Simple Summary
African swine fever, as an acute, contact-transmitted infection, has a very high mortality and infectivity, and there are no effective drugs that can treat the disease. Therefore, it is necessary to take biosecurity measures in time at the beginning of the disease outbreak. In this article, media coverage is introduced into the African swine fever model, and the results indicate that real-time media coverage of the African swine fever epidemic is beneficial for breeders to scientifically prevent the spread of the epidemic in a timely manner. Through the evaluation of specific prevention and control measures, we suggest that timely disinfection and sterilization measures taken by breeding personnel after receiving relevant reports of the swine fever epidemic can effectively control the spread of the disease.
Abstract
African swine fever is a highly contagious virus that causes pig disease. Its onset process is short, but the mortality rate is as high as 100%. There are still no effective drugs that have been developed to treat African swine fever, and prevention and control measures are currently the best means to avoid infection in pig herds. In this paper, two fractional order mathematical models with media coverage are constructed to describe the transmission of African swine fever. The first model is a basic model with media coverage, and no control measures are considered. For this model, the reproduction number is obtained by using the next generation matrix method. Then, the sufficient conditions for the existence and stability of two equilibriums are obtained. Based on the first model, the second model is established incorporating two control measures. By using Pontryagin’s maximal principle, the optimal control solution is derived. After that, some numerical simulations are performed for the two models to verify the theoretical results. Both the qualitative analysis and numerical results indicate that timely media coverage combined with disinfection control measures is crucial to preventing the spread of disease.
1. Introduction
African swine fever (ASF) is an acute, hemorrhagic and severe infectious disease caused by African swine fever virus (ASFV) infecting domestic pigs and various wild pigs. It can infect pigs of all breeds and ages, and the mortality rate can be up to 100% [1,2]. ASF has been present in sub-Saharan African countries since 1921, when it was first reported in Kenya [3,4], and spread to Western Europe and Latin America around 1975. Although most of the disease was eradicated in time, it did not fundamentally solve the problem. After 2007, ASF once again spread in many parts of the world, especially in Russia and its surrounding areas. China, the world’s largest producer and consumer of pork, was also hit by ASF in 2018. For those countries that produce and export a lot of pork every year, the ravages of ASF can be devastating to the pig industry, the most typical example of which is Denmark [5]. Because of its serious economic consequences, ASF has been designated as a notifiable animal disease by the World Organization for Animal Health [6], and has attracted the attention of relevant experts.
So far, no vaccines or drugs have been found that can effectively prevent and control ASF. Biosecurity is still the best means to fight against ASFV at present and in the future. Dione et al., in 2020 used a randomized controlled trial to assess the impact of farmers’ participatory training on ASF control-related biosafety knowledge, attitude and practice in two regions of Uganda [7]. In 2022, Stoffel et al. investigated the driving factors behind the introduction of ASF into compartments and classified compartments based on the risk of the ASF introduction. They believe that ASF is increasingly an anthropogenic problem. In order to strengthen compliance with biosecurity measures, updating compartment standards and addressing knowledge gaps among compartment personnel in ASF are the most crucial [8].
According to research, the source of infection of ASF mainly includes two parts: invisible infected pigs and diseased pigs. The body fluids and tissue fluids of these two kinds of pigs contain ASFV. Healthy pigs will be infected through direct contact with infected pigs or pollutants [9]. In addition, healthy pigs will also be infected if they eat swill containing viruses or are bitten by ticks carrying ASFV [10]. Among them, ticks play an important role as vectors in the transmission of ASF. In their 2017 work, Frant et al. proposed that the sylvatic cycle composed of wild pigs and various ticks may continue to be a major issue in controlling ASF and may cause new outbreaks of this disease in new regions of the world [11]. In 2021, Kouidere et al. established a model describing the transmission of ASF between pigs and ticks, and they used Pontryagin’s maximum principle to derive the optimal strategy for reducing the number of infected pigs and ticks [12].
According to the transmission characteristics of ASF, many mathematical models have been established to study how to effectively control the spread of the disease. For example, Barongo et al. developed a stochastic model to assess the impact of the timing of different control strategies on disease-related mortality. Through numerical simulation, it was found that if biosafety measures were implemented before 14 days after the outbreak of ASF, 74% of pigs could avoid infection. They also hypothesized that if there were effective vaccinations to enhance immunity during this period, 91% of pig deaths could be prevented [13]. In 2020, Zhang et al. established a mathematical model to study the transmission mechanism and control strategy of ASF in large farms, indicating the necessity of disinfection measures and staff management measures in pig farms [14]. In 2022, Song et al. developed a mathematical model with the asymptomatic infection and transmission of other pollution sources to investigate the impact of culling on the spread of ASF [15].
There are many mathematical models being proposed to study the control effects of different control measures on ASF [3,6,12,16,17]. All of these efforts indicate that in the early stages of the epidemic, if relevant measures can be taken in a timely manner to prevent the further spread of the epidemic, it can effectively reduce the mortality rate of pigs. It can be observed that timely reporting of disease information plays a crucial role in controlling the epidemic. We are in the era of internet information sharing, and we can fully utilize this resource to quickly and effectively let people know how to prevent and control diseases. For example, through media coverage, people can learn about the source of infection, transmission route, clinical symptoms, preventive measures and other related knowledge to keep people alert. Different people will have different reactions after receiving the news about the disease outbreak, which can be divided into two situations. Some people reduce the contact rate between healthy individuals and infected individuals by means of isolation, increasing the safe distance, wearing masks, etc., so as to reduce the infection. This is called a weak negative feedback effect of information [18,19]. However, others ensure that healthy individuals are not infected by regular disinfection of their living environment and tableware, which is known as a strong negative feedback effect of information [20,21]. There is literature demonstrating that media coverage is indeed effective in reducing the spread of disease [22,23,24]. In [25,26], the authors proposed mathematical models, including nonlinear function as the transmission rate, and they studied the influence of media coverage on infectious diseases. In 2017, Zhao et al. considered an SIR epidemic model combining time delay and media coverage [27].
Differential equations are useful mathematical tools to modeling and analyzing different problems, such as in applied science, engineering, and biological systems. The relevant research shows that many aspects of these fields have a temporal memory [28,29]. As a generalization of the integer differential equation, the fractional order differential equation is a theory about the differential and integral of any order [30]. It can be used as a tool to better describe different fields with memory and genetic characteristics. However, integer order differential equations cannot explain this feature very well. Therefore, in recent years, more and more fractional order differential equations are used to describe problems in the fields of epidemiology and other fields [28,29,30,31,32]. It has been demonstrated that fractional order models are better or more suitable than integer order models [33]. Therefore, it is of practical significance to introduce fractional order differential equations into the study of ASF.
Based on the above discussion, in this article two fractional order ASF models with media coverage are established to explore the transmission and spread of African swine fever. Compared to integer order models, fractional order models have not been widely used in the practical application of epidemic models. As an extension of integer order models, fractional order models can better interpret the memory during disease transmission. Moreover, when taking control measures in practice, the workload of the staff is not fixed, so optimal control is more practical than constant control. Thus, the research approach of this article is worth exploring.
The structure of this article is arranged as follows: In Section 2, a basic model with media coverage is proposed, and then another model with control measures is constructed, together with the meanings and value sources for the parameters in the models. The main results are listed in Section 3. Summaries and discussions are presented in Section 4. In the Section 5, some prospects for future research directions on this topic are provided.
2. Materials and Methods
ASF is mainly transmitted through direct contact with diseased pigs and ASFV contaminants in the environment. According to [34], we find that the ASF data from China mainly including the number of infected pigs and the pollution sources in the environment. It is assumed that when farmers receive the news of the local outbreak of ASF, they will reduce the probability of infection by isolating sick pigs and feeding pigs without using swill or meal waste.
2.1. Models Formulation
Motivated by [14,15,16,25,26], we establish a fractional order ASFV model with media coverage, as follows:
with initial conditions
Here, the factional order derivative is used in the Caputo sense for systems (1). The total population, denoted as , is divided into three classes: susceptible population , symptomatic infectious population , and asymptomatic infectious population . represents the density of ASFV in the environment. For this model, we will mainly consider the influence of media coverage on the spread of ASF.
In order to further study the effect of media coverage on the prevention and control of ASF, two control measures, denoted as and , are added to the basic model (1). Then, the following model with control measures is derived.
where represent the transmission rate before media alert. The term , measures the reduction of the virus transmission rate when infection is reported in the media. Because the media coverage cannot prevent the disease from spreading completely, we have , . The half-saturation constant reflects the impact of media coverage on the contact transmission. Here, the Holling II type functional response ( and ) is adopted to describe the nature of saturate transmission or psychological effects [25,35,36,37]. The detailed biological meanings of variables and parameters in systems (1) and (2) are listed in Table 1.
The values of most parameters in Table 1 are derived from references [12,15,27]. In order to adapt to the new models, this article has made appropriate modifications to the values of parameters and d within a reasonable range based on [15].
2.2. Methods
- (i)
- The next generation matrix method is used to obtain the basic reproduction number.
- (ii)
- The Descartess rule of signs is used to determine the existence of a positive equilibrium.
- (iii)
- The eigenvalue method, Routh-Hurwitz criteria and LaSalle’s invariance principle are used to prove the stability of two equilibriums.
- (iv)
- The Pontryagin’s maximum principle is used to derive the formula for the optimal solution of System (2).
- (v)
- The Adams-type predictor corrector method and MATLAB software are used for the numerical simulations.
3. Results
3.1. Qualitative Analysis Results for System (1)
To be biologically meaningful, it is important to prove that the solutions of system (1) with any nonnegative initial data are positive and bounded.
To prove our result, we list the following lemma, which is from [38].
Lemma 1.
Assume that and , then we have
- (i)
- If , for , then is non-decreasing for each .
- (ii)
- If , for , then is non-increasing for each .
Denote as the solution of system (1) with any positive initial value; then, we have the following result.
Theorem 1.
The proof of Theorem 1 requires the use of Theorem 3.4 in [39]. The details of the proof is in Appendix A.
Theorem 1 indicates that is positively invariant with respect to system (1). Thus, in the next of this section, we only need to consider the dynamics of system (1) within .
The epidemiological definition of the basic reproduction number is the average number of secondary cases produced by one infected individual introduced into a population of susceptible individuals. By using the next generation matrix method [40], the basic reproduction number of system (1) is derived as follows
where denotes the spectral radius of a matrix *, and
Remark 1.
In Equation (3), , , represent the basic reproduction number for the transmission of the symptomatic population, asymptomatic population, and environmental pollutant, respectively.
In order to obtain the equilibriums and let the right side of system (1) equal to zero, we can obtain the following algebraic equation
A simple calculation shows that in Equation (4) there always exists a trivial solution . Denote the positive solution of Equation (4) as ; then, we have
Substituting Equation (5) into the first equation of the Equation (4), we know that is the non-negative root of the following equation
where
and
According to Descartes’s rule of signs [41], we obtain the relation between the signs of the roots and the coefficients , , and , which are listed in Table 2.

Table 2.
Descartes Sign Rule for Equation (6).
When , it is easy to obtain , . According to the result in Table 2, we know that Equation (6) has exactly one positive solution, . Thus, the following result is derived.
Theorem 2.
Next, we will discuss the local and global stability of the disease-free equilibrium .
Theorem 3.
If , then the disease-free equilibrium is locally and asymptotically stable for system (1).
The proof of this theorem is in the Appendix B.
Theorem 4.
If , then the disease-free equilibrium is globally and asymptotically stable for system (1).
Proof of Theorem 4.
Consider the following Lyapunov function
and the derivative of L along the solution of system (1) is
If , we can obtain . In addition, if and only if , . The maximum invariant set of system (1) on the set is the singleton . According to the LaSalle’s invariance principle, we know that is global asymptotically stable if . This completes the proof of the theorem. □
For the endemic equilibrium of system (1), we have the following result.
Theorem 5.
If > 1, then the endemic equilibrium exists within Γ, and
- (i)
- When , the endemic equilibrium is locally asymptotically stable, provided that
- (ii)
- When , the above conditions are sufficient but not necessary for the local asymptotic stability of the endemic equilibrium . In fact, is still locally asymptotically stable if all eigenvalues of Equation (A4) satisfy
The proof of this theorem can be derived according to the Routh-Hurwitz criteria [42] and Lemma 4 in [43], and the detailed proof is in Appendix C.
3.2. Examples and Numerical Simulation Results for System (1)
In this subsection, we will establish some examples and perform some numerical simulations to verify the results obtained in the previous subsection. In addition, the sensitive analysis of some parameters is also taken for system (1). In particular, the effect of media coverage is discussed. In this paper, we use an Adams-type predictor-corrector method and MATLAB software to solve fractional order differential and integral equations.
Example 1.
Fix the following parameter values: , , , , , , , , , , , and . In this case, we obtain .
- (i)
- In Figure 1, the initial value is = [164,000, 470, 100, 300], and α have different values (). Figure 1 shows that if , then the disease-free equilibrium is always asymptotically stable for for all .
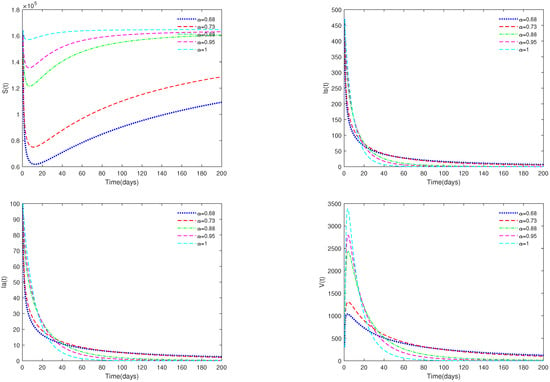 Figure 1. Time series of system (1) for different values of . Here, .
Figure 1. Time series of system (1) for different values of . Here, . - (ii)
- In Figure 2, the value of α is fixed to , and different initial values are taken. = [164,000, 470, 100, 300], [164,000, 400, 170, 300], [163,000, 370, 500, 500], [160,000, 1070, 700, 600]. Figure 2 indicates that different initial values do not affect the stability of the disease-free equilibrium of system (1).
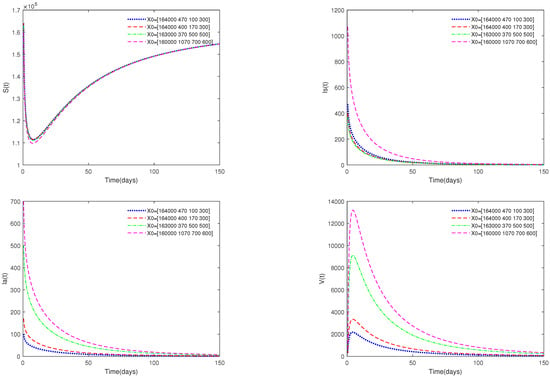 Figure 2. Time series of system (1) for different initial values. Here, .
Figure 2. Time series of system (1) for different initial values. Here, .
Example 2.
Fix the following parameter values: , , , , , , , , , , , and . In this case, we get .
- (i)
- In Figure 3, the initial value is fixed to = [164,000, 470, 100, 300], and α have different values (). Figure 3 shows that if , then the endemic equilibrium is always asymptotically stable for all .
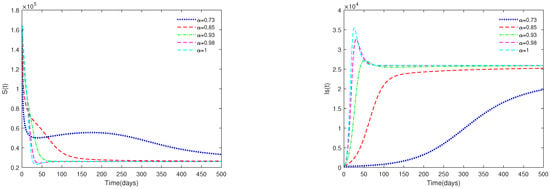
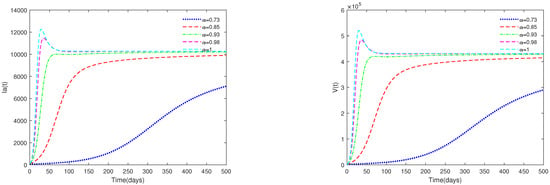 Figure 3. Time series of system (1) for different values of . Here, .
Figure 3. Time series of system (1) for different values of . Here, . - (ii)
- In Figure 4, the value of α is fixed to , and different initial values are taken as = [164,000, 470, 100, 300], [163,000, 370, 500, 500], [160,000, 1070, 700, 600]. Figure 4 indicates that different initial values do not affect the stability of the endemic equilibrium of system (1).
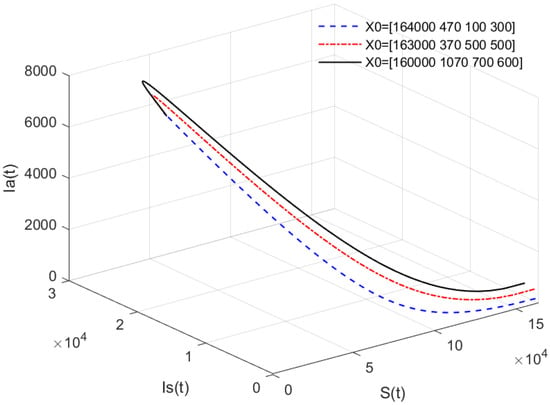 Figure 4. Phase portrait of , , and for different initial values. Here, .
Figure 4. Phase portrait of , , and for different initial values. Here, .
Example 3.
For the following parameter values: , , , , , , , , , , and .
- (i)
- In Figure 5, the value of α is fixed to 0.9, and different values for d (d = 0.0060608, 0.004060, 0.003060, 0.002060) are taken, in order to analyze the sensitivity of the mortality parameter d on system (1).
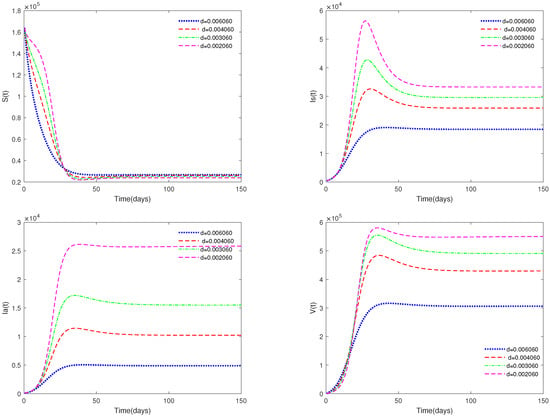 Figure 5. Time series of system (1) for different values of d.
Figure 5. Time series of system (1) for different values of d. - (ii)
- In Figure 6, the value α is fixed to 0.98, and different values for φ (φ = 0.05264, 0.04264, 0.03264, 0.02264) are taken, in order to analyze the sensitivity of parameter φ on system (1).
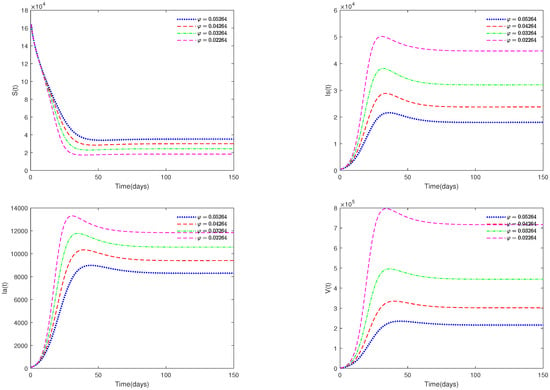 Figure 6. Time series of system (1) for different values of .
Figure 6. Time series of system (1) for different values of .
Example 4.
Fix the following parameter values: , , , , , , , , , and .
Figure 7 shows the impact of media coverage on system (1). From this figure, we can observe that the infected population (both and ) will tend to be a higher level if there is no media coverage (, ); while they will tend to be a lower level if there is media coverage (, ).
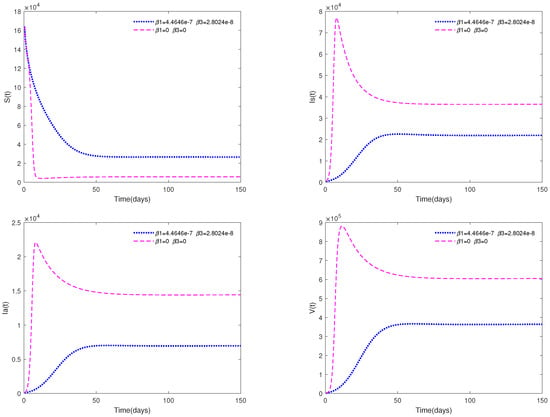
Figure 7.
Time series of system (1) with media coverage (in blue) or without media coverage (in purple).
The results of the numerical simulation are summarized, as follows.
Remark 2.
- (i)
- Figure 1 and Figure 2 show that if , then the disease-free equilibrium is always stable. If the basic reproduction number , that is, the number of healthy pigs infected by a diseased pig during its average disease period does not exceed 1, then the disease will eventually disappear, and this result is consistent with reality. The value of α can affect the speed towards the equilibrium. The initial values will not affect the stability, which is in line with Theorems 1 and 4.
- (ii)
- Figure 3 and Figure 4 indicate that if , then the disease-free equilibrium is unstable and the endemic equilibrium exists. If the basic reproduction number , that is, if the number of healthy pigs infected by a diseased pig during its average disease period is more than 1, then the disease will break out in this region and become an endemic. The value of α will affect the speed towards the endemic equilibrium . The initial values will not affect the stability, which is in accordance with Theorems 1 and 2.
- (iii)
- Figure 5 and Figure 6 show the sensitivity analysis for parameters d and φ. Through observation, it can be observed that the mortality rate d of pigs and the clearance rate φ of viruses have a significant impact on system (1). Therefore, it is reasonable for us to consider specific control measures in system (2) as removing diseased pigs and strengthening the disinfection and sterilization of pig breeding environments.
- (iv)
3.3. Qualitative Analysis Results for System (2)
In this subsection, we will analyze the fractional optimal control of system (2). Similar to the approach in Section 3.1, it is easy to prove that system (2) with any positive initial value has a unique positive solution that remains within .
Next, we will use the Hamiltonian function and Pontryagin’s Maximum Principle [44] to describe the optimal control problem.
The objective function is defined as
where T is the final time, and the parameters , are positive constants to keep a balance in the size of and ; , are positive weight parameters which are associated with the control measures and . In system (2), the control measures and , represent the clearance rate of the suspected diseased population and the elimination rate of bacteria in the environment by using alkaline disinfectant at a given time t, respectively. The goal is to minimize the populations which are already infected and the contaminants in the environment. Thus, we need to find the optimal solution and that satisfy
where
Define the Lagrangian and Hamiltonian function, as follows.
Lagrangian function
Hamiltonian function
where , , are the adjoint variables.
Theorem 6.
Given the optimal control and the corresponding solution of system (2), there exists adjoint variables satisfying the following equations.
with the transversal conditions
Furthermore, for the formula of optimal solution are given by
The proof of this theorem is in Appendix D.
3.4. Examples and Numerical Simulation Results for System (2)
Example 5.
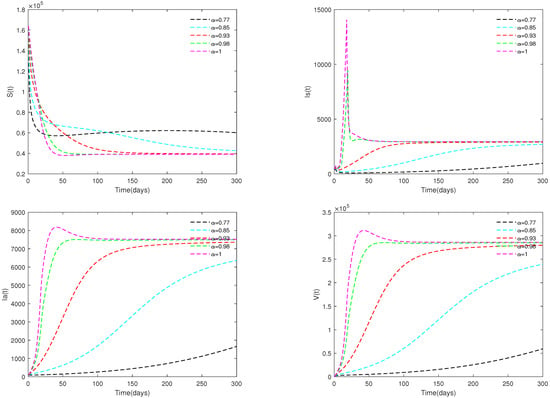
Fix the following parameter values: , , , , , , , , , , , , , , , and . In this case, we obtain .
Fix the initial value as = [164,000, 470, 100, 300], and different α values ( 0.98 and 1) are taken. Figure 8 shows that the value of parameter α affects the speed towards the stable state.

Figure 8.
Optimal solutions for system (2) with different values of . Here, .
Example 6.
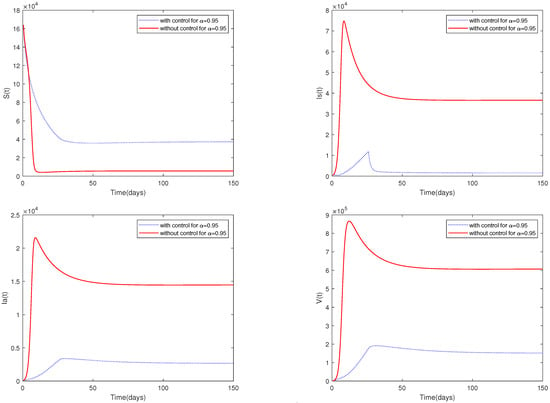
For the following parameter values: , , , , , , , , , , and initial value = [164,000, 470, 100, 300].
Figure 9 shows the dynamic behavior of system (2) with control measures and media coverage (, , , ) or without control measures and media coverage (, , , ).

Figure 9.
Optimal solutions for system (2) with control measures or without control measures.
Example 7.
Fix the following parameter values: , , , , , , , , , , , , , , , and .
- (i)
- In Figure 10, the initial value is fixed to = [164,000, 470, 100, 300]. For different values of α, this figure demonstrates the optimal solution of and when the upper limit of , is relatively small (realistically reasonable).
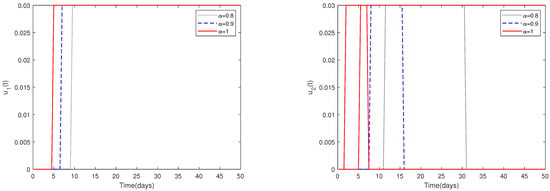 Figure 10. The optimal solutions of and for system (2) with different values of . Here, the upper limit of , is relatively small (realistically reasonable).
Figure 10. The optimal solutions of and for system (2) with different values of . Here, the upper limit of , is relatively small (realistically reasonable). - (ii)
- In Figure 11, the initial value is fixed to = [164,000, 470, 100, 300]. For different values of α, this figure shows the optimal solution of and when the upper limit of , is relatively larger (realistically unreasonable).
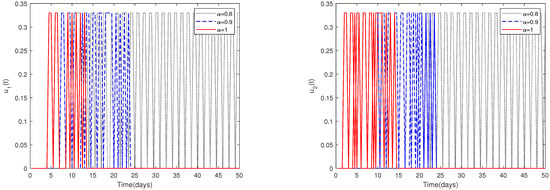 Figure 11. The optimal solutions of and for system (2) with different values of . Here, the upper limit of , is relatively big (realistically unreasonable).
Figure 11. The optimal solutions of and for system (2) with different values of . Here, the upper limit of , is relatively big (realistically unreasonable).
The numerical simulation results for system (2) are summarized as follows.
Remark 3.
- (i)
- When , Figure 8 shows that for all , the corresponding optimal solution tend to be stable with different speeds. This indicates that under the values of Example 3, ASF will outbreak in a certain region, and it will gradually become an endemic.
- (ii)
- A comparison between Figure 7 and Figure 9 shows that media coverage combined with control measures can suppress the spread of ASF more effectively. That is to say, if pig farmers take timely measures to eliminate suspected infected pigs and disinfect the environment of pig farms on a large scale after receiving media reports of the outbreak of ASF in the local area, they can greatly reduce the infection and help to prevent the spread of the epidemic.
- (iii)
- Since the magnitudes change dramatically for different parameters, choosing a suitable upper limit of , is important.Figure 10 shows that if the upper limit of , is relatively small, then the optimal control solutions and can be suitably solved. However, if the upper limit of , is relatively big, then the optimal control solutions and cannot be solved suitably, as shown in Figure 11. In fact, obvious and chaotic oscillations occur.
4. Discussion
In this paper, two fractional order African swine fever models are established to study the effect of media coverage. For the first model, no control measures are taken, and the existence and stability of equilibriums are analyzed. For the second model, the optimal solution is derived by using Pontryagin’s maximal principle.
The main results of the qualitative analysis and numerical simulation for system (1) are as follows.
- There always exists a unique positive solution for any positive initial value, and the set is positively invariant for this system. This conclusion is essential from a biological perspective.
- The basic reproduction number is obtained.
- The sufficient conditions for the existence and stability of the disease-free equilibrium and endemic equilibrium are derived.
- From Figure 1, Figure 2, Figure 3 and Figure 4, it can be observed that the initial value is not crucial and it does not affect the stability. This means that the initial value of susceptible pigs and diseased pigs is not a key factor. However, the value of is important, and it will affect the speed towards a stable state. This result indicates that the fractional order system is different from its corresponding integer order system.
- Figure 5 and Figure 6 show that both parameters d and are sensitive. In fact, d and have a significant effect on the basic reproduction number . In practice, we can reduce the value of by increasing the mortality rate of diseased pigs or increasing disinfection measures in pig houses, thereby achieving the goal of preventing the continued spread of the disease.
- Figure 7 shows that media coverage is a very useful measure to control the disease.
The main results of qualitative analysis and numerical simulation for system (2) are as follows.
- The formula of the optimal control solution and is obtained by using the Pontryagin’s maximum principle.
- Figure 9 indicates that media coverage combined with control measures (such as disinfection and sterilization) can suppress the spread of the disease more effectively.
5. Conclusions
As there are currently no effective drugs to treat ASF, preventive measures and biosecurity play an important role in preventing the outbreak of the epidemic. Research has demonstrated that the earlier control measures are taken, the easier it is to reduce the harm caused by the disease [13]. In this article, the effect of media coverage is investigated for the outbreak or elimination of ASF. Considering the memory advantage of fractional order systems, we introduced media coverage and optimal control into the mathematical model to simulate the development process of the disease. Through numerical simulations, we find that when pig farmers receive news from the media about the outbreak of ASF, timely measures such as clearing suspected diseased pigs and disinfecting pig farms can significantly reduce the number of infected pigs and reduce economic losses.
This article considers the dissemination role of media coverage on an epidemic. In fact, there are more professional tools available to monitor the spread of animal diseases, such as the Geographic Information System (GIS) software used in [45] and GIS and the Remote Sensing used in [46]. In addition, stochastic differential equations are also useful to describe the transmission of diseases. Thus, stochastic effects will be taken into consideration to construct more reasonable and realistic models in the future work.
Author Contributions
Each of the authors, R.S., Y.Z. and C.W., contributed to each part of this work equally and read and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The first author is supported by “Research Project Supported by Shanxi Scholarship Council of China (No. 2021-091)”. The second author is supported by “2022 Science and Technology Innovation Project for Shanxi Normal University”. The third author is supported by “National Natural Science Foundation of China (No. 61907027)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the manuscript.
Acknowledgments
The authors would like to thank Huaiping Zhu in York University for helpful suggestions on model formulation. We would also like to thank the anonymous reviewers for their helpful comments and suggestions, which greatly improved the quality of this paper.
Conflicts of Interest
The authors declare that they have no competing interest.
Appendix A. Proof of Theorem 1
Proof of Theorem 1.
Firstly, we will prove that the solution of system (1) with any positive initial values is always non-negative and bounded. From system (1), we easily obtain
According to Lemma 1 and the above inequalities, we have for any .
Since for any , then we have
provided that .
Since for any , then we obtain
provided that .
Secondly, we will prove that system (1) with any positive initial value has a unique solution.
Denote the right side of system (1) as vector function , and
where , , , , , , , . Then, system (1) can be written as
Obviously, the corresponding conditions (i)–(iii) of Theorem 3.1 in [39] are satisfied. Furthermore, we have
Thus, the fourth condition of Theorem 3.1 in [39] is also satisfied for system (1). According to that theorem, we know that system (1) has a unique positive solution for any positive initial value.
This completes the proof. □
Appendix B. Proof of Theorem 3
Proof of Theorem 3.
The Jacobian matrix of system (1), evaluated at the disease-free equilibrium , is given by
and the corresponding characteristic equation will be
It is easy to observe that is a negative eigenvalue, and the remaining eigenvalues satisfy the following equation
If the roots of Equation (A2) satisfy , then we have
Appendix C. Proof of Theorem 5
Proof of Theorem 5.
The corresponding characteristic equations will be
where
According to the Routh–Hurwitz criteria [42] and Lemma 4 in [43], this theorem is derived. □
Appendix D. Proof of Theorem 6
Proof of Theorem 6.
The adjoint equations and transversal conditions of optimal control problems can be easily obtained from Pontriagin’s maximum principle, as follows.
Adjoint equations
with transversal conditions
The optimal control pairs and can be derived by the following equations
Combining with the definition of optimal control solutions, we get the final formula as follows
This completes the proof. □
References
- Galindo, I.; Alonso, C. African swine fever virus: A Review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Zheng, H. Structure of African swine fever virus and associated molecular mechanisms underlying infection and immunosuppression: A Review. Front. Immunol. 2021, 12, 715582. [Google Scholar] [CrossRef] [PubMed]
- Barongo, M.B.; Karl, S.; Bernard, B.; Richard, P.B.; Eric, M.F.; Tony, A.; Edward, O.; Charles, M.; Darryn, K.; Amos, S.; et al. Estimating the basic reproductive number (R0) for African swine fever virus (ASFV) transmission between pig herds in uganda. PLoS ONE 2015, 10, e0125842. [Google Scholar] [CrossRef]
- Portugal, R.; Coelho, J.; Hoper, D.; Little, N.S.; Smithson, C.; Upton, C.; Martins, C.; Leitao, A.; Keil, G.M. Related strains of African swine fever virus with different virulence: Genome comparison and analysis. J. Gen. Virol. 2015, 96, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Halasa, T.; Btner, A.; Mortensen, S.; Christensen, H.; Boklund, A. Simulating the epidemiological and economic effects of an African swine fever epidemic in industrialized swine populations. Vet. Microbiol. 2016, 193, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Halasa, T.; Btner, A.; Mortensen, S.; Christensen, H.; Toft, N.; Boklund, A. Control of African swine fever epidemics in industrialized swine populations. Vet. Microbiol. 2016, 197, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Dione, M.M.; Dohoo, I.; Ndiwa, N.; Poole, J.; Ouma, E.; Amia, W.C.; Wieland, B. Impact of participatory training of smallholder pig farmers on knowledge, attitudes and practices regarding biosecurity for the control of African swine fever in Uganda. Transbound. Emerg. Dis. 2020, 67, 2269–3076. [Google Scholar] [CrossRef] [PubMed]
- Stoffel, C.; Buholzer, P.; Fanelli, A.; Nardi, M.D. Analysis of the drivers of ASF introduction into the officially approved pig compartments in South Africa and implications for the revision of biosecurity standards. Porc. Health Manag. 2022, 8, 43. [Google Scholar] [CrossRef]
- Boinas, F.S.; Wilson, A.J.; Hutchings, G.H.; Martins, C.; Dixon, L.J. The persistence of African swine fever virus in field-infected ornithodoros erraticus during the ASF endemic period in Portugal. PLoS ONE 2011, 6, 20383. [Google Scholar] [CrossRef]
- Jori, F.; Vial, L.; Penrith, M.; Pérez-Sánchez, R.; Etter, E.; Albina, E.; Michaud, V.; Roger, F. Review of the sylvatic cycle of African swine fever in sub-Saharan Africa and the Indian ocean. Virus Res. 2013, 173, 212–227. [Google Scholar] [CrossRef]
- Frant, M.; Woźniakowski, G.; Pejsak, Z. African swine fever (ASF) and ticks. No risk of tick-mediated ASF spread in Poland and Baltic states. J. Vet. Res. 2017, 61, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Kouidere, A.; Balatif, O.; Rachik, M. Analysis and optimal control of a mathematical modeling of the spread of African swine fever virus with a case study of South Korea and cost-effectiveness. Chaos Soliton Fract. 2021, 146, 110867. [Google Scholar] [CrossRef]
- Barongo, M.B.; Bishop, R.P.; Fèvre, E.M.; Knobel, D.L.; Ssematimba, A. A mathematical model that simulates control options for African swine fever virus (ASFV). PLoS ONE 2016, 11, e0158658. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Rong, X.; Li, J.; Fan, M.; Zhu, H. Modeling the outbreak and control of African swine fever virus in large-scale pig farms. J. Theor. Biol. 2021, 526, 110798. [Google Scholar] [CrossRef]
- Song, H.; Li, J.; Jin, Z. Nonlinear dynamic modelling and analysis of African swine fever with culling in China. Commun. Nonlinear Sci. 2023, 117, 106915. [Google Scholar] [CrossRef]
- Shi, R.; Li, Y.; Wang, C. Stability analysis and optimal control of a fractional-order model for African swine fever. Virus Res. 2020, 288, 198111. [Google Scholar] [CrossRef]
- Buonomo, B.; D’Onofrio, A.; Lacitignola, D. Globally stable endemicity for infectious diseases with information-related changes in contact patterns. Appl. Math. Lett. 2012, 25, 1056–1060. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Manfredi, P. Information-related changes in contactpatterns may trigger oscillations in the endemic prevalence of infectious diseases. J. Theor. Biol. 2009, 256, 473–478. [Google Scholar] [CrossRef]
- Vargas-De-Leon, C.; D’Onofrio, A. Global stability of infectious disease models with contact rate as a function of prevalence index. Math. Biosci. Eng. 2017, 14, 1019–1033. [Google Scholar] [CrossRef]
- Singh, S.; Chandra, P.; Shukla, J.B. Modeling and analysis of the spread of carrier dependent infectious diseases with environmental effects. J. Biol. Syst. 2008, 11, 325–335. [Google Scholar] [CrossRef]
- Gani, S.R.; Halawar, S.V. Optimal control for the spread of infectious disease: The role of awareness programs by media and antiviral treatment. Optim. Control Appl. Methods 2018, 39, 1407–1430. [Google Scholar] [CrossRef]
- Misra, A.K.; Sharma, A.; Shukla, J.B. Modeling and analysis of effects of awareness programs by media on the spread of infectious diseases. Math. Comput. Model. 2011, 53, 1221–1228. [Google Scholar] [CrossRef]
- Zuo, L.; Liu, M.; Wang, J. The impact of awareness programs with recruitment and delay on the spread of an epidemic. Math. Probl. Eng. 2015, 2015, 235935. [Google Scholar] [CrossRef]
- Juher, D.; Kiss, I.Z.; Salda, A.J. Analysis of an epidemic model with awareness decay on regular random networks. J. Theor. Biol. 2015, 365, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Sun, Y.; Zhu, H. The impact of media on the control of infectious diseases. J. Dyn. Differ. Equ. 2008, 20, 31–53. [Google Scholar] [CrossRef]
- Tchuenche, J.M.; Dube, N.; Bhunu, C.P.; Smith, R.J.; Bauch, C.T. The impact of media coverage on the transmission dynamics of human influenza. BMC Public Health 2011, 11, S5. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, M. Global Hopf bifurcation analysis of an susceptible-infective-removed epidemic model incorporating media coverage with time delay. J. Biol. Dynam. 2017, 11, 8–24. [Google Scholar] [CrossRef]
- Huang, C.; Cai, L.; Cao, J. Linear control for synchronization of a fractional-order time-delayed chaotic financial system. Chaos Soliton Fract. 2018, 113, 326–332. [Google Scholar] [CrossRef]
- Rihan, F.A.; Abdel Rahman, D.H.; Lakshmanan, S.; Alkhajeh, A.S. A time delay model of tumour-immune system interactions: Global dynamics, parameter estimation, sensitivity analysis. Appl. Math. Comput. 2014, 232, 606–623. [Google Scholar] [CrossRef]
- Vvatsala, A.L. Basic theory of fractional differential equations. Nonlinear Anal. Theor. Methods Appl. 2008, 69, 2677–2682. [Google Scholar]
- Rakkiyappan, R.; Velmurugan, G.; Cao, J. Stability analysis of fractional-order complex-valued neural networks with time delays. Chaos Soliton Fract. 2015, 78, 297–316. [Google Scholar] [CrossRef]
- Alidousti, J.; Ghaziani, R.K. Spiking and bursting of a fractional order of the modified FitzHugh-Nagumo neuron model. Math. Models Comput. Simul. 2017, 9, 390–403. [Google Scholar] [CrossRef]
- Djordjevic, V.D.; Jaric, J.; Fabry, B.; Fredberg, J.J. Fractional derivatives embody essential features of cell rheological behavior. Ann. Biomed. Eng. 2003, 31, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. 2022. Available online: http://www.moa.gov.cn/ztzl/fzzwfk/yqxx/ (accessed on 20 March 2022).
- Gao, D.; Ruan, S. An SIS patch model with variable transmission coefficients. Math. Biosci. 2011, 232, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yang, W.; Arino, J.; Khan, K. Effect of media-induced social distancing on disease transmission in a two patch setting. Math. Biosci. 2011, 230, 87–95. [Google Scholar] [CrossRef]
- Capasso, V.; Serio, G. A generalization of the Kermack-McKendrick deterministic epidemic model. Math. Biosci. 1978, 42, 43–61. [Google Scholar] [CrossRef]
- Diethelm, K. Monotonicity of functions and sign changes of their Caputo derivatives. Fract. Calc. Appl. Anal. 2016, 19, 561–566. [Google Scholar] [CrossRef]
- Wei, L. Global existence theory and chaos control of fractional differential equations. J. Math. Anal. Appl. 2007, 332, 709–726. [Google Scholar]
- Driessche, P. Reproduction numbers of infectious disease models. Infect. Dis. Model. 2017, 2, 288–303. [Google Scholar] [CrossRef]
- Akman Yıldız, T.; Arshad, S.; Baleanu, D. New observations on optimal cancer treatments for a fractional tumor growth model with and without singular kernel. Chaos Soliton Fract. 2018, 117, 226–239. [Google Scholar] [CrossRef]
- Ahmed, E.; Ama, E.S.; Haa, E.S. On some Routh-Hurwitz conditions for fractional order differential equations and their applications in Lorenz, Rssler, Chua and Chen systems. Phys. Lett. A 2006, 358, 1–4. [Google Scholar] [CrossRef]
- Pinto, C.; Carvalho, A. A latency fractional order model for HIV dynamics. J. Comput. Appl. Math. 2017, 312, 240–256. [Google Scholar] [CrossRef]
- Göllmann, L.; Kern, D.; Maurer, H. Optimal control problems with delays in state and control variables subject to mixed control–Cstate constraints. Optim. Control Appl. Methods 2010, 30, 341–365. [Google Scholar] [CrossRef]
- Catalano, S.; La Morgia, V.; Molinar Min, A.R.; Fanelli, A.; Meneguz, P.G.; Tizzani, P. Gastrointestinal Parasite Community and Phenotypic Plasticity in Native and Introduced Alien Lagomorpha. Animals 2022, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- Viani, A.; Orusa, T.; Borgogno-Mondino, E.; Orusa, R. Snow Metrics as Proxy to Assess Sarcoptic Mange in Wild Boar: Preliminary Results in Aosta Valley (Italy). Life 2023, 13, 987. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).