autophagy of the Endoplasmic Reticulum. Cell 2017, 171, 809–823.e13. [Google Scholar] [CrossRef]
- Liu, D.; Wu, H.; Wang, C.; Li, Y.; Tian, H.; Siraj, S.; Sehgal, S.A.; Wang, X.; Wang, J.; Shang, Y.; et al. STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 2019, 26, 1735–1749. [Google Scholar] [CrossRef]
- Gui, X.; Yang, H.; Li, T.; Tan, X.; Shi, P.; Li, M.; Du, F.; Chen, Z.J. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 2019, 567, 262–266. [Google Scholar] [CrossRef]
- Fischer, T.D.; Wang, C.; Padman, B.S.; Lazarou, M.; Youle, R.J. STING induces LC3B lipidation onto single-membrane vesicles via the V-ATPase and ATG16L1-WD40 domain. J. Cell Biol. 2020, 219, e202009128. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, Y.D.; Zheng, C. The crosstalk between the caspase family and the cGAS—STING signaling pathway. J. Mol. Cell Biol. 2021, 13, 739–747. [Google Scholar] [CrossRef]
- Petrasek, J.; Iracheta-Vellve, A.; Csak, T.; Satishchandran, A.; Kodys, K.; Kurt-Jones, E.A.; Fitzgerald, K.A.; Szabo, G. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl. Acad. Sci. USA 2013, 110, 16544–16549. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, D.; Sreevatsan, S.; Liu, C.; Yang, W.; Song, Z.; Yang, L.; Barrow, P.; Zhou, X. Mycobacterium bovis Induces Endoplasmic Reticulum Stress Mediated-Apoptosis by Activating IRF3 in a Murine Macrophage Cell Line. Front. Cell Infect. Microbiol. 2016, 6, 182. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.J.; Dobbs, N.; Sakai, T.; Liou, J.; Miner, J.J.; Yan, N. STING-mediated disruption of calcium homeostasis chronically activates ER stress and primes T cell death. J. Exp. Med. 2019, 216, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Sze, A.; Belgnaoui, S.M.; Olagnier, D.; Lin, R.; Hiscott, J.; van Grevenynghe, J. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe 2013, 14, 422–4234. [Google Scholar] [CrossRef] [PubMed]
- Böhnert, V.; Ritchie, C.; Li, L. IFN-Independent STING Signaling: Friend or Foe? Immunity 2020, 53, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xia, N.; Luo, J.; Zhang, Y.; Cao, Q.; Zhang, J.; Wang, Y.; Zhao, Y.; Zheng, W.; Chen, N.; et al. The Porcine Cyclic GMP-AMP Synthase-STING Pathway Exerts an Unusual Antiviral Function Independent of Interferon and Autophagy. J. Virol. 2022, 96, e0147622. [Google Scholar] [CrossRef]
- Li, S.; Yang, J.; Zhu, Y.; Ji, X.; Wang, K.; Jiang, S.; Luo, J.; Wang, H.; Zheng, W.; Chen, N.; et al. Chicken DNA Sensing cGAS-STING Signal Pathway Mediates Broad Spectrum Antiviral Functions. Vaccines 2020, 8, 369. [Google Scholar] [CrossRef]
- Jiang, S.; Luo, J.; Zhang, Y.; Cao, Q.; Wang, Y.; Xia, N.; Zheng, W.; Chen, N.; Meurens, F.; Wu, H.; et al. The Porcine and Chicken Innate DNA Sensing cGAS-STING-IRF Signaling Axes Exhibit Differential Species Specificity. J. Immunol. 2022, 209, 412–426. [Google Scholar] [CrossRef]
- Li, K.; Liu, Y.; Xu, Z.; Zhang, Y.; Luo, D.; Gao, Y.; Qian, Y.; Bao, C.; Liu, C.; Zhang, Y.; et al. Avian oncogenic herpesvirus antagonizes the cGAS-STING DNA-sensing pathway to mediate immune evasion. PLoS Pathog. 2019, 15, e1007999. [Google Scholar] [CrossRef]
- Gao, L.; Li, K.; Zhang, Y.; Liu, Y.; Liu, C.; Zhang, Y.; Gao, Y.; Qi, X.; Cui, H.; Wang, Y.; et al. Inhibition of DNA-Sensing Pathway by Marek’s Disease Virus VP23 Protein through Suppression of Interferon Regulatory Factor 7 Activation. J. Virol. 2019, 93, e01934-18. [Google Scholar] [CrossRef]
- Wang, J.; Ba, G.; Han, Y.Q.; Ming, S.L.; Wang, M.D.; Fu, P.F.; Zhao, Q.Q.; Zhang, S.; Wu, Y.N.; Yang, G.Y.; et al. Cyclic GMP-AMP synthase is essential for cytosolic double-stranded DNA and fowl adenovirus serotype 4 triggered innate immune responses in chickens. Int. J. Biol. Macromol. 2020, 146, 497–507. [Google Scholar] [CrossRef]
- Wu, J.; Dobbs, N.; Yang, K.; Yan, N. Interferon-Independent Activities of Mammalian STING Mediate Antiviral Response and Tumor Immune Evasion. Immunity 2020, 53, 115–126.e5. [Google Scholar] [CrossRef]
- Yamashiro, L.H.; Wilson, S.C.; Morrison, H.M.; Karalis, V.; Chung, J.J.; Chen, K.J.; Bateup, H.S.; Szpara, M.L.; Lee, A.Y.; Cox, J.S.; et al. Interferon-independent STING signaling promotes resistance to HSV-1 in vivo. Nat. Commun. 2020, 11, 3382. [Google Scholar] [CrossRef]
- Yum, S.; Li, M.; Fang, Y.; Chen, Z.J. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc. Natl. Acad. Sci. USA 2021, 118, e2100225118. [Google Scholar] [CrossRef]
- Liu, Y.; Gordesky-Gold, B.; Leney-Greene, M.; Weinbren, N.L.; Tudor, M.; Cherry, S. Inflammation-Induced, STING-Dependent Autophagy Restricts Zika Virus Infection in the Drosophila Brain. Cell Host Microbe 2018, 24, 57–68.e3. [Google Scholar] [CrossRef]
- Barber, G.N. Host defense, viruses and apoptosis. Cell Death Differ. 2001, 8, 113–126. [Google Scholar] [CrossRef]
- Shim, J.M.; Kim, J.; Tenson, T.; Min, J.Y.; Kainov, D.E. Influenza Virus Infection, Interferon Response, Viral Counter-Response, and Apoptosis. Viruses 2017, 9, 223. [Google Scholar] [CrossRef]
- Pujhari, S.; Rasgon, J.L.; Zakhartchouk, A.N. Anti-apoptosis in porcine respiratory and reproductive syndrome virus. Virulence 2016, 7, 610–611. [Google Scholar] [CrossRef][Green Version]
- Ren, Y.; Wang, A.; Wu, D.; Wang, C.; Huang, M.; Xiong, X.; Jin, L.; Zhou, W.; Qiu, Y.; Zhou, X. Dual inhibition of innate immunity and apoptosis by human cytomegalovirus protein UL37x1 enables efficient virus replication. Nat. Microbiol. 2022, 7, 1041–1053. [Google Scholar] [CrossRef]
- You, Y.; Cheng, A.C.; Wang, M.S.; Jia, R.Y.; Sun, K.F.; Yang, Q.; Wu, Y.; Zhu, D.; Chen, S.; Liu, M.F.; et al. The suppression of apoptosis by α-herpesvirus. Cell Death Dis. 2017, 8, e2749. [Google Scholar] [CrossRef]
- Mehta, N.; Taylor, J.; Quilty, D.; Barry, M. Ectromelia virus encodes an anti-apoptotic protein that regulates cell death. Virology 2015, 475, 74–87. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Lv, M.; Zhang, D.; Cao, Q.; Xia, N.; Luo, J.; Zheng, W.; Chen, N.; Meurens, F.; Zhu, J. The Chicken cGAS–STING Pathway Exerts Interferon-Independent Antiviral Function via Cell Apoptosis. Animals 2023, 13, 2573. https://doi.org/10.3390/ani13162573
Jiang S, Lv M, Zhang D, Cao Q, Xia N, Luo J, Zheng W, Chen N, Meurens F, Zhu J. The Chicken cGAS–STING Pathway Exerts Interferon-Independent Antiviral Function via Cell Apoptosis. Animals. 2023; 13(16):2573. https://doi.org/10.3390/ani13162573
Chicago/Turabian StyleJiang, Sen, Mengjia Lv, Desheng Zhang, Qi Cao, Nengwen Xia, Jia Luo, Wanglong Zheng, Nanhua Chen, François Meurens, and Jianzhong Zhu. 2023. "The Chicken cGAS–STING Pathway Exerts Interferon-Independent Antiviral Function via Cell Apoptosis" Animals 13, no. 16: 2573. https://doi.org/10.3390/ani13162573
APA StyleJiang, S., Lv, M., Zhang, D., Cao, Q., Xia, N., Luo, J., Zheng, W., Chen, N., Meurens, F., & Zhu, J. (2023). The Chicken cGAS–STING Pathway Exerts Interferon-Independent Antiviral Function via Cell Apoptosis. Animals, 13(16), 2573. https://doi.org/10.3390/ani13162573







