Identification of Potential miRNA-mRNA Regulatory Network Associated with Regulating Immunity and Metabolism in Pigs Induced by ASFV Infection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. RNA Extraction and Quality Detection
2.3. Establishment of an sRNA Sequencing Library and Sequencing
2.4. Bioinformatic Analysis
2.5. Target Gene Prediction and Enrichment of Differentially Expressed miRNA
2.6. Combined Analysis of Differentially Expressed miRNAs and Target Gene mRNA
2.7. Verification of Differentially Expressed miRNA by Real-Time Quantitative Polymerase Chain Reaction (qPCR)
3. Results
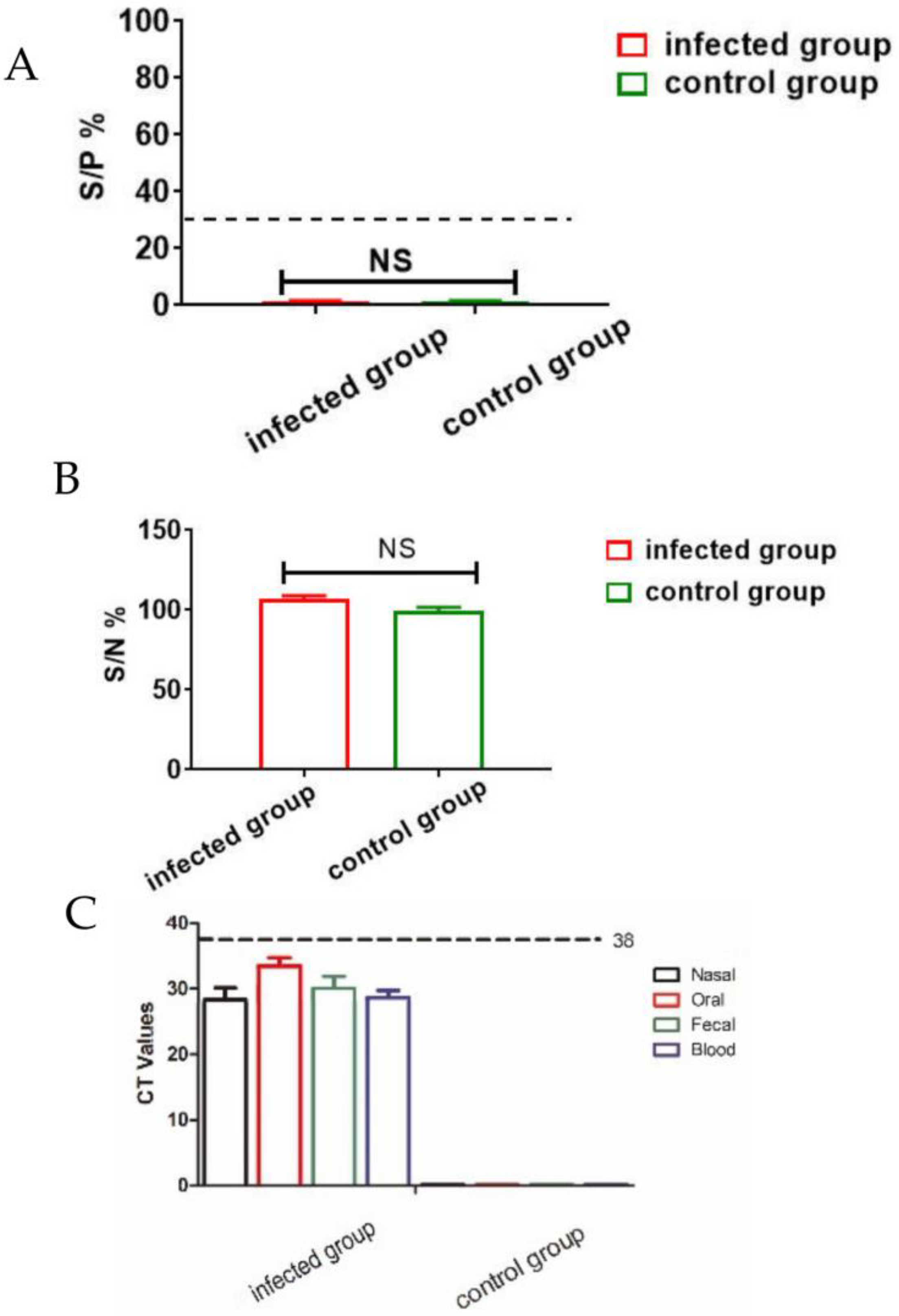
3.1. Clinical Sample Analysis via qPCR and ELISA
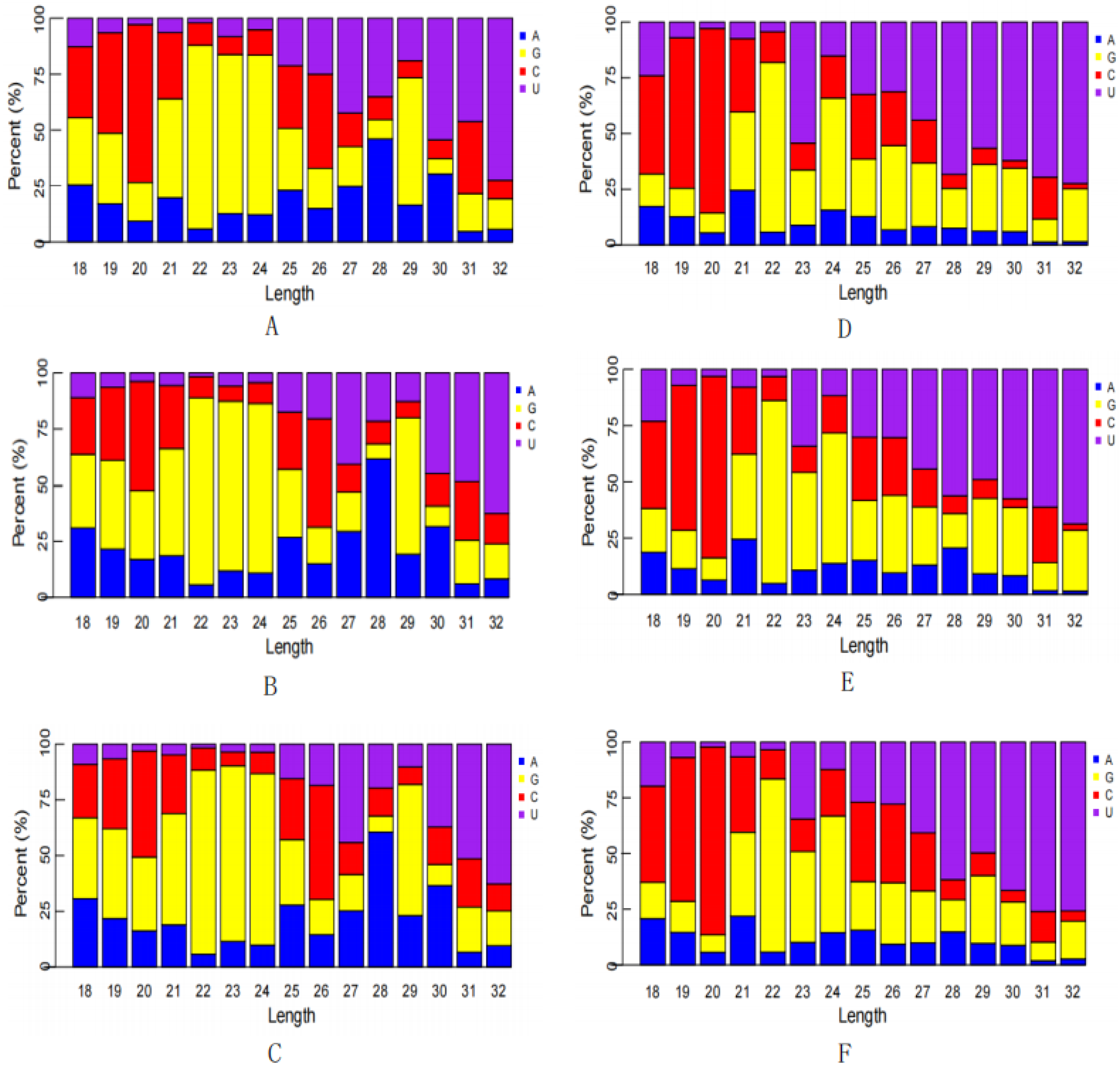
3.2. Establishment of an sRNA Sequencing Library and Sequencing
3.3. Database Comparison
3.4. miRNAs Differentially Expressed in the Transcriptome
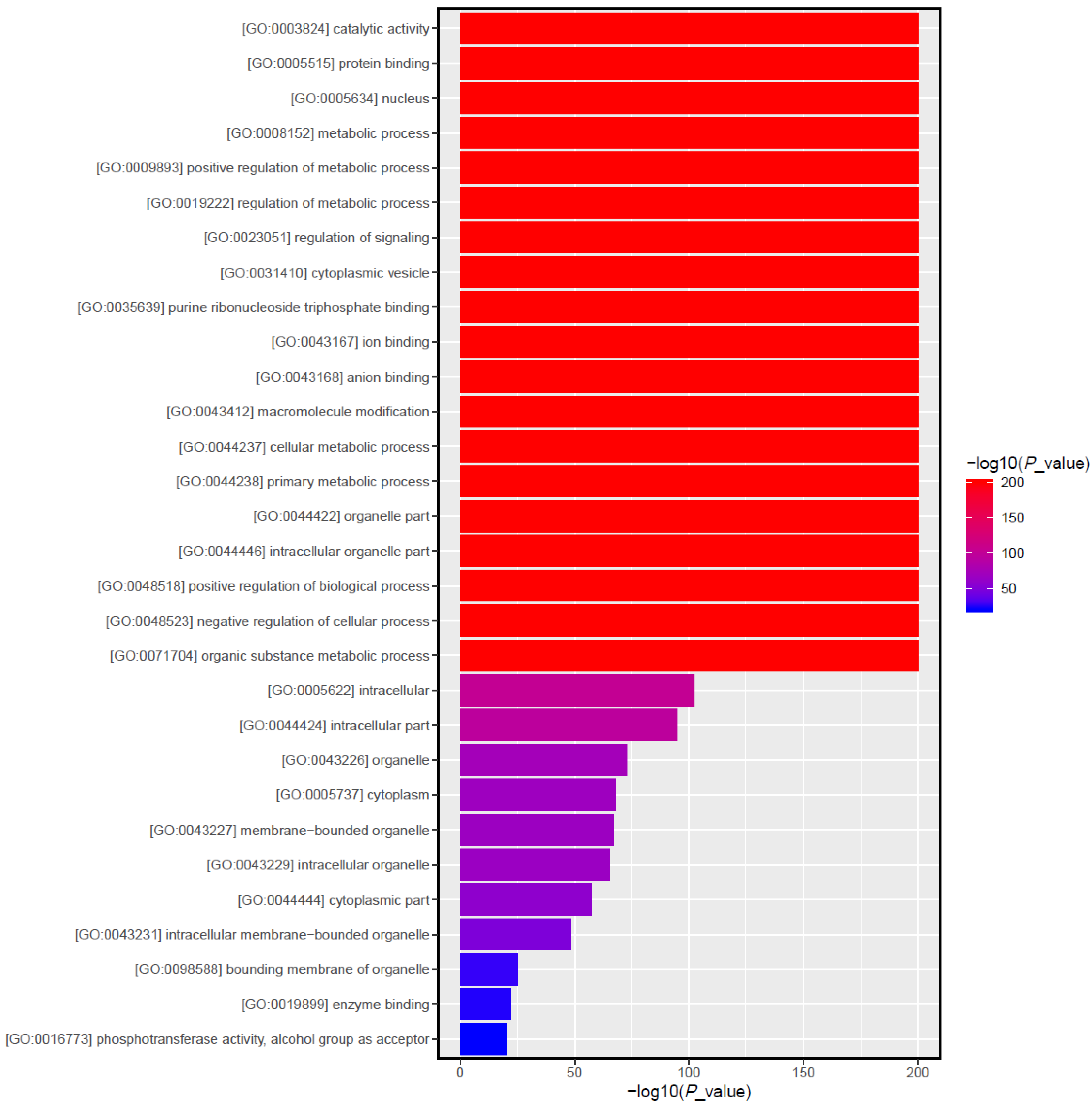
3.5. Target Gene Prediction and Function Enrichment Analyses
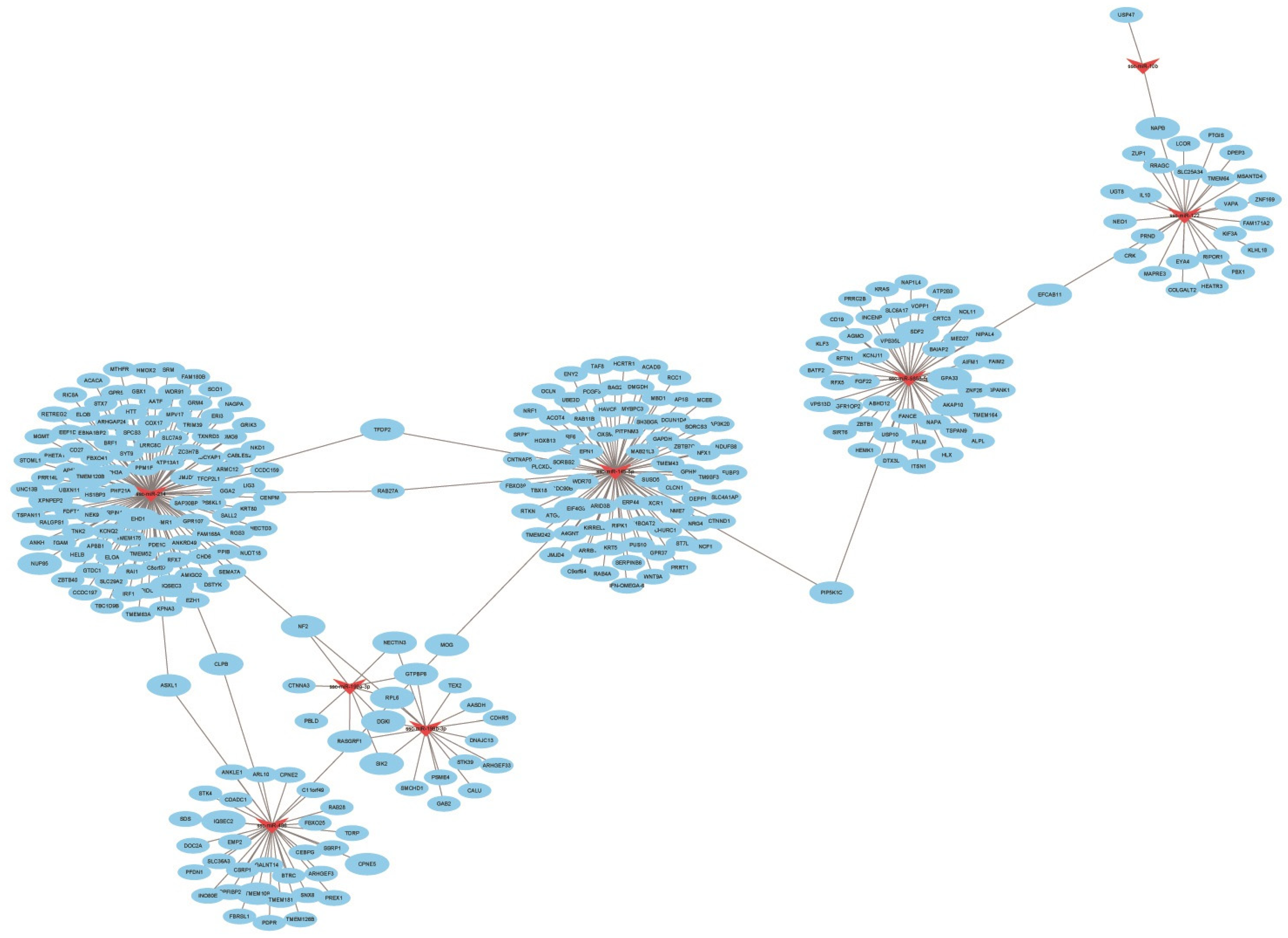
3.6. Combined Analysis of Differentially Expressed miRNAs and Target Gene mRNA
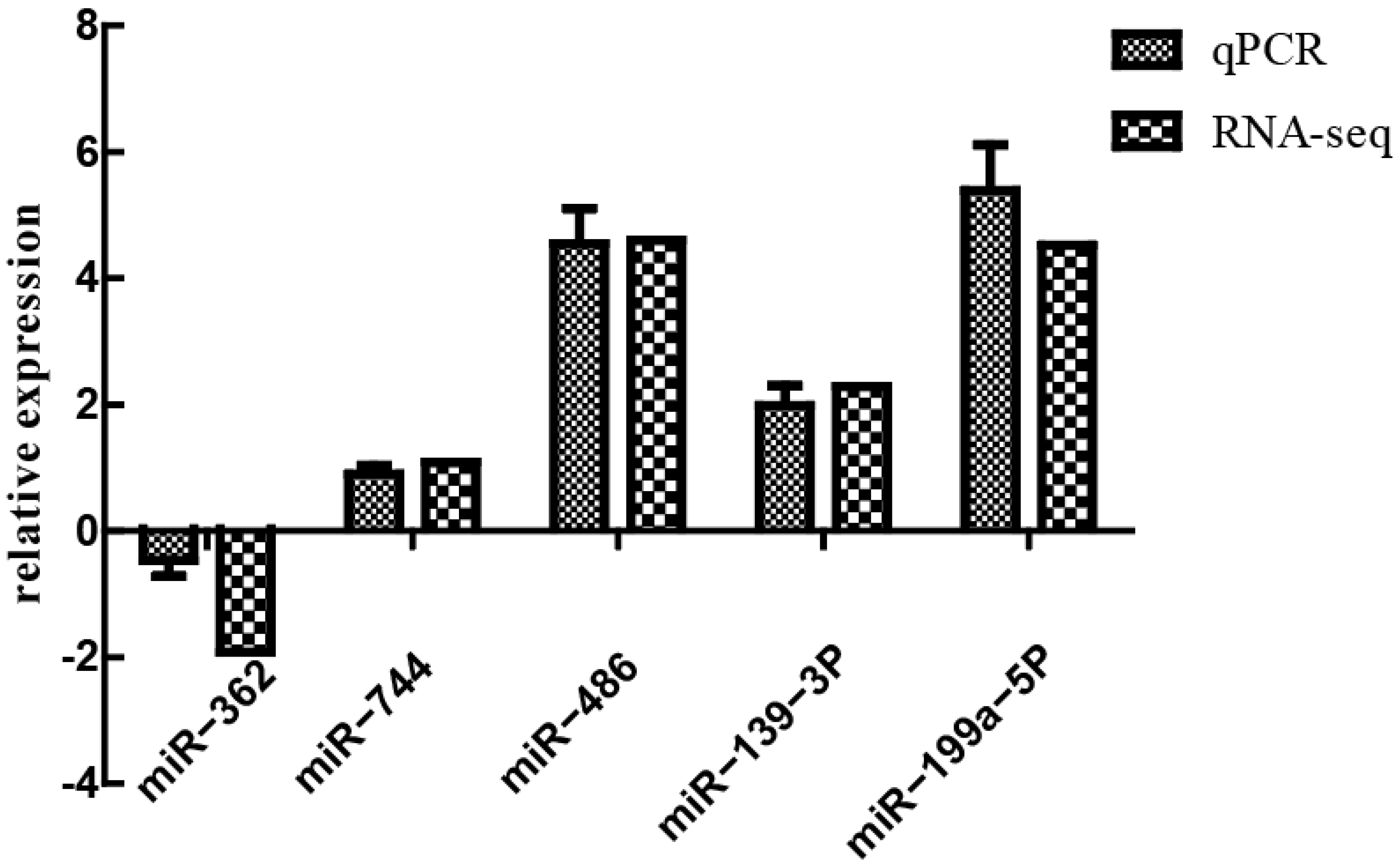
3.7. Verification of Differentially Expressed miRNA by qPCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, W.-R.; Yuan, J.; Ma, Y.-H.; Zhao, C.-Y.; Yang, Z.-Y.; Zhang, Y.; Han, S.; Wan, B.; Zhang, G.-P. Modulation of Host Antiviral Innate Immunity by African Swine Fever Virus: A Review. Animals 2022, 12, 2935. [Google Scholar] [CrossRef]
- Karger, A.; Pérez-Núñez, D.; Urquiza, J.; Hinojar, P.; Alonso, C.; Freitas, F.B.; Revilla, Y.; Lepotier, M.-F.; Montoya, M. An update on African swine fever virology. Viruses 2019, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- Jaing, C.; Rowland, R.R.; Allen, J.E.; Certoma, A.; Thissen, J.B.; Bingham, J.; Rowe, B.; White, J.R.; Wynne, J.W.; Johnson, D. Gene expression analysis of whole blood RNA from pigs infected with low and high pathogenic African swine fever viruses. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yanez, R.J.; Rodríguez J., M.; Nogal, M.L.; Yuste, L.; Enríquez, C.; Rodriguez, J.F.; Vinuela, E. Analysis of the complete nucleotide sequence of African swine fever virus. Virology 1995, 208, 249–278. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.E. On a form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Giammarioli, M.; Gallardo, C.; Oggiano, A.; Iscaro, C.; Nieto, R.; Pellegrini, C.; Del Giudici, S.; Arias, M.; De Mia, G.M. Genetic characterisation of African swine fever viruses from recent and historical outbreaks in Sardinia (1978–2009). Virus Genes 2011, 42, 377–387. [Google Scholar] [CrossRef]
- Sánchez-Vizcaíno, J.; Mur, L.; Martínez-López, B. African swine fever: An epidemiological update. Transbound. Emerg. Dis. 2012, 59, 27–35. [Google Scholar] [CrossRef]
- Cisek, A.A.; Dabrowska, I.; Gregorczyk, K.P.; Wyzewski, Z. African swine fever virus: A new old enemy of Europe. Ann. Parasitol. 2016, 62, 161–167. [Google Scholar]
- Das, S.; Deka, P.; Deka, P.; Kalita, K.; Ansari, T.; Hazarika, R.; Barman, N.N. African swine fever: Etiology, epidemiology, control strategies and progress toward vaccine development: A comprehensive review. J. Entomol. Zool. Stud. 2021, 9, 919–929. [Google Scholar]
- Núñez-Hernández, F.; Pérez, L.J.; Muñoz, M.; Vera, G.; Accensi, F.; Sánchez, A.; Rodríguez, F.; Núñez, J.I. Differential expression of porcine microRNAs in African swine fever virus infected pigs: A proof-of-concept study. Virol. J. 2017, 14, 1–13. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African swine fever virus: An emerging DNA arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Wu, Y.; Chen, H.; Zhang, S.; Li, J.; Xin, T.; Jia, H.; Hou, S.; Jiang, Y. Inhibition of cGAS-STING-TBK1 signaling pathway by DP96R of ASFV China 2018/1. Biochem. Biophys. Res. Commun. 2018, 506, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, W.; Li, L.; Li, P.; Ma, Z.; Zhang, J.; Qi, X.; Ren, J.; Ru, Y.; Niu, Q. African swine fever virus MGF-505-7R negatively regulates cGAS–STING-mediated signaling pathway. J. Immunol. 2021, 206, 1844–1857. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Ramanathan, P.; Bishop, E.A.; O’donnell, V.; Gladue, D.P.; Borca, M.V. Mechanisms of African swine fever virus pathogenesis and immune evasion inferred from gene expression changes in infected swine macrophages. PLoS ONE 2019, 14, e0223955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Li, D.; Zheng, H. Structure of African swine fever virus and associated molecular mechanisms underlying infection and immunosuppression: A review. Front. Immunol. 2021, 12, 715582. [Google Scholar] [CrossRef]
- Urbano, A.C.; Ferreira, F. African swine fever control and prevention: An update on vaccine development. Emerg. Microbes Infect. 2022, 11, 2021–2033. [Google Scholar] [CrossRef]
- Sirakanyan, S.; Arabyan, E.; Hakobyan, A.; Hakobyan, T.; Chilingaryan, G.; Sahakyan, H.; Sargsyan, A.; Arakelov, G.; Nazaryan, K.; Izmailyan, R. A new microtubule-stabilizing agent shows potent antiviral effects against African swine fever virus with no cytotoxicity. Emerg. Microbes Infect. 2021, 10, 783–796. [Google Scholar] [CrossRef]
- Bohnsack, M.T.; Czaplinski, K.; Görlich, D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. Rna 2004, 10, 185–191. [Google Scholar] [CrossRef]
- Yi, R.; Doehle, B.P.; Qin, Y.; Macara, I.G.; Gullen, B.R. Overexpression of exportin 5 enhances RNA interference mediated by short hairpin RNAs and microRNAs. RNA 2005, 11, 220–226. [Google Scholar] [CrossRef]
- Moran, Y.; Agron, M.; Praher, D.; Technau, U. The evolutionary origin of plant and animal microRNAs. Nat. Ecol. Evol. 2017, 1, 0027. [Google Scholar] [CrossRef]
- Umbach, J.L.; Cullen, B.R. The role of RNAi and microRNAs in animal virus replication and antiviral immunity. Genes Dev. 2009, 23, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Li, F.; Li, J.; Wu, C.; Xiang, G.; Zhao, X.; Nan, Y.; Zhao, D.; Ding, Q. Genome-wide transcriptomic analysis of highly virulent African swine fever virus infection reveals complex and unique virus host interaction. Vet. Microbiol. 2021, 261, 109211. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Moreno, R.; Galindo, I.; Cuesta-Geijo, M.Á.; Barrado-Gil, L.; Alonso, C. Host cell targets for African swine fever virus. Virus Res. 2015, 209, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.; Sul, H.S. Insulin signaling in fatty acid and fat synthesis: A transcriptional perspective. Curr. Opin. Pharmacol. 2010, 10, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; Matsuki, Y.; Murakami, M.; Ichisaka, T.; Murakami, H. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 2005, 307, 426–430. [Google Scholar] [CrossRef]
- Dunn, L.E.; Ivens, A.; Netherton, C.L.; Chapman, D.A.; Beard, P.M. Identification of a functional small noncoding RNA of African swine fever virus. J. Virol. 2020, 94, e01515-20. [Google Scholar] [CrossRef]
- Dell'angelica, E.C.; Mullins, C.; Caplan, S.; Bonifacino, J.S. Lysosome-related organelles. FASEB J. 2000, 14, 1265–1278. [Google Scholar]
- Chen, J.; Shi, X.; Zhang, X.; Wang, A.; Wang, L.; Yang, Y.; Deng, R.; Zhang, G.-P. MicroRNA 373 facilitates the replication of porcine reproductive and respiratory syndrome virus by its negative regulation of type I interferon induction. J. Virol. 2017, 91, e01311-16. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Zhao, J.; Fang, L.; Fang, R.; Xiao, J.; Chen, X.; Zhou, A.; Zhang, Y.; Ren, L. Difference in microRNA expression and editing profile of lung tissues from different pig breeds related to immune responses to HP-PRRSV. Sci. Rep. 2015, 5, 9549. [Google Scholar] [CrossRef]
- Takata, A.; Otsuka, M.; Kojima, K.; Yoshikawa, T.; Kishikawa, T.; Yoshida, H.; Koike, K. MicroRNA-22 and microRNA-140 suppress NF-κB activity by regulating the expression of NF-κB coactivators. Biochem. Biophys. Res. Commun. 2011, 411, 826–831. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Chen, H.; Zhu, H.; Li, J.; Guo, X. Research progress on immune evasion proteins of African swine fever virus. Chin. J. Virol. 2018, 34, 929–935. [Google Scholar]
- Zhang, F.; Li, D.; Wu, Q.; Sun, J.; Guan, W.; Hou, Y.; Zhu, Y.; Wang, J. Prepartum body conditions affect insulin signaling pathways in postpartum adipose tissues in transition dairy cows. J. Anim. Sci. Biotechnol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Song, J.-W.; Lin, J.-Y.; Miao, R.; Zhong, J.-C. Roles of microRNA-122 in cardiovascular fibrosis and related diseases. Cardiovasc. Toxicol. 2020, 20, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Shen, C.; Zhang, D.; Zhang, T.; Shi, X.; Yang, J.; Hao, Y.; Zhao, D.; Cui, H.; Yuan, X. Mechanism of interaction between virus and host is inferred from the changes of gene expression in macrophages infected with African swine fever virus CN/GS/2018 strain. Virol. J. 2021, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fang, J.; Zuo, Z.; Yin, S.; He, T.; Yang, M.; Deng, J.; Shen, L.; Ma, X.; Yu, S. Activation of porcine alveolar macrophages by Actinobacillus pleuropneumoniae lipopolysaccharide via the toll-like receptor 4/NF-κB-Mediated pathway. Infect. Immun. 2018, 86, e00642-17. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Galindo, I.; Cuesta-Geijo, M.A.; Cabezas, M.; Hernaez, B.; Munoz-Moreno, R. African swine fever virus-cell interactions: From virus entry to cell survival. Virus Res. 2013, 173, 42–57. [Google Scholar] [CrossRef]
- Jiao, Y.; Hao, L.; Xia, P.; Cheng, Y.; Song, J.; Chen, X.; Wang, Z.; Ma, Z.; Zheng, S.; Chen, T. Identification of Potential miRNA-mRNA Regulatory Network Associated with Pig Growth Performance in the Pituitaries of Bama Minipigs and Landrace Pigs. Animals 2022, 12, 3058. [Google Scholar] [CrossRef]



| miRNA | ||
|---|---|---|
| Primary Sequences (5′→3′) | Primer Sequences (5′→3′) | |
| miR-362 | AAUCCUUGGAACCUAGGUGUGAGUG | CGAATCCTTGGAACCTAGGTG |
| miR-744 | UGCGGGGCUAGGGCUAACAGCA | GTGCGGGGCTAGGGCTA |
| miR-486 | UCCUGUACUGAGCUGCCCCGAG | CGCGTCCTGTACTGAGCTGC |
| miR-139-3P | UGGAGACGCGGCCCUGUUGGAGU | GGAGACGCGGCCCTGT |
| miR-199a-5P | CCCAGUGUUCAGACUACCUGUUC | CGCGCCCAGTGTTCAGACTAC |
| miR-16 | CCCAGUGUUCAGACUACCUGUUC | CGCGTAGCAGCACGTAAATA |
| Sample | Control Group | Infected Group | ||||
|---|---|---|---|---|---|---|
| NC-1 | NC-2 | NC-3 | YD-1 | YD-2 | YD-3 | |
| Total bases | 1,427,067,450 | 1,231,831,950 | 3,334,317,150 | 3,180,968,400 | 3,126,361,800 | 4,273,526,700 |
| Clean reads | 3,526,712 | 3,820,877 | 10,695,589 | 10,430,439 | 9,873,282 | 12,949,329 |
| Error % | 0.0322 | 0.0393 | 0.0532 | 0.0524 | 0.0356 | 0.0333 |
| Q20% | 94.63 | 91.85 | 86.33 | 86.76 | 93.34 | 94.21 |
| Q30% | 88.95 | 85.02 | 80.45 | 80.5 | 86.83 | 88.23 |
| GC% | 62.41 | 62.67 | 64.17 | 67.43 | 67.41 | 67.22 |
| miRNA | Control Group | Infected Group | log2 Fold Change (Control/Experimental) | p-Value | Up- or Downregulation |
|---|---|---|---|---|---|
| ssc-miR-486 | 1520.86 | 62.58 | 4.602820854 | 1.35 × 10−20 | up |
| ssc-miR-451 | 4920.973333 | 309.1666667 | 3.992443281 | 3.20 × 10−13 | up |
| ssc-miR-199a-5p | 74.52666667 | 3.233333333 | 4.522401093 | 3.40 × 10−7 | up |
| ssc-miR-199a-3p | 116.51 | 6.91 | 4.073661759 | 8.58 × 10−7 | up |
| ssc-miR-199b-3p | 116.51 | 6.91 | 4.073661759 | 8.58 × 10−7 | up |
| ssc-miR-122 | 205.08 | 0.053333333 | 11.66100452 | 1.42 × 10−5 | up |
| ssc-miR-10b | 528.52 | 22.75333333 | 4.537201625 | 0.000154438 | up |
| ssc-miR-9858-5p | 10.74666667 | 0.556666667 | 4.246586926 | 0.000620378 | up |
| ssc-miR-2366 | 6.616666667 | 0.22 | 4.848577585 | 0.000771554 | up |
| ssc-miR-145-5p | 16.6 | 0.433333333 | 5.227516423 | 0.001947544 | up |
| ssc-miR-214 | 6.63 | 0.22 | 4.851477475 | 0.008490924 | up |
| ssc-miR-374a-3p | 100.05 | 524.2333333 | −2.389371253 | 3.63 × 10−19 | down |
| ssc-miR-142-3p | 1853.07 | 8993.783333 | −2.279004567 | 2.74 × 10−11 | down |
| ssc-miR-3613 | 4.34 | 24.9 | −2.517637716 | 1.75 × 10−6 | down |
| ssc-miR-374b-3p | 3.536666667 | 18.32 | −2.369671136 | 0.000189815 | down |
| ssc-miR-450c-5p | 26.29 | 120.34 | −2.194101437 | 0.000543707 | down |
| Downregulated miRNA after Infection | Upregulation of mRNA |
|---|---|
| ssc-miR-450c-5p | GRIA3, GTF3C1 |
| ssc-miR-374b-3p | HOOK2, STUM |
| Upregulated miRNA after Infection | Downregulation of mRNA |
|---|---|
| ssc-miR-9858-5p | FAIM2, CDC25B, MAP2K4, IL17C, NOL11, SLC27A4, IL12RB2, BCL2L12, RPS15A |
| ssc-miR-195 | MAP4, ABI2, MAPK10 |
| ssc-miR-122 | IL10, IFN-OMEGA-6, SLC8A3 |
| ssc-miR-199b-3p | CAMK4, IRAG1, STAB1 |
| ssc-miR-199a-3p | ARPC4 |
| ssc-miR-10b | CCDC88C, THAP1, CCDC88C |
| ssc-miR-145-5p | JAM2, TRAF3, CD79B, SLC1A2, IL17RB, SLC44A2, IFT80, TLE3 |
| ssc-miR-214 | SLC7A1, IL12RB1, TNK2, TLCD4, IRF1, MAPK10, TLR4, TRIM59, IARS2, TRPM7, LRATD2 |
| ID | Term | Counts | Gene Symbol |
|---|---|---|---|
| map04010 | MAPK signaling pathway | 2 | CDC25B, MAP2K4 |
| ko04620 | Toll-like receptor signaling pathway | 9 | MAP2K4, MAPK10, TRAF3, IRF7, AK4, MAP3K8, TLR4, TLR8, TLR5 |
| ko04668 | TNF signaling pathway | 4 | NFKB1, TRAF3, TNF, IRF1 |
| ko04657 | IL-17 signaling pathway | 3 | IL17B, IL17C, NFKB1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Z.; Chen, S.; Cui, S.; Zhai, W.; Huang, Y.; Gao, X.; Wang, Y.; Jiang, F.; Guo, X.; Hao, Y.; et al. Identification of Potential miRNA-mRNA Regulatory Network Associated with Regulating Immunity and Metabolism in Pigs Induced by ASFV Infection. Animals 2023, 13, 1246. https://doi.org/10.3390/ani13071246
Pang Z, Chen S, Cui S, Zhai W, Huang Y, Gao X, Wang Y, Jiang F, Guo X, Hao Y, et al. Identification of Potential miRNA-mRNA Regulatory Network Associated with Regulating Immunity and Metabolism in Pigs Induced by ASFV Infection. Animals. 2023; 13(7):1246. https://doi.org/10.3390/ani13071246
Chicago/Turabian StylePang, Zhongbao, Shiyu Chen, Shuai Cui, Wenzhu Zhai, Ying Huang, Xintao Gao, Yang Wang, Fei Jiang, Xiaoyu Guo, Yuxin Hao, and et al. 2023. "Identification of Potential miRNA-mRNA Regulatory Network Associated with Regulating Immunity and Metabolism in Pigs Induced by ASFV Infection" Animals 13, no. 7: 1246. https://doi.org/10.3390/ani13071246
APA StylePang, Z., Chen, S., Cui, S., Zhai, W., Huang, Y., Gao, X., Wang, Y., Jiang, F., Guo, X., Hao, Y., Li, W., Wang, L., Zhu, H., Wu, J., & Jia, H. (2023). Identification of Potential miRNA-mRNA Regulatory Network Associated with Regulating Immunity and Metabolism in Pigs Induced by ASFV Infection. Animals, 13(7), 1246. https://doi.org/10.3390/ani13071246





